Back to Journals » Clinical Ophthalmology » Volume 18
Implantation of Hydrophobic Acrylic Toric Intraocular Lens with High-Water Contents Using Swept-Source Optical Coherence Tomography Biometer Integrated with a Surgical Guiding System
Authors Matsumoto Y, Azuma Y, Karasawa Y, Suzuki N
Received 27 December 2023
Accepted for publication 17 April 2024
Published 25 April 2024 Volume 2024:18 Pages 1117—1124
DOI https://doi.org/10.2147/OPTH.S456609
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Yukihiro Matsumoto,1– 3 Yuichi Azuma,3 Yasue Karasawa,2 Noriyuki Suzuki3
1Eye Care Clinic, Saitama, Japan; 2Eye Care Clinic Tokyo, Tokyo, Japan; 3Eye Care Clinic Fukushima, Fukushima, Japan
Correspondence: Yukihiro Matsumoto, Eye Care Clinic, 829 Hikawa-cho, Souka, Saitama, 340-0034, Japan, Tel +81 30 6262 6100, Email [email protected]
Purpose: To evaluate postoperative outcomes after implantation of toric intraocular lenses (IOLs) made of high-water-content hydrophobic acrylic material in Japanese patients using a swept-source optical coherence tomography (SS-OCT) biometer integrated with a surgical guiding system.
Patients and Methods: In this prospective observational study, toric IOL models CNW0T3 to CNW0T9 (Alcon) were implanted in 33 eyes of 33 patients and followed-up for one month. Powers and toric models were determined using an SS-OCT biometer ARGOS® Ver 1.5 (Alcon), and the IOLs were aligned using surgical guidance. Differences between planned and actual axis positions at the end of the surgery (misalignment) and rotations from the end of surgery to one month postoperatively were measured. Additionally, postoperative uncorrected visual acuity, refraction, and residual astigmatism were evaluated.
Results: Mean and median misalignments were 2.3° (standard deviation [SD]: 1.6, 95% confidence interval [CI]: 1.7– 2.9) and 2°, and those of postoperative rotation were 2.4° (SD: 2.6, 95% CI: 1.5– 3.4) and 2°, respectively. Mean postoperative refraction was 0.06 D (SD: 0.62). Prediction errors within ± 0.5 and ± 1.0 D were 69.7% and 93.9%, respectively. Mean residual astigmatisms were 0.19 D (SD: 0.41), and mean uncorrected visual acuity was 0.00 logMAR (SD: 0.11), and 64% of the eyes scored 20/20 or better.
Conclusion: Implantation of high-water-content hydrophobic acrylic toric IOLs using SS-OCT biometry integrated with a surgical guiding system effectively corrected corneal astigmatism with accurate IOL alignment in Japanese patients.
Keywords: toric intraocular lens, swept-source optical coherence tomography, biometry, surgical guiding system
Introduction
During cataract surgery, corneal astigmatism is corrected using toric intraocular lenses (IOLs).1 Accurate correction requires precise biometric measurements, proper selection of the toric model, advanced calculation of the IOL axis position, and surgical alignment of the IOL axis. Precise biometry is necessary for determining the power of IOLs and is crucial for toric model selection and calculation of IOL axis positions, as the effect of astigmatic correction depends on the cylindrical power of each toric model and the effective lens position from the cornea.2 As toric IOLs are primarily used to provide postoperative uncorrected visual accuracy with no or less use of astigmatic corrections using spectacles, precise power calculations and toric model selection are important. Currently, swept-source optical coherence tomography (SS-OCT) is used for biometry because of its high acquisition rate and repeatability in axial length (AL) measurements. Because the surfaces of the cornea, crystalline lens, and retina can be recognized, the AL can be calculated using the refractive index of each section.3 An ARGOS® Ver 1.5 (Alcon Vision LLC, Fort Worth, TX, USA) is an SS-OCT biometry using the segmented refractive index to measure the AL with a wavelength of 1060 ± 10 nm. The ARGOS® Ver 1.5 is integrated with the Verion image guiding system (Alcon Vision LLC), thereby allowing a direct interface with the surgical guiding system, which enables seamless and accurate data transmission. Using such a sophisticated system, highly accurate toric IOL implantation is anticipated.
