Back to Journals » Journal of Multidisciplinary Healthcare » Volume 8
Implementing best practice in hospital multidisciplinary nutritional care: an example of using the knowledge-to-action process for a research program
Received 25 July 2015
Accepted for publication 27 August 2015
Published 3 October 2015 Volume 2015:8 Pages 463—472
DOI https://doi.org/10.2147/JMDH.S93103
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Celia Laur,1 Heather H Keller1,2
1University of Waterloo, 2Schlegel-University of Waterloo, Research Institute for Aging, Waterloo, Ontario, Canada
Background: Prospective use of knowledge translation and implementation science frameworks can increase the likelihood of meaningful improvements in health care practices. An example of this creation and application of knowledge is the series of studies conducted by and with the Canadian Malnutrition Task Force (CMTF). Following a cohort study and synthesis of evidence regarding best practice for identification, treatment, and prevention of malnutrition in hospitals, CMTF created an evidence-informed, consensus-based pathway for nutritional care in hospitals. The purpose of this paper is to detail the steps taken in this research program, through four studies, as an example of the knowledge-to-action (KTA) process.
The KTA process: The KTA process includes knowledge creation and action cycles. The steps of the action cycle within this program of research are iterative, and up to this point have been informed by three studies, with a fourth underway. The first study identified the magnitude of the malnutrition problem upon admission to hospital and how it is undetected and undertreated (study 1). Knowledge creation resulted in an evidence-based pathway established to address care gaps (study 2) and the development of monitoring tools (study 3). The study was then adapted to local context: focus groups validated face validate the evidence-based pathway; during the final phase, study site implementation teams will continue to adapt the pathway (studies 2 and 4). Barriers to implementation were also assessed; focus groups and interviews were conducted to inform the pathway implementation (studies 1, 2, and 4). In the next step, specific interventions were selected, tailored, and implemented. In the final study in this research program, plan–do–study–act cycles will be used to make changes and to implement the pathway (study 4). To monitor knowledge use and to evaluate outcomes, audits, staff surveys, patient outcomes, etc will be used to record process evaluations (studies 3 and 4). Finally, a sustainability plan will be incorporated into the final study of the program (study 4) to sustain knowledge use.
Discussion: Use of frameworks can increase the likelihood of meaningful and sustainable improvements in health care practice. The example of this program of research demonstrates how existing evidence has been used to identify, create, and adapt knowledge, and how multidisciplinary teams have been used to effect changes in the hospital setting.
Conclusion: Effective implementation is essential in nutritional health care, and this multidisciplinary program of research provides an example of how the KTA process can facilitate implementation and promote sustainability.
Keywords: nutrition, implementation, knowledge translation, best practice, knowledge-to-action process, hospital
Introduction
Effective implementation of current evidence is an example of knowledge translation (KT), where the new knowledge gained from research is translated into sustained improvements in health care.1,2 The process of implementing knowledge is an important consideration in order to increase the likelihood of achieving and sustaining improvements, particularly in health care.3 Effective implementation involves being aware of the likely barriers and facilitators to implementing knowledge, and the importance of prospective, planned implementation studies that use frameworks, models, or processes (henceforth all termed as frameworks) to guide implementation. Knowledge translation/implementation science (KT/IS) interventions require frameworks, because they are typically multifaceted, yet need to be flexible to adapt to various health care contexts.1
Examples of KT/IS frameworks are important in health care and specifically, nutrition. A knowledge gap currently exists regarding ideal management of malnourished patients and best practice for enhancing current nutritional practices in hospitals. Consistent with other developed nations, 45% of patients admitted to medical/surgical wards in Canadian hospitals are at risk of malnutrition.4 The additional resources required to effectively care for these patients is considerable, because the cost for treating a malnourished patient in hospital is approximately $2,000 (CAD) more than the cost to treat a well-nourished patient.5–8 To address this gap, hospitals should focus on how to incorporate evidence of best practice through methods that overcome barriers to implementation, and to adapt knowledge to their specific/local context, leading to sustained change.
