Back to Journals » Clinical Ophthalmology » Volume 18
Implantable Collamer Lens Procedure Planning: A Review of Global Approaches
Authors Thompson V, Cummings AB , Wang X
Received 23 December 2023
Accepted for publication 21 March 2024
Published 6 April 2024 Volume 2024:18 Pages 1033—1043
DOI https://doi.org/10.2147/OPTH.S456397
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Scott Fraser
Vance Thompson,1,2 Arthur B Cummings,3 Xiaoying Wang4– 6
1Vance Thompson Vision, Sioux Falls, SD, USA; 2Department of Ophthalmology, University of South Dakota Sanford School of Medicine, Vermillion, SD, USA; 3Wellington Eye Clinic, Dublin, Ireland; 4Eye Institute and Department of Ophthalmology, Eye & ENT Hospital, Fudan University, Shanghai, People’s Republic of China; 5NHC Key Laboratory of Myopia, Fudan University, Shanghai, People’s Republic of China; 6Shanghai Research Center of Ophthalmology and Optometry, Eye & ENT Hospital, Fudan University, Shanghai, People’s Republic of China
Correspondence: Vance Thompson, Vance Thompson Vision, 3101 W. 57 th St, Sioux Falls, SD, SD 57108, USA, Tel +1 480-684-4851, Email [email protected]
Abstract: More than 2 million implantable collamer lenses (ICLs) have been implanted worldwide. With a central port to improve aqueous flow through the ICL, the latest iteration of this phakic intraocular lens (pIOL) has been shown to have stable outcomes with very low rates of adverse events. However, correct planning and ICL size selection continue to be important to achieve an optimal vault. Shallow or excessive vaults are not complications in and of themselves but may increase the risk of complications. Historically, surgeons have relied on measurements of anterior chamber depth (ACD) and manual, caliper-measured white-to-white (WTW) distance to select the ICL size. New diagnostic and imaging technologies such as optical coherence tomography (OCT) and ultrasound biomicroscopy (UBM) provide additional opportunities for visualization and measurement of the intraocular dimensions involved in phakic intraocular lens implantation, including sulcus-to-sulcus (STS) and angle-to-angle (ATA) diameters. This paper reviews various approaches to ICL planning and sizing that have been published in the peer-reviewed literature, all of which produce acceptable results for predicting vault and size selection. Surgeons may also want to identify a methodology for patient evaluation and ICL size selection that best aligns with their personal preferences, diagnostic technology, and familiarity with analytical optimization tools.
Plain Language Summary: Phakic intraocular lenses (pIOLs) are one method for correcting nearsightedness, with or without astigmatism. This category of refractive surgery has been growing rapidly in the US and around the world. Implantation of the implantable collamer lens (ICL), one type of pIOL, is safe and effective, with stable outcomes and low adverse event rates. When complications do occur, they are typically associated with an inappropriate vault, or distance between the implant and the natural lens. Preoperative planning and accurate ICL sizing are required to achieve an optimal vault and varies, depending on the diagnostic technology available to the surgeon. This paper reviews the current approaches to ICL planning and sizing in order to provide guidance to surgeons implanting this pIOL.
Keywords: phakic IOL, vault, sizing, white-to-white, sulcus-to-sulcus, angle-to-angle
Introduction
The EVO Implantable Collamer Lens (ICL, STAAR Surgical, Monrovia CA, USA) is a posterior chamber phakic intraocular lens (pIOL) that was first introduced globally in 2011. It differs from its predecessor, the Visian ICL, primarily in the introduction of a 0.36-mm diameter port in the center of the optic that allows aqueous to flow from the posterior to the anterior chamber and obviates the need for preoperative peripheral iridotomies.
More than 2 million of these pIOLs (Visian and EVO) have been implanted worldwide to date.1 In the United States, ICL use has grown rapidly since 2022, when the Food and Drug Administration approved the EVO lens, its toric equivalent, and a version with a larger optic diameter (EVO+). More than 100 scientific papers analyzing the performance of EVO and EVO+ lenses have been published.2
The long history and the published literature suggest that implantation of the EVO ICL is a safe, effective, and predictable procedure widely used for the correction of myopia and myopia with astigmatism, with stable outcomes and low adverse event rates.2–4 Patients implanted with these lenses achieve high optical quality within one month of surgery.5 The safety index, or ratio of postoperative to preoperative best corrected visual acuity (BCVA), and the efficacy index, or ratio of postoperative uncorrected distance visual acuity to preoperative BCVA, have both been consistently reported as ≥1.0.1–3,6 A recent retrospective evaluation of eyes treated contralaterally with EVO ICL and LASIK found no statistically significant difference in endothelial cell loss between the two groups during three years of follow-up.7 Longer-term follow-up may be beneficial to understand any differences in stability between the two types of procedures.