Recently, toric IOLs made of hydrophobic acrylic materials with high-water content, called Clareon® (Alcon Vision LLC), were developed to reduce the incidence of glistening and increase surface light scattering due to subsurface nanoglistening (SSNG), which was observed in previous AcrySof IOLs. The reduction in glistenings and SSNG in Clareon IOLs has been confirmed experimentally4 and clinically.5,6 We anticipated that the alignment of the Clareon toric IOL would be comparable to that of the AcrySof IQ toric IOLs.7 However, the misalignment and postoperative rotation have rarely been evaluated. This prospective observational study aimed to evaluate the postoperative outcomes after implantation of toric Clareon IOLs in Japanese patients using the latest SS-OCT system integrated with surgical guidance.
Materials and Methods
Participants
The study protocol was approved by the independent investigational review board of a non-profit organization MINS in Tokyo, Japan (Approval number: 210235) and performed according to the tenets of the Declaration of Helsinki and Ethical Guidelines for Medical and Biological Research Involving Human Subjects in Japan. Written informed consent was obtained from all the participants. Inclusion criteria were patients aged ≥20 years, cataract eyes planning to undergo cataract surgery with implantation of Clareon toric IOL models CNW0T3 to CNW0T9 (Alcon), and a potential postoperative corrected distance visual acuity (CDVA) of 14/20 or better in the Snellen’s notation. Eyes with additional surgery between cataract surgery and one month postoperatively, implantation of capsule tension rings, any ocular pathology influencing postoperative vision except for cataracts, or intraoperative complications, such as disabled implantation within the capsule, were excluded. One eye of each patient was included in this study. For cases of bilateral implantation, the first eye was selected for the analysis.
A minimum sample size for obtaining the mean misalignment angle with a confidence level of 95% and confidence width of 1.2° was 27 eyes, when the standard deviation (SD) of misalignment was 1.5° (Package “presize” in R ver. 4.2.1). Assuming a dropout rate of 20%, 34 participants were included in this study.
Intraocular Lens and Surgery
Routine preoperative examinations included biometry of the participants’ eyes measured using an SS-OCT biometer ARGOS® (Ver 1.5). After capturing three SS-OCT images, the mean values of biometric parameters, such as AL, keratometry, corneal thickness, anterior chamber depth, white-to-white width, and lens thickness, were obtained. To calculate the AL, individual refractive indices of the cornea, anterior chamber, crystalline lens, and vitreous body were used. In the measurements, reference eye images were captured under illumination by a white LED (wavelength of 400–700 nm with a peak at 460 nm) for surgical guidance, which adjusted the cycle torsion during the surgery.
The CNW0T3–CNW0T9 implants were single-piece hydrophobic acrylic toric IOLs. This Clareon material was characterized as having a high water content (1.5% at 35°C) and lower glass temperature (9.1°C). The platform of the toric IOLs was the same as that of the AcrySof toric IOL (Alcon Vision LLC), except for the material. The power of the implanted IOLs was determined for emmetropia or slight myopia using the Barrett Universal II formula. The lens factor used was 2.041, which was optimized in our clinic using ARGOS. The toric models and axis positions were then determined using the Barrett Toric Calculator with a surgically induced astigmatism of 0.3 D for 2.4-mm temporal incisions. Data required for the surgical guidance were transferred from the biometer.
After removing the cataract using the phacoemulsification and aspiration technique with the Centurion® Vision System (Alcon Vision LLC), the toric IOLs were inserted into the capsule using AutoSert® IOL injectors (Alcon Vision LLC). The IOL axes were aligned according to the guidance images from the ARGOS system. At the end of the surgery, anterior segment images were captured using a slit-lamp microscope, and the positions of the IOL axes were measured.
Postoperative Examination
One month postoperatively, uncorrected distance visual acuities (UDVA) and CDVA and manifested spherical, cylindrical, and spherical equivalent (SE) refractions were examined using a Landolt-ring chart at a distance of 5 m. After mydriasis, anterior segment images were captured in the same manner as at the end of the surgery.
