Back to Journals » Journal of Pain Research » Volume 13
Improvement of Pain and Function After Use of a Topical Pain Relieving Patch: Results of the RELIEF Study
Authors Gudin JA, Dietze DT , Hurwitz PL
Received 19 April 2020
Accepted for publication 19 June 2020
Published 26 June 2020 Volume 2020:13 Pages 1557—1568
DOI https://doi.org/10.2147/JPR.S258883
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Michael Schatman
Jeffrey A Gudin,1 Derek T Dietze,2 Peter L Hurwitz3
1Englewood Hospital Medical Center, Englewood, NJ; Rutgers New Jersey Medical School, Department of Anesthesiology, Newark, NJ, USA; 2Metrics for Learning LLC, Queen Creek, AZ, USA; 3Clarity Science LLC, Narragansett, RI, USA
Correspondence: Peter L Hurwitz
Clarity Science LLC, 750 Boston Neck Road, Suite 11, Narragansett, RI 02882, USA
Tel +1917-757-0521
Fax +1855-891-8303
Email [email protected]
Purpose: Pain is the most common reason for patients to consult primary care providers. Identification of effective treatments with minimal adverse events is critical to safer opioid-sparing and multi-modal approaches to pain treatment. Topical analgesic patches target medication to peripheral sites of pain while potentially avoiding adverse effects associated with systemic medications. Opioids, prescription nonsteroidal anti-inflammatory drugs, and over-the-counter oral medications are associated with systemic toxicities, increasing morbidity and mortality. This study evaluated a topical analgesic pain-relieving patch in reducing pain severity and improving function in patients with mild to moderate arthritic, neurological, or musculoskeletal pain.
Patients and Methods: This Institutional Review Board-approved study evaluated the effectiveness of a topical pain-relieving patch in reducing Brief Pain Inventory (BPI) scores in patients. The treatment group (TG) (n=152) received patches for 14 days. A control group (CG) (n=47) did not receive the patch. After day 14, 34 CG patients crossed over to treatment (CROSSG) with the patch. Surveys were administered to patients at baseline and 14 days to assess changes in pain severity and interference. Changes in oral pain medication use, side effects, and satisfaction use were also assessed.
Results: Paired data were collected in the CG, TG and CROSSG. At day 14, TG pain severity score and pain interference score decreased (49% and 58.1%, respectively). Pain severity and interference scores decreased less in the CG (12.3% and 14.8%, respectively). In the study, 60.5% of the TG were using concomitant oral pain medications “a lot less”, and 90.8% were very/extremely satisfied with the patch. CROSSG patients showed similar reductions in pain severity and interference scores after patch treatment. No side effects of treatment were reported.
Conclusion: Results indicate that this topical analgesic pain-relieving patch can reduce BPI pain severity and interference scores in adult patients with mild to moderate arthritic, neurological, and musculoskeletal pain and should be considered as a treatment option.
Keywords: methyl salicylate, menthol, analgesic, Salonpas
Introduction
Pain is the most common reason for patients to consult primary care providers.1,14 Evaluation of effective treatments for pain relief is critical to identify safer opioid-sparing approaches to pain treatment. Chronic pain is often treated by oral opioids, yet data15 suggest that patients still do not achieve adequate pain relief. Opioid based, nonsteroidal anti-inflammatory drugs, and common, over-the-counter oral medications have been associated with end-organ dysfunction including gastrointestinal (GI), cardiovascular (CV), renal and hepatic events.16,21 Alternative pain relief options have become increasingly common and include a combination of pharmacotherapy and non-pharmacological interventions.
Successful pain treatments, including multi-modal strategies, can address negative effects associated with chronic pain conditions, including a patient’s quality-of-life,6,7,22,25 psychological well-being, and their everyday functional abilities.14,26,27
National initiatives that include new guidelines and strategies have recently been published highlighting safe and effective pain management options. One group of therapies that have been suggested as a first-line treatment option are topical analgesics, a targeted therapy aimed at the location of pain. Previous research supports that topical agents are effective and safe for patients with chronic pain.17,21 By targeting the nociceptor pathways,28 topical formulations have reduced risk of systemic adverse events because of significantly less plasma exposure, bypassing absorption in the gastrointestinal (GI) track, while delivering effective concentrations in the targeted tissues.29,30 Limited drug–drug interactions, less potential for misuse, ease of application, direct application to the targeted site of pain, patient satisfaction, and success in treating the patient’s pain can provide advantages over systemically delivered oral medications.17,21,31,35
Although adverse GI and renal side effects appear reduced with topical versus oral diclofenac, the US Food and Drug Administration (FDA) mandates that warnings be placed on all NSAID products about the risks of GI and CV adverse effects.