In the FDA-monitored clinical trial that led to US approval of the EVO ICL, there were no cases of pupillary block, angle closure glaucoma, pigment dispersion or anterior subcapsular cataract.6 Clinicians find that complications, when they do occur, are most often associated with excessive or insufficient vault, which may require rotation of the lens or an exchange for a lens of a different size. The ICL is designed to vault anteriorly over the crystalline lens. The manufacturer recommends an optimal postoperative vault of 250–900 µm or 50% to 180% of corneal thickness,8 others recommend 250–750 µm,9 and some assert an even wider range, from 90 µm to 1000 µm or greater, may be adequate in the absence of clinical sequelae.2,10,11
Postoperative vault (Figure 1) is influenced by ocular anatomy and the size of the ICL. It is a dynamic measurement, influenced as well by accommodation, pupil dynamics and light conditions.12,13 Based on measurements of the eye and anticipated vaulting, surgeons must choose one of four available ICL sizes: 12.1, 12.6, 13.2, or 13.7 mm in diameter. A lens that is too large may result in excessive vault, increasing the risk of endothelial cell loss, elevated IOP, mydriasis, angle closure or pupillary-block glaucoma, and iris chafing with pigment dispersion.14–16 Implanting an undersized lens with inadequate vault may increase the risk of anterior subcapsular cataract, as well as dislocation or rotation of the ICL.14,17 Shallow or excessive vaults are not complications in and of themselves, but may increase the risk of complications. Suboptimal lens size with inadequate vault, in particular, has been reported as the leading cause for ICL exchange.9 In one very large series of consecutive ICL cases, only 0.21% of the eyes (22/10,258) underwent secondary surgical intervention, including realignment of toric ICLs or lens exchange.17
 |
Figure 1 Anterior segment OCT image of postoperative vault with caliper values. Courtesy of Francisco Pastor-Pascual, Oftalvist Valencia. Abbreviation: OCT, optical coherence tomography. |
In the FDA clinical trial for the EVO and EVO+ ICLs, investigators were required to use the ICL size recommended by the manufacturer’s Online Calculation and Ordering System (OCOS) after entering the anterior chamber depth (ACD) and white-to-white (WTW) distance; the methods for measuring ACD and WTW were at the surgeon’s discretion. New imaging technologies such as optical coherence tomography (OCT) and ultrasound biomicroscopy (UBM) provide additional opportunities for visualization and measurement of the intraocular dimensions involved in phakic intraocular lens implantation. Methods for ICL size selection based on these imaging modalities have been widely used around the world, with very good outcomes. Moshirfar et al recently reviewed nomograms for ICL size selection and offered a flow chart for nomogram selection based on available technology.18
In order to help surgeons who are implanting or are about to begin implanting the ICL, this paper reviews alternative approaches to ICL planning and sizing that have been published in the peer-reviewed literature, including WTW, angle to angle (ATA) and sulcus to sulcus (STS) methodologies. Much as the choice of lamellar flap thickness, phaco device settings, or crosslinking protocols depends on regional norms and technological availability, surgeons may also want to identify a methodology for patient evaluation and ICL size selection that best aligns with their personal preferences, diagnostic technology, and familiarity with analytical optimization tools. The influence of pupil size and surgical placement will also be discussed.
Planning Methods
Indications
Approved indications for the ICL vary slightly by geographic location and ICL model. Key anatomical parameters include refraction, ACD, and anterior chamber angle grade. In all cases, the anterior chamber angle, as determined by gonioscopic exam, should be Grade III or larger; narrow angles are contraindicated. ACD should be at least 2.8 mm, although in the US, the lens is contraindicated in eyes with ACD <3.00 mm. EVO ICLs may be used in eyes with −0.5 to −20.0 D of myopia and up to 6.0 D of astigmatism at the spectacle plane, although some parts of this range would be considered off-label in the US The Visian ICL may be implanted in hyperopic eyes with between +0.50 and +16.0 D of hyperopia, provided the ACD and other requirements are also met. All ICL candidates should have a history of at least 1 year of stable refraction.
White-to-White
Conventionally, ICL size has been determined based on the horizontal corneal diameter, or WTW, and the ACD. These variables are entered into OCOS (http://www.ocos.staar.com/) to determine recommended ICL size according to the manufacturer’s nomogram.
WTW is an external measurement that serves as a surrogate for the internal ciliary sulcus where the ICL will be positioned after surgery. While the corneal diameter is often described as being measured from the 3 o’clock limbus to the 9 o’clock limbus, the limbus itself is a 1.5-mm to 2.0-mm “gray zone” which introduces up to 4.0 mm of variability in a WTW measurement depending on whether the inner or outer limbus is selected as a starting point. For the best reproducibility, surgeons should measure from mid gray zone to mid gray zone. The WTW can be measured inexpensively with calipers (Figures 2 and 3) or with widely available biometry, topography, and tomography devices. However, the correlation between WTW corneal measurements and the internal sulcus to sulcus measurement is weak.11,15,19
 |
Figure 3 Calipers setting during precision white-to-white measurement. Courtesy of Vance Thompson. |