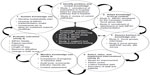
A program of research has been undertaken in Canada to address the issue of in-hospital malnutrition. The first study within this research program (study 1) was the Nutrition Care in Canadian Hospitals (NCCH) cohort study conducted by the Canadian Malnutrition Task Force (CMTF) from 2010 to 2013.4 In the second and third studies (studies 2 and 3), tools to address hospital malnutrition and to improve detection and treatment were developed.9 This included the development of the Integrated Nutrition Pathway for Acute Care (INPAC), an evidence-based algorithm for the detection, treatment, and monitoring of malnutrition among acute care medical and surgical patients.9 The current phase of this research program (study 4) is a 2-year project, called More-2-Eat (M2E), which has been designed to test the implementation of INPAC in five hospitals across Canada. The entire research program is grounded in KT/IS frameworks including the knowledge-to-action (KTA) process, and M2E specifically employs the plan–do–study–act (PDSA) cycles and the Model for Improvement for implementation.1,10–13 The overall program of research is outlined in Figure 1. Results from the initial studies can be found elsewhere.4,9
  | Figure 1 Overview of the program of research. |
The aim of the current work is to detail the iterative process of KT/IS within this program of research, which consists of four key studies, as an example of the KTA process. Each step within the KTA process will be discussed within the context of the specific research studies conducted (studies 1–3) and underway (study 4).
Selection of implementation framework(s)
Several frameworks exist to support translating new knowledge into practice. A common framework used in health care is the Promoting Action on Research Implementation in Health Services (PARIHS) framework.14 PARIHS highlights the need to consider evidence, context, and facilitation.14 Each of these three components is integral to effective implementation, and leaves significant room for adaptation to the needs of the intervention. Evidence, context, and facilitation are all considered in the overall program of research.
The Quality Implementation Framework is commonly used, and as it provides a series of steps for implementation, it has been integral in the M2E study (study 4).15 These steps include: 1) initial considerations regarding the host setting; 2) creating a structure for implementation; 3) ongoing structure once implementation begins; and 4) improving future applications.15 The safer health care now version of the Model for Improvement framework also addresses implementation by asking three key questions, and includes the use of PDSA cycles to test small changes in processes.10 The key questions included in the Model for Improvement include: 1) What are we trying to accomplish? 2) How will we know a change is an improvement? 3) What changes can we make that will result in improvement?10 The Quality Implementation Framework provided a general structure of testing and implementing change for the M2E project, but is not sufficiently comprehensive as a theoretical framework to guide the entire program of research.
The KTA process was chosen as the primary framework to follow for the overall program of research, as it captures the essence of the PARIHS and Quality Implementation Framework models, but is more comprehensive. In the KTA framework, several steps in the process are detailed, providing a logical order to follow that is consistent with PARIHS, but goes beyond PARIHS by considering evaluation and sustainability. KTA is also flexible in that implementation techniques, such as PDSA cycles can fit readily within the model. These PDSA cycles, which are intuitive, as they recognize that sustained change tends to happen following many trials and modifications to make the improvement. These cycles allow the user to gain an understanding of what works and what does not, with room to try different approaches until the improvement is fully incorporated into practice. These sub processes within KTA result in specific tailoring and increased potential for sustainable change. PDSA and other cyclical frameworks can be repeatedly applied to both small and larger phases of implementation.13 The steps do not necessarily need to be completed in order, as work done at various steps can be complementary. The following sections will outline how the studies in this program of research have followed the iterative KTA framework.
Overview of the KTA process
The KTA process was published by Graham et al, and provides a cyclical, stepwise sequence which can be followed by either clinicians or researchers.12 The KTA process encompasses two phases: 1) the knowledge creation cycle and 2) the action cycle.12 The knowledge creation cycle leads to the identification of a “problem”, and includes the development of the evidence base to support implementation. The subsequent action cycle tailors and implements the evidence created in the knowledge creation cycle to overcome the problem. The steps of the action cycle include: 1) identify problem/identify, review, and select knowledge; 2) adapt knowledge to local context; 3) assess barriers to knowledge use; 4) select, tailor, and implement interventions; 5) monitor knowledge use; 6) evaluate outcomes; 7) sustain knowledge use; and 8) identify new problem. Figure 2 displays the KTA process, using the example of this program of research, and demonstrates that although the cycle has a logical stepwise flow, the initial phases (ie, studies 1–3, identified as the double-ended arrows between the action and knowledge creation cycles) were revisited several times before moving on to later phases.