This Relieving Pain: Evaluating Patient Quality of Life Improvement – Perceptions, Experience and Feedback After Use of A Topical Pain Relief Patch (RELIEF) study evaluated patients with mild to moderate and acute and chronic pain conditions and their overall perceptions of pain treatment and associated symptoms with the use of a specific topical pain-relieving patch (Salonpas® Pain Relieving Patch. Hisamitsu Pharmaceutical Company, Inc, Japan). This formulation is an over-the-counter (OTC) analgesic topical pain patch that includes menthol, camphor, and methyl salicylate as the active ingredients. Menthol and camphor are topical analgesics which provide analgesia by activating and then desensitizing epidermal nociceptors29,36 including transient receptor potential vanilloid subtype 1 (TRPV1) channels.37 Methyl salicylate has been associated with significant pain relief with minimal adverse events38 and is hydrolyzed to salicylic acid, in the skin and surrounding tissue, providing local anti-inflammatory effects, inhibiting the Cox-1 and Cox-2 enzyme receptor pathways of the Arachidonic Acid cascade increasing the threshold for nerve firing and pain.39
This study assessed changes in Brief Pain Inventory short form (BPI) pain severity scores and pain interference scores, as well as a change in the use of pain medications other than the patch within and between control, treatment, and crossover groups.
Methods
Study Design
This study was a prospective, Institutional Review Board-approved Observational Study used to evaluate patients treated with an OTC topical pain-relieving patch containing methyl salicylate 10%, menthol 6%, and camphor 3.1%. Following informed consent, patients who met the eligibility criteria and who were treated with the pain-relieving patch comprised the study’s test groups (treatment group—TG, and control to treatment group–CROSSG), and patients who met the eligibility criteria but were not treated with the pain-relieving patch comprised the study’s control group (CG). Patient answers to the baseline and day 14 surveys were used to evaluate pain relief by comparing answers to validated pain measurement scales (eg, BPI) as well as other survey questions that consider patient satisfaction.
This study was performed in full accordance with the rules of the Health Insurance Portability and Accountability Act of 1996 (HIPAA) and the principles of the declaration of Helsinki and the international council of Harmonisation/GCP. The study protocol was approved by IntegReview institutional review board.
Patients
Clinicians at five United States (US) investigator sites invited patients who were eligible to enroll in the study. For the treatment groups, inclusion criteria were as follows: 1) ages 18 to 64 years, inclusive; 2) ability to provide written informed consent; 3) received a topical pain-relieving patch from their treating physician; 4) had been diagnosed with a mild to moderate pain condition. Patients who were a beneficiary of a government-funded healthcare program, patients who currently or who have had a history of use of illicit or prescription drugs of abuse, and patients who were pregnant were ineligible to participate in the study. A CG of patients who met inclusion criteria but did not receive the medication were also enrolled and subsequently evaluated alongside the TG patients. A sub-group of CG patients who were observed for 14 days while not being treated with the pain-relieving patch crossed over to the TG (CROSSG) and were observed for another 14 days while on treatment.
Each site provided patients an identification number to keep personal information confidential in compliance with the HIPAA, and a confidential file containing the informed consent forms and patient identification numbers were kept and maintained in a secured cabinet only accessible to the principal investigator and authorized personnel. Patient survey responses were provided to Clarity Science with no identifying information about patients.
Patients could withdraw from this study at any time with the assurance of no harmful or unfavorable impact on their medical care. All diagnostic tests and treatment decisions were made at the discretion of physicians, with no tests, treatments, or investigations performed as part of this study.
Topical Intervention
The study aimed to evaluate patients who had been prescribed an OTC topical pain-relieving patch, the Salonpas® Pain Relieving Patch (Hisamitsu Pharmaceutical Company, Inc, Japan) containing methyl salicylate 10%, menthol 6%, and camphor 3.1% from their physician or clinician. Patient consent and compliance, as well as treatment outcomes, were collected for the duration of the study. Study subjects were instructed to apply the patch every 8 hours, except during overnight hours for 14 days.
Study Procedures and Assessments
At the time of enrollment, patients were asked to complete a baseline survey and were then asked to complete follow-up surveys on days 3, 7, and 14 of the study period. Baseline to day 14 results are reported here.
The baseline and follow-up surveys are comprised of questions to address the primary pain complaint of the patient. The primary pain complaint was indicated as the follows: 1) arthritis; 2) neuropathy or radiculopathy; 3) myofascial or musculoskeletal pain or spasm; or 4) other. Once patients chose their primary pain complaint, and confirmed by the clinician, they indicated the location of that pain in areas such as hands, feet, hips, knees, neck, shoulders, and back, among others. Patients indicated only one pain complaint and location of pain for which the medication was primarily being used.
Patients completed the BPI as part of each survey. The BPI is often used as a measure of pain for a wide range of conditions including cancer, musculoskeletal disorders, depressive conditions, and surgical pain. The BPI is commonly recommended for use in clinical trials of patients with acute and chronic pain conditions26,40 and has adequate internal consistency, acceptable-to-excellent test–retest reliability, satisfactory-to-good construct validity, criterion validity, and is sensitive to change.41,44 Ratings on the BPI are based on a 0–10 numerical scale. For the questions about pain severity, 0 is “no pain” and 10 is “pain as bad as you can imagine.” For the questions about pain interference with activities of daily living, 0 is “does not interfere” and 10 is “completely interferes.” Patient responses to questions regarding pain severity and pain interference were compiled to yield the overall score for pain severity and pain interference.
