Back to Journals » Patient Preference and Adherence » Volume 16
Impairment of Quality of Life in Patients with Implanted Subcutaneous Cardioverter Defibrillator (S-ICD) Compared to Implanted Transvenous Cardioverter Defibrillator Therapy
Authors Thienel M, Haum M, Sadoni S, Novotny J, Estner HL, Fichtner S, Lackermair K
Received 15 July 2022
Accepted for publication 5 October 2022
Published 5 November 2022 Volume 2022:16 Pages 3027—3033
DOI https://doi.org/10.2147/PPA.S378741
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Johnny Chen
Manuela Thienel,1,* Magda Haum,1,* Sebastian Sadoni,2 Julia Novotny,1 Heidi L Estner,1 Stephanie Fichtner,1 Korbinian Lackermair1
1Department of Medicine I, Munich University Hospital, Ludwig-Maximilian-University, Munich, Germany; 2Department of Cardiac Surgery, Munich University Hospital, Ludwig-Maximilian-University, Munich, Germany
*These authors contributed equally to this work
Correspondence: Korbinian Lackermair, Department of Medicine I, Munich University Hospital, Ludwig-Maximilian-University, Marchioninistr. 15, Munich, 81377, Germany, Email [email protected]
Background: The subcutaneous cardioverter defibrillator (S-ICD) has been shown to be a viable alternative to transvenous ICDs (TV-ICD) in all patients at risk of sudden cardiac death (SCD) but without pacing indication.
Aim: The aim of this study was to examine the impact of therapy with current S-ICD devices on quality of life (QoL) in comparison to patients with TV-ICD devices.
Methods: In our single-centre study, 52 consecutive patients with S-ICD and 52 matched patients with TV-ICD were analysed. QoL has been assessed by a standardized questionnaire (EQ-5D-3L, modified). Additionally, clinical baseline and follow-up data were evaluated.
Results: Two-thirds of the total study population reported restrictions in daily routine compared to their life before ICD implantation. A total of 27.7% of S-ICD patients stated to expect an improvement of QoL by deactivation or explantation of their defibrillator compared to only 6.4% of patients with TV-ICD (p=0.006), which was mainly caused by discomfort and pain from the S-ICD pocket (relevant discomfort and pain in 32.6% vs 11.5%; p< 0.01).
Limitations: Main limitation of the study is that quality of life was assessed for one single time point only and time since implantation differed significantly between S-ICD and TV-ICD. Furthermore our collective is younger, and, due to the high proportion of patients without cardiomyopathy, the mean EF is better than usual ICD collective. The absence of heart failure in about the half of our patients might have relevant impact on our QoL analysis.
Conclusion: A relevant proportion of S-ICD patients expects an improvement of QoL by explantation of the device. Of note, this impression was not driven by the fear of receiving shocks but mainly by discomfort and pain caused by the pulse generator.
Keywords: subcutaneous cardioverter defibrillator, S-ICD, transvenous cardioverter defibrillator, quality of life
Introduction
An implantable cardioverter defibrillator (ICD) enables sufficient protection from sudden cardiac death (SCD) in patients at high risk for sustained ventricular arrhythmia.1–5 Due to its intravascular lead standard transvenous ICD (TV-ICD) therapy is associated with acute complications such as implantation-related pneumothorax and cardiac perforation as well as long-term issues like lead dysfunction, displacement, and infection.6–8 To avoid premature defect of leads, TV-ICD implicates restrictions of flexibility of the shoulder, which may cause relevant restrictions of daily activity especially in younger patients.
The subcutaneous cardioverter defibrillator (S-ICD) sought to overcome primarily lead-related complications and has been shown to be a viable alternative to traditional TV-ICDs for all patients in need of an ICD but without pacing indications.9,10 Due to its entirely extra-thoracic system especially younger S-ICD patients might benefit from fewer restrictions in mobility compared to TV-ICDs. On the other hand, the larger pulse generator and its position between M. serratus anterior and M. latissimus dorsi may cause discomfort and pain in particular in slim and female S-ICD patients.11 The rate of inappropriate shocks has decreased over time,12 but it is still higher in S-ICD compared to the transvenous systems, which might lead to anxiety and fear of shocks in patients.13 This may obviously have an impact on patients’ mental well-being and their quality of life (QoL), but still the effect of S-ICD therapy with contemporary 2nd- and 3rd-generation S-ICD generators on QoL has not been studied methodically yet. In our current study, we present our experience with a cohort of S-ICD patients with a special focus on the influence on QoL.
Methods
Study Design
The current study reports on a single-centre investigation of consecutive patients with implantation of an S-ICD (Emblem™, Boston Scientific) between June 2015 and November 2020 in our hospital and follow-up in our outpatient department. Longitudinal data were extracted from routine clinical management without additional examinations or treatment beyond routine clinical care in a retrospective manner. Baseline characteristics were collected within the routine follow-up outpatient visit. The study conformed to the 1975 Declaration of Helsinki and was approved by the ethics committee of the Ludwig-Maximilians-University of Munich (accession number: 20–641). All participants gave written informed consent.