The devices used for measuring WTW rely on a variety of optical principles for the automatic detection of physical landmarks, so there is some variation in their measurements, and they should not be used interchangeably.20 In our experience, automated Lenstar (Haag Streit, Köniz, Switzerland) and IOLMaster (Carl Zeiss Meditec, Dublin, CA, USA) WTW measurements overestimate WTW by 0.5 mm compared to Pentacam (Oculus Optikgeräte, Wetzlar, Germany), which overestimates WTW compared to manual caliper measurements. Ang et al found that mean WTW measurements were significantly different among four tested measurement devices (P < 0.0001), affecting the reproducibility of ICL sizing.14 They determined that, of all combinations of WTW and ACD measurements tested, an OCOS calculation based on IOLMaster ACD and IOLMaster WTW was the least likely to lead to an ICL size calculation that achieved the desired vault range, while caliper WTW plus any measure of ACD (Orbscan, IOLMaster, or Pentacam) was the most likely to achieve the desired vault.14
Caliper measurement of WTW can be optimized by performing it under moderate magnification at the microscope and using a lid speculum after topical anesthesia, with the patient in a supine position fixating on a fixation light. The WTW method has a high success rate. It has been reported to be the most predictable method available21 and to result in an achieved vault that is not clinically or statistically different from other methods of size calculation (Figure 4).3,22 In the FDA clinical trial of the EVO ICLs, mean vault at 6 months was 492 ± 227 µm, and there were only two eyes (0.3%, 2/629) that required secondary surgical intervention for anterior chamber angle narrowing related to excessive vault, neither of which had elevated IOP.6 In earlier meta-analyses conducted by Packer, the overall rate of secondary surgical intervention for EVO/EVO+ lenses was 0.47%3 to 0.80%.22 Recently, in comparing two ICL sizing nomograms and four vault prediction formulas, Tang et al determined that calculating the ICL size with the OCOS nomogram using Pentacam-derived WTW had the highest concordance rate with the ideal ICL size (ie, the size that would produce a vault between 250–750 um).20
 |
Figure 4 Pooled normal distribution of vault based on means and standard deviations of STS and WTW sizing methods.22 Source: Mark Packer, MD, previously printed in Clinical Ophthalmology. Abbreviations: STS, sulcus-to-sulcus diameter; WTW, white-to-white diameter. |
UBM Sulcus-to-Sulcus
High-frequency UBM with a wide scanning field enables direct measurement of the ciliary STS diameter (Figure 5). Given that the EVO ICL is implanted behind the iris in the ciliary sulcus, direct measurement of the STS would seem to be ideal for choosing the appropriate ICL size. This method has been in use in some form since at least 2011.
 |
Figure 5 Sulcus-to-sulcus diameter (light blue line) measurement with ultrasound biomicroscopy. Courtesy of STAAR Surgical. |
An early UBM nomogram developed by Dougherty et al used only ICL power and STS, measured with the VuMax-II (Sonomed Escalon, Lake Success, NY, USA), to determine ICL size.11 A table to look up the recommended size by STS and ICL power is available (Table 1) Using this nomogram, they achieved a mean postoperative vault of 340 μm ± 174 in 72 eyes (range 90 to 952 μm); there were no cases of inadequate or excessive vault.11 The ICL size using the nomogram was different from the size that would have been selected with the OCOS/WTW method in 69% of cases.11
 |
Table 1 ICL Size Selection According to Dougherty nomogram11 |
Also using the VuMax-II, Kojima et al developed an equation (K formula) for vault prediction and lens sizing using ACD, STS, and the STS-lens rise (STSL), or the distance between the STS plane and the anterior crystalline lens surface. Among 81 eyes studied, the mean postoperative vault was 640 µm, with 74.1% of eyes in the 250–750 µm range.15 Although there were no vault-related complications, 11.1% (9/81 eyes) had a vault higher than 1000 µm. The subjects in this study were all Japanese and highly myopic. The authors posited that ICL power might have had a stronger influence on results if the study had included a broader population.15
Reinstein et al used multivariate regression analysis to evaluate a variety of UBM measurements for ICL vault prediction.23 They found that STS itself was no longer statistically significant after including a new UBM measurement of the ciliary body inner diameter (CBID). A smaller CBID, they theorized, means the lens is under more compression and will consequently have a higher vault. Scotopic pupil diameter also significantly predicted postoperative ICL vault. The highest predictive power was achieved by combining CBID and pupil size with STSL, ICL size, and ICL power. With the formula they developed, Reinstein et al found that 94% of eyes achieved a vault within 300 μm of their target.23 Surgeons can use an online calculator with this formula at www.ICLSizing.com. Its use requires a number of data points available only from the Artemis Insight 100 (ArcScan, Golden, CO, USA) UBM device. Once these variables are inputted, the calculator provides the predicted vault for each of the four ICL sizes.
Another online calculator that makes use of UBM variables is available at http://www.zzcal.com/calc/en/icl. It incorporates the Zhang and Zheng (ZZ) ICL formula and relies on LT, vertical STS, and horizontal STS to predict vault and calculate IOL size.24
A promising new UBM-based planning methodology tool, “ICLguru”, was first presented in the Zaldivar Institute International Course in Mendoza, Argentina in November, 2023. It predicts central and peripheral vault and postoperative iridocorneal angles, with >97% of eyes being within 250 µm of the predicted vault. Published data on this methodology is anticipated in the near future.