Patients were asked to indicate any other medications that they had been taking for pain relief at the time of the baseline and the day 14 survey. Categories of medications that patients could choose included OTC agents (eg, ibuprofen, naproxen, acetaminophen, acetylsalicylic acid, and other pain medications such as creams, gels, roll-ons, sprays, patches or rubs), prescription NSAIDs (eg, naproxen sodium, celecoxib, meloxicam or diclofenac), prescription opioids (eg, fentanyl, hydrocodone, hydromorphone, morphine or oxycodone), or prescription anticonvulsants (eg, gabapentin or pregabalin). Patients could indicate more than one type of pain relief medication in each of the four categories of pain medication.
Study End Points
There were five primary endpoints:
- Changes from baseline to day 14 in BPI mean pain severity and interference scores within the CG, TG and CROSSG for the primary pain complaint.
- Differences in changes from baseline to day 14 in BPI mean pain severity and interference scores between the CG, TG and CROSSG for the primary pain complaint.
- Changes from baseline to day 14 in the use of prescription and OTC medications (other than the OTC pain relieving patch) within the CG and TG.
- Patient satisfaction with the topical pain-relieving patch treatment.
- Any side effects reported by patients.
Statistical Analysis
For all variables, descriptive statistics were calculated, including frequencies and percent for categorical variables and means with standard deviation (SD) for continuous variables. The maximum sample size available was used for each statistical analysis.
Changes from baseline to day 14 in BPI mean pain severity and pain interference scores were analyzed using the paired t-test to identify any statistically significant differences within each of the CG, TG and CROSSG. The unpaired t-test was used to identify any statistically significant differences in the amount of change in BPI mean pain severity and pain interference scores from baseline to day 14 between the CG, TG and CROSSG.
Each survey collected the numbers and types of prescription and OTC oral/topical medications being used for pain relief; statistically significant differences in the use of these types of medications from baseline to day 14 were determined using the McNemar test and χ2 test for binomial paired and unpaired data, respectively. Descriptive statistics were used to determine patient satisfaction with the pain-relieving patch within those treated. Descriptive statistics were also used to report any side effects experienced by patients.
A two-tailed alpha was set to 0.05 for all statistical comparisons. SPSS v. 23 was used for all analyses.
Results
Baseline Demographic and Clinical Characteristics of Patients
A total of 199 patients at 5 US investigator sites were enrolled in the study and completed the baseline and day 14 surveys. The total number of patients in the TG receiving the pain-relieving patch was 152, and the total number of patients in the CG who did not receive the pain-relieving patch was 47. The total number of patients in the CROSSG was 34.
Demographic results were similar for gender and age at the baseline survey for the CG, TG, and CROSSG (Table 1). Of the 47 patients in the CG, 20 (42.6%) were male and 27 (57.4%) were female. The 152 patients in the TG had a total of 52 (34.2%) male and 100 (65.8%) female patients. The mean age was 48.4 years for the CG and 45.4 years for the TG. For the 34 patients in the CROSSG, 14 (41.2%) were male and 20 (58.8%) were female. Mean age at baseline was 47.3 years for this crossover group. The number of females in the TG is not statistically significantly different from the number of females in the CG or the CROSSG (P = 0.303 and 0.437, respectively, Fisher’s Exact Test).
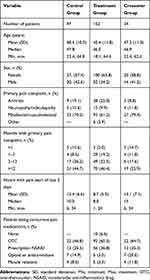 |
Table 1 Baseline Demographic and Clinical Characteristics of the Control, Treatment and Crossover Groups |
The primary pain complaint for the CG, TG, and CROSSG patients was recorded at baseline (Table 1). Myofascial/musculoskeletal pain was the most prominent pain complaint indicated by 70.2% (n=33) patients in the CG, 61.2% (n=93) in the TG and 79.4% (n=27) in the CROSSG patients. Arthritis was the next most common pain complaint for 19.1% (n=9) CG patients and 25.0% (n=38) of patients in the TG. Just 10.6% (n=5) of the CG patients and 9.9% (n=15) of the TG patients indicated that neuropathy/radiculopathy was their primary pain complaint. Only 3.9% (n=6) of the TG patients listed “other” as their primary pain complaint. For the CROSSG, 11.8% (n=4) had a primary neuropathy or radiculopathy complaint and 8.8% (n=3) an arthritis complaint, with no other complaints noted.
At baseline, CG study participants who indicated myofascial/musculoskeletal pain as their primary complaint noted that their back was the most common location of pain (n=15). In the TG, the most commonly noted musculoskeletal pain complaint location included back and lower extremities (n=72 patients).
The length of time patients had the primary pain complaint was similar for all three groups. The greatest proportion within each group had the pain for more than 1 year: CG=44.7%, TG=46.4%, and CROSSG=55.9%, indicating a chronic pain condition. Mean amount of time in hours of pain per day for the last 3 days were: CG=13.4 (SD=6.6), TG=8.7 (SD=5.5), and CROSSG=15.1 (SD=7.1).