KTA knowledge creation cycle
Knowledge inquiry
CMTF conducted the NCCH study (study 1), the first project in this program of research, from 2010 to 2013, to determine the prevalence of malnutrition in Canadian hospitals, to determine the outcomes of malnutrition, and to describe the current nutritional care practices and perceptions of hospital staff.4 For NCCH data collection, the universities of Toronto, Guelph, and Waterloo provided ethical approval, as did the research ethics boards of each of the 18 hospitals involved in the study.4 The challenges and barriers to appropriate nutritional care for malnourished patients were identified through focus groups, interviews, and surveys with patients, nurses, and physicians.4,16–18 Key process gaps were that patients were not identified as malnourished or at-risk of malnutrition upon admission to hospital, and that few patients who were identified as malnourished were referred to a dietitian for specialized nutritional care. Poor food intake was common, and limited strategies, including monitoring, were used to improve food intake. Many barriers were identified as being amenable to intervention, such as opening packages and making food trays more accessible to patients.4,16–18
Knowledge synthesis
In study 2, a literature review was conducted on best practices (or “better-than-current” practices) to increase the food intake of patients in hospital, and the most appropriate ways to make hospitals “food aware”. The literature review resulted in a list of strategies to improve practices that incorporate all hospital staff, management, patients, and their families in the solution.19 These strategies were categorized into organization, staff, and patient/family levels. An example of organizational strategies included recommendations for the use of KT/IS frameworks to develop and implement policies/protocols for enhanced nutritional care. At the staff level, a recommendation was to clarify the roles and responsibilities of all staff in nutritional care. Patients and families were encouraged to participate in nutritional care (ie, intake monitoring, advocating for nutritional needs, and making the dining area as pleasant as possible).19
These results, and that of the NCCH study, suggested a need for a multilevel approach to make hospitals more food aware.4,19–24 Therefore, a pathway that delineated the ideal actions of staff, and the roles of multidisciplinary teams to prevent, detect, and treat malnutrition and to monitor food intake and body weight was considered a key mechanism for promoting “food awareness” and changing the philosophy of care to that of “food as medicine”. In study 2, a modified Delphi25,26 process was conducted to develop and attain consensus among a multidisciplinary panel of experts on the pathway, which resulted in INPAC.9 Barriers and facilitators to this knowledge use were also attained through focus groups with health care professionals in four hospitals. Face validation of INPAC was also a key result of these focus groups.
Knowledge tools/products
The process of developing INPAC highlighted the need for tools that could support implementation of key aspects of the pathway. In study 3, tools that were developed included a Mealtime Audit Tool and a My Meal Intake Tool, which have undergone validation and reliability testing (Keller HH et al. Unpublished data, 2015). The Mealtime Audit Tool was designed to identify barriers to food intake and patient perceptions of the meal and food. The My Meal Intake Tool was used to assess intake of foods and fluids provided in a single meal, as well as reasons for poor consumption. INPAC is already publicly available,9 and when the other tools are finalized, they will be available for use from the CMTF website (http://nutritioncareincanada.ca), and the details published.