UBM has strong theoretical advantages for measuring the actual size of the ciliary sulcus. However, several authors have found no relationship between UBM measurement of the horizontal STS and postoperative vault.23,25 This may be due in part to problems with inter-examiner variability and poor reproducibility increasing the error of STS measurements.15,22,26,27 Additionally, UBM can be time consuming in the clinic and uncomfortable for patients.26,28
Other characteristics of the ciliary sulcus structure discernable with UBM and/or OCT may also contribute to vault surprises. Chen et al reported that every 1° reduction in the iris-ciliary angle (ICA) was associated with 4% increased odds of vault greater than 1000 µm (OR = 0.96; 95% CI = 0.93 to 0.99; P < 0.001) and that an anteriorly positioned ciliary body was also associated with an increased risk of excessive vault after ICL implantation (OR = 3.57; 95% CI = 1.67 to 7.63; P < 0.001).29 Tan et al further explore the role of preoperative ICA in postoperative vault and conclude that assessment of ICL haptic-related parameters adds significant information to interpret the vault after surgery.30 The positioning of the ICL haptic outside the ciliary sulcus and features of ciliary sulcus morphology that affect the resting position of the ICL are strongly suspected to influence vault.27,30,31
OCT Angle-to-Angle
Swept-source optical coherence tomography can precisely measure a variety of anterior chamber parameters thought to be relevant to ICL sizing, including the angle-to-angle (ATA) distance, crystalline lens rise (CLR, or the distance between the anterior crystalline lens surface and the angle recess-to-angle recess line), and anterior chamber width (ACW, or the distance between the scleral spurs on the nasal and temporal sides) (Figure 6). An advantage of formulas that use ATA and related parameters is that OCT devices are widely available and familiar to cataract surgeons. Intra- and inter-examiner reproducibility are very good for anterior segment OCT.32 As with other types of ICL vault prediction and sizing approaches, devices cannot be used interchangeably. For example, Kim et al have noted that using anatomic values from Anterion OCT (Heidelberg) in formulas designed for the CASIA-2 OCT (Tomey, Nuremberg, Germany) does not guarantee accurate prediction.28
The NK formula was developed by Nakamura et al and has been revised several times,32–34 uses the ACW and CLR parameters from the CASIA-2 OCT device to calculate the predicted vault and select the ICL size.32 The CASIA-2 automatically identifies the position of the scleral spur and the angle recess and calculates other variables from those positions.32 The NK-1 formula was determined by its authors to achieve a 70% or greater probability of a vault within the range of 250–1,000 µm. However, because investigators intentionally selected larger ICL sizes based on age and biometry, the proportion of high vaults was higher than desired with the first iteration of the formula. The formula was then improved (NK-2) and a table was published to convert the optimal ICL size to one of the four commercially available sizes (Table 2).33 With the NK-2 formula, 91.2% of eyes achieved a vault in the 250–1,000 µm range, with a mean vault of 670 ± 223 µm. A third iteration (NK-3) achieved a similar percentage within the desired range and a mean vault of 533 ± 203 µm.34 The NK-3 formula uses a smaller horizontal compression-vault coefficient than has previously been assumed for most formulas. The NK-3 equation is: Optimal ICL size (mm) = 5.307 + 0.0617 x (ACW)[mm] +0.501 x (CLR) [mm].
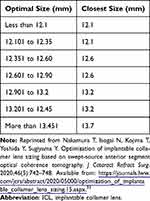 |
Table 2 ICL Size Selection Based on Optimal Size Calculation Using the NK-2 formula33 |
The KS formula, developed by Igarashi et al is a true ATA formula that also relies on data from the CASIA-2.35 The equation used to predict the ICL vault with the KS formula is Vault (μm) = 660.9 × (ICL size [mm] – ATA [mm]) + 86.6. Both the NK and KS formulas are integrated into the CASIA-2 device.
Most ICL vault prediction and ICL sizing formulas are based on the linear regression of a few variables. A limitation of linear regression is that it cannot always explain the relationships among various measurements.36 Machine learning models capable of integrating many variables and continually refining algorithms over time as more data are included may overcome this limitation and be helpful in choosing an appropriate size ICL.37 A group of researchers, including clinicians led by Ik Hee Ryu from B&V IIT Eye Center in Seoul, Korea, and VisuWorks machine learning specialists, have been working to develop vault prediction models using various machine learning methods and data from CASIA-2 and Anterion OCT devices.26,28,36,37
Kim et al found that Anterion measurements of aqueous depth (AQD), anterior chamber volume, anterior chamber angle (ACA) distance, spur-to-spur distance, crystalline lens thickness (LT), and WTW distance were highly associated with ICL vault.28 Ultimately, the least absolute shrinkage and selection operator (LASSO) regression model with the best vault prediction performance included only three variables: AQD, ACA, and LT.28 While similar to the NK formula using CASIA-2 OCT, the authors note that all measurements used in the LASSO model were captured using a fully automated process.
Kamiya et al reported that ICL size was the most important parameter in vault prediction, followed by the ICL power and CASIA-2 measurements of crystalline lens vault (LV), ACD, CLR, and ATA.26 All the machine learning methods tested showed significantly less mean absolute error and higher percentages of eyes within 200 μm of the targeted ICL vault than the conventional OCOS nomogram, although the mean vaults were similar. In this large study population of 1,745 eyes, extremely low vault (9 eyes) was more likely than extremely high vault (1 eye) to lead to ICL exchange. Additionally, the predictive model worked better on a validation data set from Korea than a second data set from Japan, highlighting the importance of optimizing ICL vault calculation formulas.26
A web-based application (http://loocus-iolcalc.ai) that enables clinicians to use an ensemble machine learning calculator developed by this research group has been reported to significantly improved ICL size selection accuracy.36
Much of the work in developing OCT- and ATA-based formulas has been done in highly myopic Asian eyes and thus may not be applicable to European populations or lower myopes. The smaller size ICLs predominate in Asian eyes; models trained on Asian eyes have almost none of the largest-size (13.7 mm) ICLs in their training data sets.9 Rocamora et al suggest that the degree of myopia affects several anterior chamber parameters relevant to sizing. They have recently published a suite of ICL sizing formulas evaluated in a white European population with a range of myopia from low to high.9 These formulas use OCT and biometry variables in a LASSO model capable of performing predictive tasks with a large number of independent variables. With the “LASSO-full” formula, the authors report achieving a mean vault of 491.78 ± 253.47 µm, and reducing the maximal absolute error (the largest absolute difference between the estimated and achieved vault) from as high as 841 µm with other formulas to just 347.6 µm with their LASSO-full model.9 The suite of formulas can be accessed at http://icl.emmetropia.be.