Baseline BPI Severity and Interference Scores
The mean BPI pain severity score at baseline was higher for the CG (5.7) compared to the TG (4.9), 95% CI: 0.2, 1.5 (Table 2). The baseline mean BPI interference score was also higher for the CG (5.4) compared to the TG (4.3), 95% CI: 0.2, 1.9. In the CROSSG, the severity and interference scores were 5.4 and 5.5, respectively, at the beginning of the treatment period—similar to the CG scores.
 |
Table 2 Baseline and Day 14 Brief Pain Inventory Scores for Overall Severity, Severity Questions, Overall Interference, and Interference Questions for the Control, Treatment, and Crossover Groups |
BPI Pain Severity Scores
Changes from Baseline to Day 14 Within and Between the CG, TG and CROSSG
The TG showed a 49.0% decrease (4.9 to 2.5, 95% CI: 2.1, 2.6) and the CG showed a 12.3% decrease in mean BPI pain severity scores from baseline to day 14 (5.7 to 5.0, 95% CI: 0.4, 1.0), see Figure 1A. The mean pain severity score decrease was significantly greater in the TG as compared to the control group (95% CI: 1.3, 2.0), see Figure 2A. The CROSSG reported a 15.6% decrease in BPI pain severity score from baseline to day 14 before receiving treatment (6.4 to 5.4, 95% CI: 0.8, 1.2); after crossing over, a 48.1% decrease in mean BPI pain severity score was noted from treatment baseline to treatment day 14 (5.4 to 2.8, 95% CI: 0.8, 1.2). The mean pain severity score decrease in the CROSSG was significantly greater during their treatment period as compared to their control period (95% CI: 1.1, 2.0).
For each of the questions which comprise the BPI pain severity score (pain at its worst and least in the last 24 hours, pain right now, and how much pain on average), see Table 2, changes in mean scores from baseline to day 14 decreased statistically significantly for both the CG and the TG, except for pain at its least in the CG. The amount of decrease from baseline to day 14 was greater for the TG compared to the CG for each of these questions.
BPI Pain Interference Scores
Changes from Baseline to Day 14 Within and Between the CG, TG and CROSSG
BPI pain interference scores (which include quality of life (QoL) parameters and ratings of: general activity, mood, walking ability, normal work, relations with other people, sleep, and enjoyment of life) decreased statistically significantly from baseline to day 14 for both the CG and TG (Figure 1B). The TG had a 58.1% decrease from 4.3 to 1.8, 95% CI: 2.3, 2.8, and the CG had a 14.8% decrease in mean BPI interference score from 5.4 to 4.6, 95% CI: 0.6, 1.0. The amount of decrease in mean pain interference scores from baseline to day 14 was statistically significantly greater for the TG compared to the CG (95% CI: 1.5, 2.1), see Figure 2B. The CROSSG reported a 14.1% decrease in mean BPI pain interference score from baseline to day 14 before receiving treatment (6.4 to 5.5, 95% CI: 0.7, 1.2). After crossing over to treatment, these 34 patients reported a 54.5% decrease in mean BPI pain interference score from treatment baseline to treatment day 14 (5.5 to 2.5, 95% CI: 2.4, 3.4). The mean pain interference score decrease in the CROSSG was significantly greater during their treatment period as compared to their control period (95% CI: 1.4, 2.5).
For each of the questions which comprise the BPI pain interference score, changes in mean scores from baseline to day 14 decreased statistically significantly for both the CG and the TG. The amount of decrease from baseline to day 14 was statistically significantly greater for the TG compared to the CG for each of these questions.
Changes in Self-Perceived Pain Relief from Medications
One of the BPI questions (not part of the pain severity or interference scores) asks the patient how much pain relief (from 0%=no relief to 100%=complete relief in increments of 10%) they have experienced from treatments or medications within the last 24 hours. In the TG, the 87.5% increase in relief was statistically significant (39.9% at baseline to 74.8% at day 14, 95% CI: −39.9, −29.9). In the CG, the 12.0% increase in relief was not statistically significant (26.6% relief at baseline to 29.8% at day 14, 95% CI: −6.81, 0.44). The amount of change in percent relief from baseline to day 14 was statistically significantly greater for the TG compared to the CG (95% CI: −37.8, −25.6).
Concurrent Pain Medications
Changes from Baseline to Day 14 Within the CG and TG
In each survey, patients indicated the type of medication they were taking for pain relief including OTC pain medications, prescription anti-inflammatory medications, opioids or anticonvulsants, or muscle relaxants. In the CG there were 34 patients (72.3%) taking at least one medication at baseline with no reported change in the number of patients taking at least one medication at day 14. At baseline in the TG, there were 142 (93.4%) patients taking at least one medication. At day 14, that number had decreased by 37.3% to 89 (58.6%) patients taking at least one medication (P<0.001, McNemar test).
The total number of patients taking OTC pain medication at baseline in the CG was 22 (46.8%) while in the TG it was 92 (60.5%). There was no change in OTC pain medication within the CG; the 22 patients who reported use at baseline also reported use at day 14 (see Figure 3A). OTC pain medication use decreased by 29.3% in the TG, decreasing from 92 patients (60.5%) to 65 patients (42.8%), see Figure 3B. Ibuprofen was the highest reported OTC pain medication in use at baseline (38.3% in the CG and 41.4% in the TG). Acetaminophen was the second most common OTC pain medication used by 19.1% of the CG and 17.1% of the TG. As far as prescription anti-inflammatory medication, diclofenac (CG=19.1%, TG=19.1%) was reported most often. When evaluating changes in ibuprofen, acetaminophen, and Voltaren at day 14 of the TG, there was a reduction in these adjuvant analgesics of between 24%, 39%, and 62%, respectively.