Statistical Analysis
The positions of the IOL axis were measured from the anterior segment images at the end of surgery and one month postoperatively. Guided images acquired during the surgery were overlaid by adjusting the image size for the corneal diameter and rotating it to meet the positions of the subconjunctival blood vessels and iris patterns. Absolute differences in positions between the planned position and the end of the surgery were calculated as misalignments. Rotations at the end of the surgery and one month postoperatively were also calculated. Means, 95% confidence intervals (95% CI), and medians of the misalignments and rotations were calculated. The eyes within 5° and 10° were also counted.
Postoperative UCVA and DCVA were converted to logMAR values, and the mean and SD values were calculated. Prediction errors in the use of ARGOS biometry together with the Barrett Universal II formula were evaluated. Prediction errors were calculated with the prediction of postoperative refraction and manifest SE refraction at one month postoperatively. The arithmetic mean, median of absolute prediction errors, and rate of eyes within ±0.5 D and ±1.0 D were obtained.
Preoperative corneal and postoperative refractive astigmatism were analyzed in the same manner as previously suggested.8 Cumulative histogram of the magnitude of the preoperative corneal and postoperative refractive (residual) astigmatisms were obtained with astigmatism ≤0.25 D, ≤0.5 D, ≤0.75 D, ≤1.0 D, ≤1.5 D, and ≤2.0 D. Residual astigmatisms were vertexed to the corneal plane. Vectors of the preoperative corneal and residual astigmatisms were also plotted in double-angle coordinates with the centroids, SDs of the centroids, and 95% confidence ellipses of astigmatism.
Results
Although 38 participants were enrolled, 33 patients (33 eyes) completed follow-up for one month postoperatively. Demographic data of the participants are presented in Table 1. Three eyes had an epiretinal membrane. Predicted postoperative refractions ranged between −3.21–0.10, and the mean was −0.30 (SD: 0.55). No intraoperative or postoperative complications were observed.
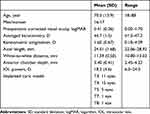 |
Table 1 Demographic Data of the Study Participants |
The mean and median of the misalignment angles were 2.3° (SD: 1.6, 95% CI: 1.7–2.9) and 2°, respectively, and the range was between 0 and 6. Figure 1 shows the distribution of misalignment and postoperative rotation angles. Twenty-nine (87.9%) and 33 (100%) eyes achieved misalignments within 5° and 10°, respectively. Rotation was measured in 32 of the 33 eyes. The mean and median were 2.4 (SD: 2.6, 95% CI: 1.5–3.4) and 2°, respectively, and the range was from 0° to 12°. In 27 (84.4%) and 31 (96.9%) eyes, the rotations were less than 5° and 10°, respectively. In the 12° eye, the AL was 22.12 mm, but there was no singularity in the biometric parameters.
 |
Figure 1 Distributions of intraoperative misalignment (left) and postoperative rotation (right) angles. |
At one month postoperatively, the mean UDVA and CDVA were 0.00 (SD: 0.11) and −0.04 (SD: 0.07) logMAR. The mean of SE refractions and residual astigmatisms were 0.06 D (SD: 0.62) and 0.19 D (SD: 0.41). Cumulative percentages of eyes achieving UDVA and CDVA are shown in Figure 2. There were 64% of eyes obtaining UCVA of 0.0 logMAR (20/20 in Snellen notation) or better. Using the ARGOS biometry and Barrett Universal II formula, the mean prediction error was 0.37 D (SD: 0.32), and the median of the absolute errors was 0.31 D. The prediction errors within ±0.5 and ±1.0 D were obtained in 23 and 31 eyes, respectively (69.7% and 93.9%, respectively).