Some authors advocate using ATA to modify results of the OCOS nomogram. Beltrán-Murcia et al recently reported that the best results in sizing and vault ICL calculation are obtained using the OCOS online calculator (OCOS), as long as Pentacam-derived ACW and CLR are used as accessory measurements to help the surgeon decide when to reduce the OCOS-recommended ICL by one size.21
Discussion
Much as surgeons typically rely on measures of astigmatism from multiple devices for toric intraocular lens selection, ICL selection may benefit from the incorporation of measures from multiple diagnostic devices. Wu et al recently reported on a new formula (the WH formula) that combines conventional biometric information with OCT and UBM parameters.27 When ICL size, ATA, CLR, and CSA were all used to predict the vault with this formula, 92% of eyes achieved a postoperative vault in the targeted range. The authors suggest that ciliary sulcus angle (CSA), which they measured manually using ImageJ software and Suoer SW-3200 UBM images, represents a good quantitative assessment of the ciliary sulcus morphology, which they posit affects postoperative vault in ways that are difficult to predict.27 They do not use ACD in the prediction formula, believing that ACD does not directly affect the postoperative vault, but rather, influences the individual’s tolerable vault range. A deeper anterior chamber, therefore, can accommodate a larger vault but does not cause one, and a shallow chamber, by this logic, would have a tighter ideal range for the vault.27
Existing formulas may also need to be modified by ICL size,20,38 particularly for the largest and smallest ICL sizes. In one comparison of different prediction formulas, predicted and achieved vaults were within the desired range of 250–750 µm for the 12.6- and 13.2-mm ICLs, but not for the 12.1 and 13.7 mm ICLs.38
Preoperative pupil diameter is a factor in several ICL vault prediction formulas23,27,39 and may be an independent risk factor for abnormal vault.40 Shen et al hypothesize that a smaller preoperative pupil diameter may be associated with iris tension, which pushes the ICL back towards the crystalline lens after ICL implantation, resulting in a lower vault.39 After analyzing a very large data set of 6,297 eyes, they reported that pupil size was one of the most important features for vault prediction.39 Moshirfar et al also reported that pupil size, along with ACD and CBID, was significantly correlated with vault.41 Pupil movement is also a factor in vault change,42 suggesting that the consistency of lighting conditions may be important in the accuracy of both preoperative OCT imaging and measurement of postoperative vault.23,36 Cycloplegic drops that stimulate pupil change may also affect ICL vault.43 Lighting conditions may affect vault, pupil diameter, iris thickness, and angle opening distance.36 A wide variety of lighting conditions during OCT capture for ICL planning have been described,27,28,34 while some studies do not mention the lighting conditions under which OCT imaging was obtained.
In choosing a given formula or nomogram for ICL sizing, surgeons should take into consideration the demographics of the population on which the formula or nomogram is based. Formulas derived primarily in Asian eyes may underestimate the need for the largest ICL size, while those derived from European populations may overestimate ICL size for relatively smaller Asian eyes. A very recent paper retrospectively evaluating vault predictability in a US (Utah) patient population found that the Kim, Rocamora, Russo and Reinstein nomograms were the most predictive in this population.41 However, controlled, prospective comparisons are needed to validate this finding. The patient’s age at the time of ICL implantation may also be a consideration when using formulas that factor in CLR, as CLR may continue to increase over time due to natural lens aging.
Surgical technique is also likely a critical factor, as ICL footplate placement plays a significant role in lens vault and may be contributing to inaccuracies in vault prediction.12,23,27,44 The haptics or footplates of the ICL should ideally be positioned fully in the ciliary sulcus. However, considerable variability in haptic positioning has been seen in UBM studies.30,31 Wu et al posit that this is largely due to the ciliary sulcus angle (CSA). A small CSA, they note, prevents adequate contact between the foot plate and the ciliary sulcus, which causes the foot plate to lie above the ciliary sulcus and increase the vault. When the CSA is large, it reduces the support of the ciliary sulcus to the ICL lens and may cause the foot plate to move downward postoperatively, which results in a reduction in vault.27 In comparing eyes with low vs normal vault, Yiming et al found that malposition of the ICL haptics behind the ciliary process may lead to shallow vault.45 It has been suggested that adjustments to surgical technique, such as the use of intraoperative OCT to review the vault and angle anatomy during the procedure, may help to achieve more consistent ICL positioning.23 Footplate position can be a consideration during postoperative analysis of larger or smaller than anticipated achieved vault. The use of UBM imaging to visualize footplate positioning can be helpful in these cases.