Naproxen was the next highest reported prescription (CG=2.1%, TG=7.9%). The total number of patients taking prescription anti-inflammatory medication at baseline in the CG was 12 (25.5%), with an 8.3% decrease at day 14 to 11 patients (23.4%). The TG reported 56 patients (36.8%) taking these prescription pain medications at baseline and showed a 64.3% decrease in use to 20 patients (13.2%) by day 14. Use of diclofenac in the CG remained the same (19.1%) from baseline to day 14, while TG use decreased from 19.1% to 9.2%.
Although a minority of patients reported using opioids, anticonvulsants, and muscle relaxants, there was a clear trend in the treatment group toward a decrease in these agents as compared to baseline.
Separate from indicating use of specific medications for pain, patients in the TG and CROSSG were asked how their use of oral pain medications had changed (scale: 1=A lot more, 2=More, 3=No change, 4=Less, 5=A lot less). At day 14 the mean rating was 4.5 (SD=0.7, n=151) for the TG with 88.8% reporting “less” or “a lot less.” For the CROSSG at treatment day 14, the mean rating was 4.4 (SD=0.5, n=34) with 100% reporting “less” or “a lot less.”
Satisfaction with Use of the Topical Pain-Relieving Patch
Day 14 satisfaction ratings in the TG related to specific aspects of use of the topical pain-relieving patch (scale: 1=Strongly disagree, 2=Disagree, 3=Neutral, 4=Agree, 5=Strongly agree) were 4.9 for each of “easy to apply” and “convenient,” and 4.6 for each of “preferred over pills/oral medication” and “preferred over other pain-relieving treatments (creams, gels, roll-ons, sprays, patches, and rubs).” Overall satisfaction was 4.6 out of 5 (scale: 1=not at all, 2=Not very, 3=Somewhat, 4=Very, 5=Extremely) in the TG. In the CROSSG, the mean ratings were 5.0 for “easy to apply,” 4.9 for “convenient,” and 4.7 for “preferred over pills/oral medication” and “preferred over other pain-relieving treatments.” Overall mean satisfaction was 4.3.
Safety
Patients in the TG and CROSSG reported no adverse events or serious adverse events while being treated with the pain-relieving patch.
Discussion
The RELIEF study reported here is a prospective, non-randomized study of patients experiencing mild to moderate acute arthritic, neurological, and musculoskeletal pain. Patients indicated a variety of medical treatments for pain at baseline and at 14 days. Medical treatments included OTC oral agents, prescription NSAIDs, opioids, anticonvulsants, skeletal muscle relaxants or a combination of the above. BPI scores indicated that patients in the treatment group were experiencing mild, moderate, or more severe pain.
In the present study, data were collected from patients at baseline and day 14 of the two-week study period. The CG showed more moderate to severe pain at baseline compared to the TG which can occur when clinical trial subjects are not specifically randomized to treatment groups. A portion of subjects opted to be in the CG initially; some investigators noted that this may have been due to a patient lack of confidence from preconceived notions about the effectiveness of a topical treatment (The higher pain scores in the CG may have influenced those patients not to enroll in a topical analgesic study for fear of treatment failure). The notable analgesic responses seen in the CROSSG group challenges those preconceived notions and offers some insights as to the potential benefit of topical treatments to patients with higher pain scores.
Changes in BPI pain severity and pain interference scores and use of concurrent pain medications from baseline to day 14 were evaluated to assess effectiveness of topical analgesics for the treatment of acute pain. This study showed that treatment with a pain-relieving patch led to a reduction in mean scores for BPI pain severity and pain interference. The comparison of changes from baseline BPI pain severity (49% v 12%) and pain interference (58% v 14%) between the TG and CG was statistically significant. A high percentage of patients in the TG reported significantly lower use of concurrent pain medications at day 14. When evaluating changes in ibuprofen, acetaminophen, and Voltaren at day 14, there was a reduction in these adjuvant analgesics in the TG of 24%, 39%, and 62%, respectively. There were no side effects reported with the topical pain-relieving patch. Although there were no adverse events reported by patients during the 14 days of treatment, any adverse events, such as skin irritation, which has been noted in some studies after longer-term use of a topical patch, require further study for appropriate evaluation.