KTA action cycle
Identifying the problem and the knowledge solution
As demonstrated in Figure 2, there is an iterative link between the knowledge creation and the action cycles. Knowledge creation cycle leads to identification of the problem, and as the action cycle continues, it can lead to further questions for the knowledge creation cycle. The problem demonstrated by the NCCH study (study 1), revealed that the prevalence of malnutrition upon admission to medical and surgical wards in Canadian hospitals was 45%, with elderly patients more likely to be malnourished.4,22 Nutritional practices in these hospitals, including diagnosis, treatment, and monitoring of malnourished patients, were inconsistent. The lack of a systematic approach to nutritional care for malnourished patients was identified as the problem, and this problem demonstrated the need for knowledge translation of best practice within hospitals. Given that INPAC was created in study 2 as a potential mechanism to facilitate hospitals to be more food aware and to enhance the nutritional care provided to malnourished patients,9 planning for the M2E project (ongoing study 4) began as the mechanism for implementing INPAC. Three of the five hospitals currently involved in M2E (study 4) were originally involved in NCCH (study 1),4 and the other two hospital sites had also identified the problem of malnutrition and its detection and treatment as an area for improvement. Details of the selected M2E hospitals are provided in Adapting knowledge to local content.
Consistent with PARIHS, “facilitation” of the INPAC implementation was recognized as a key step. To assist facilitators, a project team was created consisting of national and international experts, as well as “coaches” to assist sites with implementation. “Site implementation teams”, including a “site champion” and a research associate, are responsible for the main components of implementation in their hospital. Distinct stages of the ongoing M2E project (study 4) include: the developmental phase; the testing and implementation phase; and the sustainability phase.
Adapting knowledge to local context
Focus groups with dietetic staff in eight hospitals in study 1 identified that a culture change was needed to raise awareness and to adapt knowledge, such as screening protocols, to the local setting. INPAC was developed to be applicable in the Canadian context, although many of the principles are transferable to other countries. To determine potential applicability of INPAC to local contexts, in study 2, focus groups were formed at four hospital sites across Canada.9 The focus groups investigated how to enhance the interpretation of this knowledge tool by considering visual appearance, layout, and instructions. Their feedback was used to streamline the pathway into a simple and easy-to-follow tool.9 Participants in the focus groups indicated that INPAC was consistent with what they considered quality nutritional care practices and that the steps in the pathway were feasible. However, the participants also reported that further work was required to determine how INPAC could be implemented, what resources were required for implementation, and what would be involved in changing job routines, or how accountability could be assured.9 For example, the notion of having trays and food products accessible to patients appears to be a simplistic action; however, it is relatively complex to ensure that this happens in a safe and appropriate manner. Is the person who delivers the meal tray trained to ask the patient about the need for tray setup and to provide this assistance? What about issues with food safety and handling of multiple trays and food products with each patient? What safety issues need to be considered for the patient with dysphagia or those with self-feeding difficulties? If a nurse is not available to assist with eating, do the packages get opened for the patient? Thus, a seemingly simple problem of trays and food packages being inaccessible cannot be resolved simply by identifying that there is such a problem and that it needs to be fixed; rather, a process for implementing change that is feasible and sustainable is needed. Tools to identify these barriers were therefore created in study 3.
The current M2E project (study 4) is focused on these “how” aspects of implementation of INPAC, considering the local context and ensuring it is aligned with local and regional policies. For example, hospitals in Western Canada do not typically have dietary technicians, and thus, some roles such as nutritional screening, which could be done by this level of personnel, would need to be done by others, such as nurses. Union rules and roles of employees, as well as unit culture, also need to be considered, resulting in a locally tailored innovation (ie, INPAC) specific to hospital unit circumstances.
With the recognition that implementation needs to be tailored, five diverse hospitals across four provinces in Canada were selected as the sites for INPAC implementation (note: these are separate from the four focus group sites used in the development of the INPAC study discussed earlier). Sites were selected to promote study diversity, with academic and community hospitals included, as well as variation in region and size of the hospital. The five M2E hospitals are located in 1) Ontario, a community hospital with 150 beds, 2) an Ontario academic hospital with 1,100 beds; 3) Alberta, an academic hospital with 798 beds; 4) Saskatchewan, an academic hospital with 430 beds; and 5) Manitoba, a community hospital with 186 beds. Capacity for readiness of the hospital to undertake implementation was a key factor in selection and three of five sites had previously been in the NCCH study (study 1), thus problem identification in the KTA framework was already present in these settings.