Conclusion
In his 2016 review of sizing methodologies, Packer found a great deal of similarity in achieved vault, regardless of the sizing methodology employed.22 Similarly, in a 2018 review, Packer found no clinically meaningful or statistically significant difference in achieved vault based on WTW- or STS-based sizing methodologies.3
Although a target vault of 500 µm is typically considered ideal, the range of acceptable vault is quite broad, from at least 250 to 750 µm and likely well beyond. As Yang et al argue, achieving a perfect 500-µm vault or accurately calculating the precise vault may be less important than identifying risk factors for abnormal vault.40 The size of the CSA and perhaps other, as yet undefined, aspects of ciliary sulcus morphology that influence whether the ICL haptics can be correctly positioned in the ciliary sulcus may in fact be the greatest risk factors for abnormal vault. The influence of confounding morphological factors and the risks of abnormal vault are amplified when larger ICLs are implanted.40
A vault outside of the desired range does not necessarily cause complications or require an ICL exchange. With predecessors to the EVO ICL, a shallow vault was associated with the development of anterior subcapsular cataract.16,46 However, the central port introduced with the EVO ICLs helps to maintain metabolism and reduce the likelihood of lens opacification compared to prior lens designs without a central port, even with a shallow vault.10,21,22,42,46 Recent reviews have found rates of anterior subcapsular cataract of 0.0%3 and 0.17%4 for EVO ICL, with some cases in the latter review likely due to factors other than vault. Even in studies of EVO ICL eyes with very low vault of <250 µm or <100 µm, there have been few or no cases of anterior subcapsular cataract and no significant differences in lens density compared to a control.47,48
In summary, innovations in ICL design have significantly improved safety and efficacy across a wide range of measured vaults. All currently employed methods, including those based on WTW, STS, and ATA for predicting vault and selecting EVO ICL sizing produce acceptable results. Therefore, experienced surgeons should investigate the best method for their own personal use, based on the available measurement technology and their analytical optimization tools.
Acknowledgments
Jan Beiting (Wordsmith Consulting, Cary, NC) provided assistance in preparing this manuscript.
Disclosure
A. Cummings and X. Wang have no conflicts of interest to disclose for this work. V. Thompson reports personal fees from Acufocus, AdOM, Alcon, Allergan, Allotex, Amring, Avisi Technologies, Balance Ophthalmics, Bausch and Lomb, BRIM Biotechnology, BVI, Carl Zeiss Meditec, Centricity, Conjtac, Crystilex, CSO, D&D Biopharmaceuticals, DelSiTech, Euclid Vision Group, Avellino, Expert Opinion, eyeBrain Medical Inc, Eyedetec, Eyesafe, Forsight Robotics, Glaukos, iVeena, Johnson and Johnson, LayerBio, Lightfield Medical, Medevise, Melt Pharmaceuticals, NanoDrops, Ocular Innovations, Oculotix, Oyster Point Pharma, Rayner, Reopia, RxSight, Singular Strategies, Stuart Therapeutics, Tarsus Rx, TearClear, TearOptix, TherOptix, Treehouse Health, Trukera, Visus, 2EyesVision, Staar, Stepwise Medical, and Visant Medical, outside the submitted work.
References
1. Wannapanich T, Kasetsuwan N, Reinprayoon U. Intraocular implantable collamer lens with a central hole implantation: safety, efficacy, and patient outcomes. Clin Ophthalmol. 2023;17:969–980. doi:10.2147/OPTH.S379856
2. Martínez-Plaza E, López de la Rosa A, López-Miguel A, Holgueras A, Maldonado MJ. EVO/EVO+ visian implantable collamer lenses for the correction of myopia and myopia with astigmatism. Expert Rev Med Devices. 2023;20(2):75–83. doi:10.1080/17434440.2023.2174429
3. Packer M. The implantable collamer lens with a central port: review of the literature. Clin Ophthalmol. 2018;12:2427–2438. doi:10.2147/OPTH.S188785
4. Montés-Micó R, Ruiz-Mesa R, Rodríguez-Prats JL, Tañá-Rivero P. Posterior-chamber phakic implantable collamer lenses with a central port: a review. Acta Ophthalmol. 2021;99(3):e288–e301. doi:10.1111/aos.14599
5. Miao H, Chen X, Tian M, Chen Y, Wang X, Zhou X. Refractive outcomes and optical quality after implantation of posterior chamber phakic implantable collamer lens with a central hole (ICL V4c). BMC Ophthalmol. 2018;18:141. doi:10.1186/s12886-018-0805-3
6. Packer M. Evaluation of the EVO/EVO+ sphere and toric Visian ICL: six month results from the United States Food and Drug Administration clinical trial. Clin Ophthalmol. 2022;16:1541–1553. doi:10.2147/OPTH.S369467
7. Choi H, Ryu IH, Lee IS, Kim JK, Yoo TK. Comparison of implantation of posterior chamber phakic IOL implantation and laser vision correction in terms of corneal endothelial cells: 3-year observational paired-eye study. J Cataract Refract Surg. 2023;49(9):936–941. doi:10.1097/j.jcrs.0000000000001246