In this study, patients were asked to provide their response to treatment and their perceptions of improvement in various QoL parameters, satisfaction of treatment, and functionality. Patients in the TG group demonstrated an 87.5% increase in pain relief with a statistical separation from the 12% improvement in control group. It is often difficult for clinicians to determine what level of analgesia constitutes clinically meaningful relief for patients. Farrar et al45 have reported on patient responder rates and noted that a 30% improvement in pain intensity represents changes that are clinically meaningful to patients. In addition, a measure known as the Minimal Clinically Important Improvement (MCII) has been used to define meaningful relief with various medications including topical nonsteroidal analgesics.46,47 The MCII represents a patient’s perception of what is an important improvement. It can be defined as the smallest change in measurement that signifies an important improvement. Patient reported outcome measures such as responder rate and MCII should be included in analgesic studies as changes in function and QoL are difficult to measure with numeric pain ratings. This type of information would conform to recent FDA guidance which promotes the use of data from observational studies to capture real-world evidence of changes in patient perception – which is often lacking in traditional clinical trials.48
The potential benefits of opioid and NSAID sparing are critical in our current climate of analgesic overuse. Although prescribing of opioids has plateaued or decreased in recent years, from 1999–2018, more than 232,000 people died in the United States from overdoses involving prescription opioids.49,50 In addition to abuse, addiction, and overdose-related deaths with opioids, oral NSAIDs can also cause serious AEs including gastrointestinal bleeding, cardiovascular and renal complications.51,56
Results from this IRB-approved observational study suggest that topical pain-relieving patches may provide an effective and safe treatment alternative to conventional therapies including opioids, prescription/OTC NSAIDs, and acetaminophen for the management of mild-moderate, and chronic pain. Controlled trials are suggested to confirm these results.
Limitations
This was an observational study based on a sample of patients attending diverse clinical settings for the treatment of acute arthritic, neurological, and musculoskeletal pain who consented to participate in this study. There were differences between the CG and TG in baseline measures of type of primary pain complaint, BPI mean pain severity and interference scores, and self-reported use of concurrent pain medications. Generalizability of the findings may be limited to the TG only because those patients received topical therapies.
Pain complaints and changes in use of concurrent pain medications were reported by patients in both the CG and TG. In a few instances, there were patients who did not complete the follow up surveys after baseline. These patients’ data were removed from evaluation. The direct comparison between the CG and TG is not matched regarding type and location of primary pain complaints. The lack of consistency and availability of matched data in the composition of the CG and TG could affect the accuracy of these results.
Conclusion
These results suggest that the topical pain-relieving patches studied were effective and safe for the relief of mild to moderate pain attributed to arthritis, neurological conditions, and musculoskeletal disorders. Reductions in the interference of pain were noted as well as an overall decrease in concomitant medications. These results support the use of this analgesic pain-relieving patch as a first-line treatment and should be considered for future pain management guidelines as part of multimodal pain treatment regimens.
Disclosure
Jeffrey A Gudin has received compensation from Clarity Science LLC for his role as principal investigator and for providing protocol-required services for the study. He is also a consultant for Hisamitsu America, Inc. Peter L Hurwitz is President of Clarity Science LLC and reports grants from Hisamitsu America, Inc. Derek T Dietze received compensation for study statistical analyses from Clarity Science LLC. The authors report no other conflicts of interest in this work.
References
1. Goldberg DS, McGee SJ. Pain as a global public health priority. BMC Public Health. 2011;11(1):770. doi:10.1186/1471-2458-11-770
2. Harker J, Reid KJ, Bekkering GE, et al. Epidemiology of chronic pain in Denmark and Sweden. Pain Res Treat. 2012;2012:371248.
3. Langley PC. The prevalence, correlates and treatment of pain in the European Union. Curr Med Res Opin. 2011;27(2):463–480. doi:10.1185/03007995.2010.542136
4. Mansfield KE, Sim J, Jordan JL, Jordan KP. A systematic review and meta-analysis of the prevalence of chronic widespread pain in the general population. Pain. 2016;157(1):55–64. doi:10.1097/j.pain.0000000000000314
5. Ohayon MM, Stingl JC. Prevalence and comorbidity of chronic pain in the German general population. J Psychiatr Res. 2012;46(4):444–450. doi:10.1016/j.jpsychires.2012.01.001
6. Reid KJ, Harker J, Bala MM, et al. Epidemiology of chronic non-cancer pain in Europe: narrative review of prevalence, pain treatments and pain impact. Curr Med Res Opin. 2011;27(2):449–462. doi:10.1185/03007995.2010.545813
7. Reitsma ML, Tranmer JE, Buchanan DM, Vandenkerkhof EG. The prevalence of chronic pain and pain-related interference in the Canadian population from 1994 to 2008. Chronic Dis Inj Can. 2011;31(4):157–164.