Site implementation teams and site champion(s) lead the implementation testing of this knowledge product. These multidisciplinary teams and champions include a mix of dietitians, physicians, nurses, food service professionals, hospital management, and many others, as selected by the hospital to meet their local needs. The M2E research associate is typically a nurse or nutritional professional selected by the hospital to lead on data collection for the study and facilitate actions of the implementation team. Key opinion leaders from any profession were included in the project team, and at test sites, these individuals were recruited to facilitate implementation.1
Each M2E test site is encouraged to adapt INPAC to their local context, while still maintaining the core components of the pathway across sites. For example, choice of which clinical group completes screening, or is involved in supporting standard nutritional care practices, is based on local context that considers work routines. In one province, the M2E champion is a dietitian, the research associate is a nurse, and screening is to be piloted by a nurse upon admission. A lesson learned in this selection process was the importance of incorporating nurses into the site implementation team, as they provide a lot of direction regarding how to improve nutritional culture throughout the daily activities in the hospital. Although it is difficult to include hospital staff representing all health care professions, it is important for implementation and sustainability that the implementation team be as inclusive and as multidisciplinary as possible. The site implementation team is influential in tailoring INPAC, and in considering feedback from team members through focus groups conducted prior to implementation. PDSA cycles, described in more detail in the Selecting, tailoring, and implementing interventions section, facilitate this adaptation and testing out of how INPAC needs to be tailored to the local site.
Assessing barriers to knowledge use
To assess barriers to knowledge use during implementation, a combination of qualitative and quantitative data are/were collected. In study 2, INPAC developmental focus groups and stakeholder meetings highlighted potential issues with the tool itself, such as confusion in terminology and strategies to overcome these issues, and other potential barriers to suggested solutions.9 For example, the difficultly in having a “whole-hospital” approach meant that the project must be presented or “marketed” in several different ways. For staff working directly with patients, the message is about patient safety and treating food as medicine. For hospital management, the message is and was initially presented in terms of cost and utilizing background data,5 as well as what new information will be collected regarding cost, resource utilization, etc, as INPAC is implemented. In the M2E study (study 4), focus groups and interviews conducted pre- and post-INPAC implementation identify further details regarding potential barriers to implementation, including use of specific tools, auditing processes, and other topics that are relevant to the site undertaking full implementation.
A staff survey is used to assess pre- and post-implementation changes in knowledge, attitudes, and self-perceived practices (KAP). The survey is designed to investigate staff knowledge of malnutrition prevalence, use of screening tools, monitoring processes including food intake and patients’ weight, and practices regarding promotion of food intake of malnourished patients. All of these practices are consistent with the core components of INPAC. Results from the pre-implementation survey identified gaps in staff knowledge, and continues to inform education delivered during implementation. The results from this survey within M2E are still to be published.
To track fidelity to implementation in M2E, INPAC audits conducted regularly track progress regarding INPAC implementation, and are used by sites to target key areas for improvement. It is anticipated that as the project progresses and implementation becomes ingrained in care routines, greater proportions of patients will have received the core components of the care pathway. As a way to highlight gaps and to address barriers to using the care pathway, audit data are summarized monthly by the research team and are disseminated to the hospital through an indicator report. Scorecards will be used to collect the planning ideas of the site implementation team and to collect the stepwise improvements they undertake with PDSA cycles. This scorecard will also track training and other activities undertaken to implement and sustain INPAC. These tools will be available for hospitals to use upon request through the CMTF website.
Selecting, tailoring, and implementing interventions
For M2E, baseline data were collected, including the proportion of malnourished patients identified through the Canadian Nutrition Screening Tool (CNST), barriers to food intake experienced by patients, and their quality of life and food intake. The staff KAP and a site survey were used to establish current processes and activities with respect to nutritional care. These data are currently being used to lay the groundwork to address gaps in nutritional care and to determine how consistent or inconsistent current practices are with INPAC. Raising awareness of the implementation teams on these gaps specific to their unit can help to establish buy-in for implementation.