 |
Figure 2 Cumulative percentages of eyes achieving uncorrected and corrected distance visual acuities (UDVA and CDVA, respectively) at one month postoperatively. |
Figure 3 shows the cumulative histogram of the magnitude of preoperative and postoperative residual astigmatism in the corneal plane (N = 33). Residual astigmatisms within 0.5 D and 1.00 D were achieved in 82% and 91% eyes, respectively. Figure 4 shows the double-angle plots of the preoperative corneal (left) and postoperative refractive (center) astigmatisms and postoperative refractive astigmatism prediction errors (right). There was no with-the-rule postoperative refractive astigmatism. Results of the vector analysis indicated that the astigmatic prediction errors within 0.5 D and 1.00 D were obtained in 82% and 94% eyes, respectively.
 |
Figure 3 Cumulative histogram of the magnitude of preoperative corneal and postoperative residual astigmatisms on the corneal plane (N=33). |
 |
Figure 4 Double-angle plots of preoperative corneal (left) and postoperative refractive (center) astigmatisms and postoperative refractive astigmatism prediction errors (right). |
Discussions
In the current study, implantation of Clareon toric IOLs using the SS-OCT biometry system integrated with surgical guidance was effective in correcting corneal astigmatism with accurate IOL alignment. To the best of our knowledge, this is the first assessment of postoperative outcomes and IOL misalignment for the Clareon toric IOL using SS-OCT biometry integrated with surgical guidance. While accurate positioning and stability of toric IOLs are critical for preferred outcomes, current misalignment and postoperative rotation are quite small. Several studies have evaluated IOL misalignment and rotation. In a study by Webers et al, the mean IOL misalignment based on preoperative images and images taken 1 h after surgery with surgical guidance was 1.3° (versus 2.8° with manual marking).9 In a study by Walter et al7 the mean IOL rotation at one day after implantation of Clareon IOLs (N = 127) was 1.85°, and 4.7% eyes were over 5°. In an evaluation of alignments after surgery of 110 Japanese eyes with AcrySof IQ toric IOLs using image guidance, the mean misalignment one day postoperatively was 2.35°.10 The current misalignments were similar to the previous results. The postoperative rotations were also stable, as indicated in the previous results.7 Furthermore, Slade et al reported residual astigmatism within 0.5 D and 1.0 D in eyes implanted with AcrySof IQ toric IOL using Verion guiding system were achieved in 74.5% and 94% patients, respectively.8 Consequently, the current residual astigmatism with the Clareon toric and integrated ARGOS systems was similar to the use of AcrySof IQ toric IOLs and the Verion guiding system.11 Compared with the previous results, it was concluded that the current misalignment and rotation were comparable with the AcrySof or Clareon toric IOLs using the surgical guidance system.
In the use of toric IOLs for correcting corneal astigmatism and proving postoperative outcomes with the least additional astigmatic corrections,1 both accurate IOL power calculation and toric model selection are required. In IOL power calculations, SS-OCT biometry is preferred in terms of accuracy and repeatability in AL measurements, and the current prediction errors with the use of Barrett Universal II were similar to those obtained using other SS-OCT biometry and advanced power calculation formulas.12 A significant effect was not found in the current results, while the prediction accuracy using ARGOS was equal to or greater than the accuracy using other SS-OCT biometers that use an equivalent refractive index.3
In the study with the limited number of subjects, the follow-up period was relatively short. However, the current results demonstrate intraoperative IOL misalignment and early postoperative outcomes in the use of toric IOL of Clareon material and surgical guidance system. Future studies with long follow-up time and a larger sample size would be warranted to confirm our outcomes. In addition, the impact of axial length on the toric IOL rotation was not evaluated in the current study, and further studies should assess the influence of axial length on the toric IOL rotation.
Conclusion
The implantation of high-water-content hydrophobic acrylic toric IOLs using SS-OCT biometry integrated with a surgical guiding system effectively corrected corneal astigmatism with accurate IOL alignment among Japanese patients.
Data Sharing Statement
The data used and analyzed for this study are available from the corresponding author on reasonable request.
Ethics Approval and Informed Consent
The study protocol was approved by the independent investigational review board of a non-profit organization MINS in Tokyo, Japan, (Approval number: 210235) and performed according to the tenets of the Declaration of Helsinki and Ethical Guidelines for Medical and Biological Research Involving Human Subjects in Japan. Written informed consent was obtained from all the participants.