8. Tsang A, Von Korff M, Lee S, et al. Common chronic pain conditions in developed and developing countries: gender and age differences and comorbidity with depression-anxiety disorders. J Pain. 2008;9(10):883–891. doi:10.1016/j.jpain.2008.05.005
9. Friessem CH, Willweber-Strumpf A, Zenz MW. Chronic pain in primary care. German figures from 1991 and 2006. BMC Public Health. 2009;9(1):299. doi:10.1186/1471-2458-9-299
10. Hasselström J, Liu-Palmgren J, Rasjö-Wrååk G. Prevalence of pain in general practice. Eur J Pain. 2002;6(5):375–385. doi:10.1016/S1090-3801(02)00025-3
11. Hensler S, Heinemann D, Becker MT, et al. Chronic pain in German general practice. Pain Med. 2009;10(8):1408–1415. doi:10.1111/j.1526-4637.2009.00735.x
12. Jordan KP, Kadam UT, Hayward R, Porcheret M, Young C, Croft P. Annual consultation prevalence of regional musculoskeletal problems in primary care: an observational study. BMC Musculoskelet Disord. 2010;11(1):144. doi:10.1186/1471-2474-11-144
13. Lalonde L, Choinière M, Martin E, Berbiche D, Perreault S, Lussier D. Costs of moderate to severe chronic pain in primary care patients – a study of the ACCORD program. J Pain Res. 2014;7:389–403. doi:10.2147/JPR.S55388
14. Mills S, Torrance N, Smith BH. Identification and management of chronic pain in primary care: a review. Curr Psychiatry Rep. 2016;18(2):22. doi:10.1007/s11920-015-0659-9
15. Krebs EE, Gravely A, Nugent S, et al. Effect of opioid vs nonopioid medications on pain-related function in patients with chronic back pain or hip or knee osteoarthritis pain: the space randomized clinical trial. JAMA. 2018;319(9):872–882. doi:10.1001/jama.2018.0899
16. Argoff CE, Albrecht P, Irving G, Rice F. Multimodal analgesia for chronic pain: rationale and future directions. Pain Med. 2009;10(Suppl 2):S53–S66. doi:10.1111/j.1526-4637.2009.00669.x
17. Casale R, Symeonidou Z, Bartolo M. Topical treatments for localized neuropathic pain. Curr Pain Headache Rep. 2017;21(3):15. doi:10.1007/s11916-017-0615-y
18. Flores MP, Castro AP, Nascimento Jdos S. Topical analgesics. Rev Bras Anestesiol. 2012;62(2):244–252. doi:10.1016/S0034-7094(12)70122-8
19. Haroutiunian S, Drennan DA, Lipman AG. Topical NSAID therapy for musculoskeletal pain. Pain Med. 2010;11(4):535–549. doi:10.1111/j.1526-4637.2010.00809.x
20. McCarberg BH, D’Arcy Y. Target pain with topical peripheral analgesics. Nurse Pract. 2007;32(7):44–49. doi:10.1097/01.NPR.0000279572.01195.84
21. Sawynok J. Topical and peripherally acting analgesics. Pharmacol Rev. 2003;55(1):1–20. doi:10.1124/pr.55.1.1
22. Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain. 2006;10(4):287–333. doi:10.1016/j.ejpain.2005.06.009
23. Breivik H. A major challenge for a generous welfare system: a heavy socio-economic burden of chronic pain conditions in Sweden – and how to meet this challenge. Eur J Pain. 2012;16(2):167–169. doi:10.1002/j.1532-2149.2011.00025.x
24. Mäntyselkä PT, Turunen JH, Ahonen RS, Kumpusalo EA. Chronic pain and poor self-rated health. JAMA. 2003;290(18):2435–2442. doi:10.1001/jama.290.18.2435
25. Torrance N, Elliott AM, Lee AJ, Smith BH. Severe chronic pain is associated with increased 10-year mortality. A cohort record linkage study. Eur J Pain. 2010;14(4):380–386. doi:10.1016/j.ejpain.2009.07.006
26. Dworkin RH, Turk DC, Wyrwich KW, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9(2):105–121. doi:10.1016/j.jpain.2007.09.005
27. Jackman RP, Purvis JM, Mallett BS. Chronic nonmalignant pain in primary care. Am Fam Physician. 2008;78(10):1155–1162.
28. Dubin AE, Patapoutian A. Nociceptors: the sensors of the pain pathway. J Clin Invest. 2010;120(11):3760–3772. doi:10.1172/JCI42843
29. Barkin RL. The pharmacology of topical analgesics. Postgrad Med. 2013;125(4 suppl 1):7–18. doi:10.1080/00325481.2013.1110566911
30. Kendroud S, Hanna A. Physiology, nociceptive pathways. [Updated 2019 Apr 24]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020.
31. Cline AE, Turrentine JE. Compounded topical analgesics for chronic pain. Dermatitis. 2016;27(5):263–271. doi:10.1097/DER.0000000000000216
32. Peppin JF, Albrecht PJ, Argoff C, et al. Skin matters: a review of topical treatments for chronic pain. Part one: skin physiology and delivery systems. Pain Ther. 2015;4(1):17–32. doi:10.1007/s40122-015-0031-0
33. Peppin JF, Albrecht PJ, Argoff C, et al. Skin matters: a review of topical treatments for chronic pain. Part two: treatments and applications. Pain Ther. 2015;4(1):33–50. doi:10.1007/s40122-015-0032-z
34. Gudin JA, Brennan MJ, Harris ED, et al. Changes in pain and concurrent pain medication following compounded topical analgesic treatment for chronic pain: 3- and 6-month follow-up results from the prospective, observational optimizing patient experience and response to topical analgesics study. J Pain Res. 2017;Volume 10(10):2341–2354. doi:10.2147/JPR.S143513
35. Gudin JA, Brennan MJ, Harris ED, et al. Reduction of opioid use and improvement in chronic pain in opioid-experienced patients after topical analgesic treatment: an exploratory analysis. Postgrad Med. 2018;130(1):42–51. doi:10.1080/00325481.2018.1414551
36. Barkin RL. The pharmacology of topical analgesics. Postgrad Med. 2013;125(4 Suppl 1):7–18.
37. Xu H, Blair NT, Clapham DE. Camphor activates and strongly desensitizes the transient receptor potential vanilloid subtype 1 channel in a vanilloid-independent mechanism. J Neurosci. 2005;25(39):8924–8937. doi:10.1523/jneurosci.2574-05.2005.