Throughout implementation and the KTA process, the implementation teams will initiate a series of PDSA cycles, with data captured by scorecards. These cycles promote the use of an iterative approach, which uses small-scale cycles to rapidly assess change and to adapt to feedback, thereby providing a flexible approach to delivery.13 Data regarding the tailoring and implementation process provide suggestions and examples to other hospitals on methods of implementation, which are consistent and are therefore perceived as effective by site staff. Site staff currently have the opportunity to network and share their experiences at monthly teleconferences, as well as a LISTSERV™, which enhances site-specific tailoring of solutions.
Education for staff (physicians, nurses, dietitians and other allied health care providers) and patients is conducted throughout the implementation based on barriers/needs identified in the KAP survey and as highlighted at a local level. Education is also conducted with appropriate staff regarding their role in carrying out components of INPAC. The project team has created educational materials regarding prevalence of malnutrition in Canadian hospitals, barriers to food intake, strategies to address barriers, and malnutrition screening and assessment. The study sites can tailor and select those aspects of training they consider most relevant for their team/hospital. After the completion of the research project, all material including tools and education will remain available for the hospital to use and adapt thus increasing the likelihood of sustainable change. Once finalized, all education materials will be available from the CMTF website.
Monitoring and evaluating knowledge use
For effective implementation, it is important that monitoring and evaluation strategies are in place to determine when a change is having an effect. In M2E, the research team has created a series of tools to monitor and evaluate the implementation process. This package includes comparing pre- and post-implementation scores on the staff KAP questionnaire; length of stay data collected from hospital administrative data; trends in the INPAC process collected through the audit tool; and patient-reported outcomes collected through the Mealtime Audit Tool and the My Meal Intake Tool (tools created and validated in study 3). A context assessment conducted pre-, during and post-implementation determines if there is a change in acceptance toward implementation, and this assessment is a way of scoring how each site is progressing in their ability to implement change.9
Resource utilization measures are currently being used to determine how implementation affects current work. For example, the amount of time required for a nurse to conduct nutritional screening, the number of additional referrals received by the dietetic team, and the additional time dietitians spend on implementing INPAC are all key resource implications of INPAC implementation.
Sustained knowledge use
Within the KTA process, sustainability of knowledge use is one of the most important aspects of implementation, yet is the least often completed or reported.3 By incorporating hospital staff in the implementation and by continuously collecting data regarding plans for sustainability, it is anticipated that this phase will be successful in the five test sites selected for study 4. To test this assumption, in the final 6 months of M2E (study 4), data collection will focus on monitoring the key elements of INPAC to determine if they are sustained without continual support from the research and site implementation teams. Other approaches for promoting sustainability include the “housing” of the project; for example, the INPAC implementation toolkit (the final outcome of the M2E project) will be available on the CMTF website. This availability will increase access to implementation tools so that they are readily available for uptake by other interested parties. Additional investments will also allow for sustained support for auditing, etc; however, each project should have a sustainability plan regardless of this additional investment.
Discussion
This article presents a program of research, consisting of four studies, as an example of the KTA process. Although implementation projects require adaptation based on the needs of the process, it is integral to have examples to foster development of new implementation projects, particularly in nutrition. The use of existing implementation frameworks, such as KTA, is integral to effective implementation, as they can be used as a guide to ensure that all steps are considered and that there is a clear plan of action.1,12 Overall, implementation frameworks increase the likelihood that successful nutritional interventions can translate into improved patient care and increased food intake.
Implementation is an iterative process, and as many factors need to be considered, reporting all steps can be a challenge. Emphasis on reporting is typically placed on the outcome and results, rather than on the process. Yet, without understanding the how of better practices with respect to implementation, KT/IS research in the area of nutrition will be limited. For this reason, describing the process of prospectively selecting KTA as the KT/IS framework, and describing the studies undertaken and how they are linked, provides a unique yet important example of process reporting. Reproducibility of implementation studies is key for scaling up or “rolling out” of the plan, and increases the strength of the evidence produced by these studies. The current report serves as not only an example of the KTA process, but also demonstrates the research activities, knowledge creation, and early action cycle steps required before an implementation project, such as M2E, can be conducted. The current report also provides an audit of the entire M2E implementation process and promotes future uptake of INPAC in other settings beyond the test sites. This paper and supporting evidence emphasizes the need for prospective selection of KT/IS frameworks in implementation studies.