Consent for Publication
This is not applicable as the manuscript does not contain any participant-identifying information.
Acknowledgments
We thank Dr Kaori Sato for her support in designing the concept of this study and helpful discussion.
Author Contributions
All authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The study was supported by an investigator-initiated study grant (IIT#69775041) from Alcon Japan Ltd. The funder had no role in the study design; in the collection, analysis or interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Disclosure
Y. Matsumoto has received grants from Alcon Japan Ltd. The other authors report no conflicts of interest in this work.
References
1. Keshav V, Henderson BA. Astigmatism management with intraocular lens surgery. Ophthalmology. 2021;128(11):e153–e163. doi:10.1016/j.ophtha.2020.08.011
2. Holladay JT. Standardizing constants for ultrasonic biometry, keratometry, and intraocular lens power calculations. J Cataract Refract Surg. 1997;23(9):1356–1370. doi:10.1016/S0886-3350(97)80115-0
3. Tamaoki A, Kojima T, Hasegawa A, et al. Clinical evaluation of a new swept-source optical coherence biometer that uses individual refractive indices to measure axial length in cataract patients. Ophthalmic Res. 2019;62(1):11–23. doi:10.1159/000496690
4. Werner L, Thatthamla I, Ong M, et al. Evaluation of clarity characteristics in a new hydrophobic acrylic IOL in comparison to commercially available IOLs. J Cataract Refract Surg. 2019;45(10):1490–1497. doi:10.1016/j.jcrs.2019.05.017
5. Oshika T, Fujita Y, Inamura M, Miyata K. Mid-term and long-term clinical assessments of a new 1-piece hydrophobic acrylic IOL with hydroxyethyl methacrylate. J Cataract Refract Surg. 2020;46(5):682–687. doi:10.1097/j.jcrs.0000000000000142
6. Kinoshita K, Miyata K, Nejima R, Honbo M, Mori Y, Minami K. Surface light scattering from 1-piece hydrophobic acrylic intraocular lenses with hydroxyethyl methacrylate: contralateral observation for 7 years. J Cataract Refract Surg. 2021;47(6):702–705. doi:10.1097/j.jcrs.0000000000000621
7. Walters TR, Lehmann R, Moyes A, French JW, Sreenivasan V, Modi SS. Rotational stability of the clareon monofocal aspheric hydrophobic acrylic intraocular lens 6 months after implantation. Clin Ophthalmol. 2022;16:401–409. doi:10.2147/OPTH.S348551
8. Abulafia A, Koch DD, Holladay JT, Wang L, Hill W. Pursuing perfection in intraocular lens calculations: IV. Rethinking astigmatism analysis for intraocular lens-based surgery: suggested terminology, analysis, and standards for outcome reports. J Cataract Refract Surg. 2018;44(10):1169–1174. doi:10.1016/j.jcrs.2018.07.027
9. Webers VSC, Bauer NJC, Visser N, et al. Image-guided system versus manual marking for toric intraocular lens alignment in cataract surgery. J Cataract Refract Surg. 2017;43(6):781–788. doi:10.1016/j.jcrs.2017.03.041
10. Sasaki K, Eguchi S, Miyata A, et al. Anterior capsule coverage and rotational stability of an acrylic toric intraocular lens. J Cataract Refract Surg. 2021;47(5):618–621. doi:10.1097/j.jcrs.0000000000000489
11. Slade S, Lane S, Solomon K. Clinical outcomes using a novel image-guided planning system in patients with cataract and IOL implantation. J Refract Surg. 2018;34(12):824–831. doi:10.3928/1081597X-20181115-01
12. Savini G, Hoffer KJ, Balducci N, Barboni P, Schiano-Lomoriello D. Comparison of formula accuracy for intraocular lens power calculation based on measurements by a swept-source optical coherence tomography optical biometer. J Cataract Refract Surg. 2020;46(1):27–33. doi:10.1016/j.jcrs.2019.08.044
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