38. Higashi Y, Kiuchi T, Furuta K. Efficacy and safety profile of a topical methyl salicylate and menthol patch in adult patients with mild to moderate muscle strain: a randomized, double-blind, parallel-group, placebo-controlled, multicenter study. Clin Ther. 2010;32(1):34–43. doi:10.1016/j.clinthera.2010.01.016.
39. Amman R, Peskar BA. Anti-inflammatory effects of aspirin and sodium salicylate. Eur J Pharmacol. 2002;447(1):1–9. doi:10.1016/S0014-2999(02)01828-9
40. Mendoza TR, Chen C, Brugger A, et al. The utility and validity of the modified brief pain inventory in a multiple-dose postoperative analgesic trial. Clin J Pain. 2004;20(5):357–362. doi:10.1097/00002508-200409000-00011
41. Erdemoglu AK, Koc R. Brief pain inventory score identifying and discriminating neuropathic and nociceptive pain. Acta Neurol Scand. 2013;128(5):351–358. doi:10.1111/ane.12131
42. Keller S, Bann CM, Dodd SL, Schein J, Mendoza TR, Cleeland CS. Validity of the brief pain inventory for use in documenting the outcomes of patients with noncancer pain. Clin J Pain. 2004;20(5):309–318. doi:10.1097/00002508-200409000-00005
43. Mendoza T, Mayne T, Rublee D, Cleeland C. Reliability and validity of a modified brief pain inventory short form in patients with osteoarthritis. Eur J Pain. 2006;10(4):353–361. doi:10.1016/j.ejpain.2005.06.002
44. Tan G, Jensen MP, Thornby JI, Shanti BF. Validation of the brief pain inventory for chronic nonmalignant pain. J Pain. 2004;5(2):133–137. doi:10.1016/j.jpain.2003.12.005
45. Farrar JT, Young JP, LaMoreaux L, et al. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. PAIN. 2001;94(2):149–158. doi:10.1016/S0304-3959(01)00349-9
46. Kvien TK, Heiberg T, Hagen KB. Minimal clinically important improvement/difference (MCII/MCID) and patient acceptable symptom state (PASS): what do these concepts mean? Ann Rheum Dis. 2007;66(Suppl 3):iii40–41. doi:10.1136/ard.2007.079798
47. Tubach F, Ravaud P, Martin-Mola E, et al. Minimum clinically important improvement and patient acceptable symptom state in pain and function in rheumatoid arthritis, ankylosing spondylitis, chronic back pain, hand osteoarthritis, and hip and knee osteoarthritis: results from a prospective multinational study. Arthritis Care Res (Hoboken). 2012;64(11):1699–1707. doi:10.1002/acr.21747
48. Upton FHR 6—21st century cures act. 114th congress (2015–2016) Jul 13, 2015. Available from: www.congress.gov/bill/114th-congress/house-bill/6/text.
49. Wide-ranging online data for epidemiologic research (WONDER). Atlanta, GA: CDC, National Center for Health Statistics; 2020. Available at http://wonder.cdc.gov.
50. Atluri S, Sudarshan G, Manchikanti L. Assessment of the trends in medical use and misuse or opioid analgesics from 2004 to 2011. Pain Med. 2015;16(9):1745–1758. doi:10.1111/pme.12773
51. Farkouh ME, Greenberg BP. An evidence-based review of the cardiovascular risks of nonsteroidal anti-inflammatory drugs. Am J Cardiol. 2009;103(9):1227–1237. doi:10.1016/j.amjcard.2009.01.014
52. Harirforoosh S, Jamali F. Renal adverse effects of nonsteroidal anti-inflammatory drugs. Expert Opin Drug Saf. 2009;8(6):669–681. doi:10.1517/14740330903311023
53. John R, Herzenberg AM. Renal toxicity of therapeutic drugs. J Clin Pathol. 2009;62(6):505–515. doi:10.1136/jcp.2008.058271
54. Lazzaroni M, Porro GB. Management of NSAID-induced gastrointestinal toxicity: focus on proton pump inhibitors. Drugs. 2009;69(1):51–69. doi:10.2165/00003495-200969010-00004
55. Scarpignato C, Hunt RH. Nonsteroidal anti-inflammatory drug-related injury to the gastrointestinal tract: clinical picture, pathogenesis, and prevention. Gastroenterol Clin North Am. 2010;39(3):433–464. doi:10.1016/j.gtc.2010.08.010
56. Trelle S, Reichenbach S, Wandel S, et al. Cardiovascular safety of non-steroidal anti-inflammatory drugs: network meta-analysis. BMJ. 2011;342:c7086. doi:10.1136/bmj.c7086
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