8. Alfonso JF, Fernandez-Vega L, Lisa C, Fernandes P, Jorge J, Montés Micó R. Central vault after phakic intraocular lens implantation: correlation with anterior chamber depth, white-to-white distance, spherical equivalent, and patient age. J Cataract Refract Surg. 2012;38(1):46–53. doi:10.1016/j.jcrs.2011.07.035
9. Rocamora L, Orlando JI, Lwowski C, Kohnen T, Mertens E, Van Keer K. Postoperative vault prediction for phakic implantable collamer lens surgery: LASSO formulas. J Cataract Refract Surg. 2023;49(2):126–132. doi:10.1097/j.jcrs.0000000000001079
10. Gonvers M, Bornet C, Othein-Girard P. Implantable Contact Lens for moderate to high myopia; relationship of vaulting to cataract formation. J Cataract Refract Surg. 2003;29:918–924. doi:10.1016/S0886-3350(03)00065-8
11. Dougherty PJ, Rivera RP, Schneider D, Lane SS, Brown D, Vukich J. Improving accuracy of phakic intraocular lens sizing using high-frequency ultrasound biomicroscopy. J Cataract Refract Surg. 2011;37(1):13–18. doi:10.1016/j.jcrs.2010.07.014
12. Gonzalez-Lopez F, Mompean B, Bilbao-Calabuig R, Vila-Arteaga J, Beltran J, Baviera J. Dynamic assessment of light-induced vaulting changes of implantable collamer lens with central port by swept-source OCT: pilot study. Transl Vis Sci Technol. 2018;7(3):4. doi:10.1167/tvst.7.3.4
13. Kato S, Shimizu K, Igarashi A. Vault changes caused by light-induced pupil constriction and accommodation in eyes with an implantable collamer lens. Cornea. 2019;38(2):217–220. doi:10.1097/ICO.0000000000001785
14. Ang RET, Reyes EKF, Ayuyao FAJ, Umali MIN, Cruz EM. Comparison of white-to-white measurements using four devices and their determination of ICL sizing. Eye and Vision. 2022;9:36–49. doi:10.1186/s40662-022-00308-z
15. Kojima T, Yokoyama S, Ito M, et al. Optimization of an Implantable Collamer Lens sizing method using high-frequency ultrasound biomicroscopy. Am J Ophthalmol. 2012;153(4):632–637. doi:10.1016/j.ajo.2011.06.031
16. Fernandes P, Gonzalez-Meijome JM, Madrid-Costa D, Ferrer-Blasco T, Jorge J, Montés Micó R. Implantable collamer posterior chamber intraocular lenses: a review of potential complications. J Refract Surg. 2011;27(10):765–776. doi:10.3928/1081597X-20110617-01
17. Wei R, Li M, Aruma A, et al. Factors leading to realignment or exchange after implantable collamer lens implantation in 10,258 eyes. J Cataract Refract Surg. 2022;48:1190–1196. doi:10.1097/j.jcrs.0000000000000950
18. Moshirfar M, Santos JM, Cha DS, Herron M, Stoakes IM, Hoopes PC. Exploring nomograms for implantable collamer lens size selection in myopia: a literature-based compilation. Clin Ophthalmol. 2023;17:3307–3322. doi:10.2147/OPTH.S427815
19. Reinstein DZ, Archer TJ, Silverman RH, Rondeau MJ, Coleman DJ. Correlation of anterior chamber angle and ciliary sulcus diameters with white-to-white corneal diameter in high myopes using Artemis VHF digital ultrasound. J Refract Surg. 2009;25:185–194. doi:10.3928/1081597X-20090201-03
20. Tang C, Sun T, Duan H, Liu Y, Qi H. Evaluation of the performance of two nomograms and four vault prediction formulas for Implantable Collamer Lens size selection. J Refract Surg. 2023;39(7):456–461. doi:10.3928/1081597X-20230605-01
21. Beltrán-Murcia J, Capelo LÁ, Blázquez-Sánchez V. Analysis of vault prediction in phakic implantable phakic collamer lenses: manufacturer’s calculator vs theoretical formulae vs. clinical practice. Graefes Arch Clin Exp Ophthalmol. 2023;261(8):2403–2409. doi:10.1007/s00417-023-06016-1
22. Packer M. Meta-analysis and review: effectiveness, safety, and central port design of the intraocular collamer lens. Clin Ophthalmol. 2016;10:1059–1077. doi:10.2147/OPTH.S111620
23. Reinstein DZ, Archer TJ, Vida RS, Piparia V, Potter JG. New sizing parameters and model for predicting postoperative vault for the Implantable Collamer Lens posterior chamber phakic intraocular lens. J Refract Surg. 2022;38(5):272–279. doi:10.3928/1081597X-20220302-01
24. Zhang J, Shao J, Zheng L, Zhao X, Chen S. Implantable collamer lens sizing based on measurement of the sulcus-to-sulcus distance in ultrasound biomicroscopy video clips and ZZ ICL formula. BMC Ophthalmol. 2022;22(1):363. doi:10.1186/s12886-022-02583-9
25. Lee D-H, Choi S-H, Chung E-S, Chung T-Y. Correlation between preoperative biometry and posterior chamber phakic Visian implantable Collamer lens vaulting. Ophthalmology. 2012;119(2):272–277. doi:10.1016/j.ophtha.2011.07.047
26. Kamiya K, Ryu IH, Yoo TK, et al. Prediction of phakic intraocular lens vault using machine learning of anterior segment optical coherence tomography metrics. Am J Ophthalmol. 2021;226:90–99. doi:10.1016/j.ajo.2021.02.006
27. Wu H, Zhong D-J, Luo D-Q, Zhang L-Y, Liu J, Wang H. Improvement in the ideal range of vault after implantable collamer lens implantation: a new vault prediction formula. Front Med Lausanne. 2023;10:1132102. doi:10.3389/fmed.2023.1132102