It is important to acknowledge that this example of the KTA process does not include all aspects of this program of research, only those relevant to the KTA process. Detail has not been provided in the current report regarding INPAC or results of each study, as other publications focus on these results.4,9 However, using an implementation framework prospectively allows for greater structure and consistency, and increases the potential that an implementation project will be sustainable.1 It is also important to consider that “new” knowledge from the early phases of KTA may require several iterations before it is suitable to continue through the other phases of KTA. The KTA process is not fully linear and relies on many iterations of each of the steps within the cycle to allow for incorporation of new knowledge and ideas, and ensure that a strong plan is taken forward and be adapted as required.
The field of nutrition needs to conduct implementation studies prospectively by using appropriate frameworks to ensure that evidence emerging from nutritional research is translated and implemented effectively. Learning by example is a key way to move forward, and supports the effective translation of knowledge into practice, particularly in health care.
Conclusion
Using this nutritional program of research as an example of the KTA process demonstrates the need for project leaders to adapt to the needs of the audience, to encompasses local context, and to prospectively plan for potential ways to create sustainable change. Incorporation of KT/IS framework is particularly important in the field of nutrition, and aims to address the gap between evidence and practice. The steps of the KTA process are key to facilitating implementation and in promoting potential sustainability in future KT/IS projects. Publications such as the current work, which outline the steps within a well-accepted implementation framework, are essential in the field of nutrition in order to promote the use of such frameworks in future implementation initiatives.
Acknowledgments
The authors would like to thank Bridget Davidson for her work with the overall project, as well as James McCullough for his work in creating some of the implementation tools. Acknowledgment is also given to CMTF for their continued work, as well as to the M2E team, who are currently implementing this project. The authors would also like to thank Dr Lauren Ball for editorial assistance and for strategic direction with this publication process. This research is funded by the Technology Evaluation in the Elderly Network (TVN), which is supported by the Government of Canada through the Networks of Centres of Excellence program.
Author contributions
CL and HK both contributed to the conceptualization of the manuscript, CL led the drafting of the manuscript, and HK contributed to manuscript development. Both authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Grimshaw JM, Eccles MP, Lavis JN, Hill SJ, Squires JE. Knowledge translation of research findings. Implement Sci. 2012;7:50. | |
Straus S, Tetroe J, Graham ID. Knowledge Translation in Health Care. Moving from Evidence to Practice. Selecting, Tailoring, and Implementing Knowledge Translation Interventions. 2nd ed. Oxford: Wiley-Blackwell BMJ Books; 2013. | |
Wiltsey Stirman S, Kimberly J, Cook N, Calloway A, Castro F, Charns M. The sustainability of new programs and innovations: a review of the empirical literature and recommendations for future research. Implement Sci. 2012;7:17. | |
Allard JP, Keller H, Jeejeebhoy KN, et al. Malnutrition at hospital admission-contributors and effect on length of stay: a prospective cohort study from the Canadian Malnutrition Task Force. JPEN J Parenter Enteral Nutr. Epub 2015 Jan 26. | |
Curtis L, Keller H H, Allard J, et al. The costs of malnutrition in acute care: a report of the Canadian Malnutrition Task Force. In press. 2015. | |
Norman K, Pichard C, Lochs H, Pirlich M. Prognostic impact of disease-related malnutrition. Clin Nutr. 2008;27(1):5–15. | |
Barker LA, Gout BS, Crowe TC. Hospital malnutrition: prevalence, identification and impact on patients and the healthcare system. Int J Environ Res Public Health. 2011;8(2):514–527. | |
Correia MI, Waitzberg DL. The impact of malnutrition on morbidity, mortality, length of hospital stay and costs evaluated through a multivariate model analysis. Clin Nutr. 2003;22(3):235–239. | |
Keller H, McCullough J, Davidson B, et al. The Integrated Nutrition Pathway for Acute Care (INPAC): building consensus with a modified Delphi. Nutr J. 2015;14:63. | |
Canadian Patient Safety Institute [webpage on the Internet]. Improvement frameworks: getting started kit. Edmonton, Alberta: Canadian Patient Safety Institute; 2011. Available from: http://www.patientsafetyinstitute.ca/en/toolsResources/ImprovementFramework/Documents/Improvement%20Frameworks%20GSK%20EN.PDF. Accessed June 01, 2015. | |
Field B, Booth A, Ilott I, Gerrish K. Using the Knowledge to Action Framework in practice: a citation analysis and systematic review. Implement Sci. 2014;9:172. | |
Graham ID, Logan J, Harrison MB, et al. Lost in knowledge translation: time for a map? J Contin Educ Health Prof. 2006;26(1):13–24. | |
Taylor MJ, McNicholas C, Nicolay C, Darzi A, Bell D, Reed JE. Systematic review of the application of the plan-do-study-act method to improve quality in healthcare. BMJ Qual Saf. 2014;23(4):290–298. | |
Rycroft-Malone J. The PARIHS framework–a framework for guiding the implementation of evidence-based practice. J Nurs Care Qual. 2004;19(4):297–304. | |
Meyers DC, Durlak JA, Wandersman A. The quality implementation framework: a synthesis of critical steps in the implementation process. Am J Community Psychol. 2012;50(3–4):462–480. | |
Keller H, Allard J, Vesnaver E, et al. Barriers to food intake in acute care hospitals: a report of the Canadian Malnutrition Task Force. J Hum Nutr Diet. Epub 2015 Apr 20. | |
Duerksen DR, Keller HH, Vesnaver E, et al. Physicians’ perceptions regarding the detection and management of malnutrition in Canadian hospitals: results of a Canadian Malnutrition Task Force survey. JPEN J Parenter Enteral Nutr. 2015;39(4):410–417. | |
Duerksen DR, Keller HH, Vesnaver E, et al. Nurses’ perceptions regarding the prevalence, detection, and causes of malnutrition in Canadian hospitals: results of a Canadian Malnutrition Task Force survey. JPEN J Parenter Enteral Nutr. Epub 2014 Sep 4. | |
Laur C, McCullough J, Davidson B, Keller H. Becoming food aware in hospital: a narrative review to advance the culture of nutrition care in hospitals. Healthcare. 2015;3(2):393–407. | |
Bell JJ, Bauer JD, Capra S, Pulle RC. Multidisciplinary, multi-modal nutritional care in acute hip fracture inpatients–results of a pragmatic intervention. Clin Nutr. 2014;33(6):1101–1107. | |
Cheung G, Pizzola L, Keller H. Dietary, food service, and mealtime interventions to promote food intake in acute care adult patients. J Nutr Gerontol Geriatr. 2013;23(3):175–212. | |
Davidson B. An Inter-professional Approach to Malnutrition in Hospitalized Adults: Dietitians Leading the Way. Toronto, Ontario: Dietitians of Canada; 2014. Available from: http://www.dietitians.ca/ Downloads/Public/Interprofessional-Approach-to-Malnutrition-in-Hosp.aspx. Accessed on June 01, 2015. | |
Keller HH, Vesnaver E, Davidson B, et al. Providing quality nutrition care in acute care hospitals: perspectives of nutrition care personnel. J Hum Nutr Diet. 2014;27(2):192–202. | |
Tappenden KA, Quatrara B, Parkhurst ML, Malone AM, Fanjiang G, Ziegler TR. Critical role of nutrition in improving quality of care: an interdisciplinary call to action to address adult hospital malnutrition. J Parenter Enteral Nutr. 2013;37(4):482–497. | |
Fink A, Kosecoff J, Chassin M, Brook RH. Consensus methods: characteristics and guidelines for use. Am J Public Health. 1984; 74(9):979–983. | |
Hsu CC, Sandford BA. The Delphi Technique: making sense of consensus. Prac Assess Res Eval. 2007; 12(10):1–8. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.