28. Kim T, Kim SJ, Lee BY, et al. Development of an implantable collamer lens sizing model: a retrospective study using ANTERION swept-source optical coherence tomography and a literature review. BMC Ophthalmol. 2023;23:59. doi:10.1186/s12886-023-02814-7
29. Chen Q, Tan W, Lei X, et al. Clinical prediction of excessive vault after Implantable Collamer Lens implantation using ciliary body morphology. J Refract Surg. 2020;1(6):380–387. doi:10.3928/1081597X-20200513-02
30. Tan W, Wang Z, Zeng Q, et al. The influence of iris-ciliary angle (ICA) on the vault after implantation of V4c implantable collamer lens: a chain mediation model of ICL haptic related factors. BMC Ophthalmol. 2023;23(1):403. doi:10.1186/s12886-023-03122-w
31. Zhang X, Chen X, Wang X, Yuan F, Zhou X. Analysis of intraocular positions of posterior implantable collamer lens by full-scale ultrasound biomicroscopy. BMC Ophthalmol. 2018;18(1):114. doi:10.1186/s12886-018-0783-5
32. Nakamura T, Isogai N, Kojima T, Yoshida Y, Sugiyama Y. Implantable collamer lens sizing method based on swept-source anterior segment optical coherence tomography. Am J Ophthalmol. 2018;187:99–107. doi:10.1016/j.ajo.2017.12.015
33. Nakamura T, Isogai N, Kojima T, Yoshida Y, Sugiyama Y. Optimization of implantable collamer lens sizing based on swept-source anterior segment optical coherence tomography. J Cataract Refract Surg. 2020;46(5):742–748. doi:10.1097/j.jcrs.0000000000000134
34. Nakamura T, Nishida T, Isogai N, Sugiyama Y, Yoshida Y, Yoshida Y. Evaluation of implantable collamer lens sizing developed by reviewing the horizontal compression-vault coefficient. J Cataract Refract Surg. 2023;49(5):525–530. doi:10.1097/j.jcrs.0000000000001140
35. Igarashi A, Shimizu K, Kato S, Kamiya K. Predictability of the vault after posterior chamber phakic intraocular lens implantation using anterior segment optical coherence tomography. J Cataract Refract Surg. 2019;45(8):1099–1104. doi:10.1016/j.jcrs.2019.02.020
36. Kang EM, Ryu IH, Lee G, et al. Development of a web-based ensemble machine learning application to select the optimal size of posterior chamber phakic intraocular lens. Transl Vis Sci Technol. 2021;10(6):5. doi:10.1167/tvst.10.6.5
37. Choi H, Kim T, Kim SJ, et al. Predicting postoperative anterior chamber angle for phakic intraocular lens implantation using preoperative anterior segment metrics. Transl Vis Sci Technol. 2023;12(1):10. doi:10.1167/tvst.12.1.10
38. Ando W, Kamiya K, Hayakawa H, Takahashi M, Shoji N. Comparison of phakic intraocular lens vault using conventional nomogram and prediction formulas. J Clin Med. 2020;9(12):4090. doi:10.3390/jcm9124090
39. Shen Y, Wang L, Jian W, et al. Big-data and artificial-intelligence-assisted vault prediction and EVO-ICL size selection for myopia correction. Br J Ophthalmol. 2023;107(2):201–206. doi:10.1136/bjophthalmol-2021-319618
40. Yang J, Zou Z, Wu M, et al. Development and validation of a new multivariable prediction model to estimate risk of abnormal vault. BMC Ophthalmol. 2023;23(1):203. doi:10.1186/s12886-023-02956-8
41. Moshirfar M, Han KD, Jaafar MA, et al. Comparative evaluation of multiple nomograms for predicting postoperative vault after implantable collamer lens surgery. J Cataract Refract Surg. 2024;50(1):64–71. doi:10.1097/j.jcrs.0000000000001304
42. Chen X, Wang X, Xu Y, et al. Long-term comparison of vault and complications of implantable collamer lens with and without a central hole for high myopia correction: 5 years. Curr Eye Res. 2022;47(4):540–546. doi:10.1080/02713683.2021.2012202
43. Gargallo-Martinez B, Garcia-Medina JJ, Rubio-Velazquez E, et al. Vault changes after cyclopentolate instillation in eyes with posterior chamber phakic intraocular lens. Sci Rep. 2020;10(1):9646. doi:10.1038/s41598-020-66146-y
44. Russo A, Filini O, Savini G, et al. Predictability of the vault after implantable collamer lens implantation using OCT and artificial intelligence in White patient eyes. J Cataract Refract Surg. 2023;49(7):724–731. doi:10.1097/j.jcrs.0000000000001182
45. Yiming Y, Xi C, Huan Y, et al. Evaluation of ciliary body morphology and position of the implantable collamer lens in low-vault eyes using ultrasound biomicroscopy. J Cataract Refract Surg. 2023;49:1133–1139.
46. Nakamura T, Isogai N, Kojima T, Yoshida Y, Sugiyama Y. Posterior chamber phakic intraocular lens implantation for the correction of myopia and myopic astigmatism: a retrospective 10-year follow-up study. Am J Ophthalmol. 2019;206:1–10. doi:10.1016/j.ajo.2019.04.024
47. Gonzalez-Lopez F, Bouza-Miguens C, Tejerina V, Mompean B, Ortega-Usobiaga J, Bilbao-Calabuig R. Long-term assessment of crystalline lens transparency in eyes implanted with a central-hole phakic collamer lens developing low postoperative vault. J Cataract Refract Surg. 2021;47(2):204–210. doi:10.1097/j.jcrs.0000000000000425
48. Kato S, Shimizu K, Igarashi A. Assessment of low-vault cases with an implantable collamer lens. PLoS One. 2020;15(11):e0241814. doi:10.1371/journal.pone.0241814
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