Study Population
Patients with S-ICD therapy attending our outpatient department to receive routine follow-up were consecutively recruited to this analysis. S-ICD therapy was prescribed either due to primary or secondary prevention in accordance to current guidelines.14 Additionally, an age-, sex-, and disease-matched control cohort of patients with conventional TV-ICD was equally recruited in a retrospective manner from our outpatient department.
Quality of Life Assessment
Quality of life was evaluated within the routine follow-up in our outpatient department using a standardized questionnaire (EQ-5D-3L)15 comprising five dimensions of QoL: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. Each dimension is assessed in three levels: no problems, some problems, and extreme problems. In addition, dichotomous questions (see Supplemental Figure 1) about fear of shock, sense of security, disturbance of sleep, and impairment of usual activities subjectively caused by the ICD were posed in accordance to our previous work.16
Statistics
Data are presented as mean ± SEM for continuous variables. Number of cases or percentage were reported for categorical variables. Normality testing was performed using Shapiro–Wilk test, and comparison of continuous variables was done with Student's t-test. Categorical variables were compared using a chi-square test. A p-value <0.05 was considered statistically significant. Statistical analysis was conducted using SPSS software, version 28.0, IBM Corp., Armonk, NY, USA.
Results
A total of 52 consecutive patients with S-ICD and a cohort of 52 patients with TV-ICD, matched for age, sex, and underlying disease, were included. Baseline characteristics of the study population are depicted in Table 1. The predominant indication for ICD therapy was secondary prophylactic in patients without structural cardiomyopathy and non-ischemic cardiomyopathy (Figure 1). The mean age was 43.5 years in the S-ICD group compared to 46.0 years in the TV-ICD population (p=0.270). Patients with primary prophylactic indication had symptomatic heart failure with reduced left ventricular ejection fraction. Correspondingly, current medication consisted of pharmacological heart failure therapy.
 |
Table 1 Baseline Characteristics |
 |
Figure 1 Comparison of the indication for ICD-implantation for implanted subcutaneous ICD and implanted transvenous ICD: This figure demonstrates differences in the indication for ICD therapy. |
Device was 2nd- or 3rd-generation S-ICD (Boston Scientific A209 or A219, identical in hardware construction) in 42 patients (81%). Mean time since implantation of the defibrillator was 2.4 years in the S-ICD group and 3.6 years in the TV-ICD group (p=0.05).
Overall shock was without significant between-group differences (28.8% vs 36.5%; p=0.4). Nevertheless, inappropriate shock (iAS) rate was significantly higher in S-ICD patients (4.8% vs 0.6%/patient year; p<0.01). Although patients in both groups felt equally protected by their defibrillator (86.5% in the S-ICD group, 94.1% in the TV-ICD group; p=0.19), 27.7% of S-ICD patients expected an improvement of quality of life by a deactivation or explantation of the defibrillator compared to only 6.4% of patients with TV-ICD (p<0.01).
This differences could not be explained by fear of receiving shocks (17.3% in S-ICD patients, 13.5% in TV-ICD patients; p=0.15) but rather by discomfort and pain caused by the generator itself (medium or severe pain in 32.6% of S-ICD patients vs 11.5% of TV-ICD patients; p<0.01).
When asked about accomplishing their daily life in general, more than two-thirds of the total study population felt restricted compared to their life before implantation of the defibrillator (73.1% in the S-ICD-group and 63.5% in the ICD group; p=0.32). Patients stated restrictions of physical flexibility in 69.2% without a relevant between-group difference (73.1% in the S-ICD group vs 65.4% in the TV-ICD group; p=0.69). The ability of self-care (hygiene) was reduced in 73.1% in the S-ICD group and in 63.5% in the TV-ICD group (p=0.29). Numerically more patients with S-ICD reported having restrictions of mental wellbeing (73.1%) compared to TV-ICD patients (63.5%). Nevertheless, this was without statistical significance (p=0.47).
All levels of impairment of daily life asked in the EQ-5D-3L questionnaire are depicted in Table 2 and Figure 2
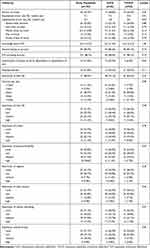 |
Table 2 Results |
Discussion
In accordance with current studies, the S-ICD has experienced an expansion of indication for all defibrillator patients without need for additional pacing function, regardless of the underlying cardiac disease.9,10 However, crucial aspects such as the impact of current 2nd- and 3rd-generation S-ICD on the quality of life remain unstudied yet. Since the initial proof of concept with the 1st-generation S-ICD available since 2002, technical development of hard- and also software of the S-ICD led to a relevant reduction of generator size with the 2nd-generation generator (about 20% reduction of thickness, launched in 2015) and additionally significant reduction of iAS shock rate as the Smart Pass technology was introduced since 3rd-generation devices in 2016.9,10 Thus, S-ICD therapy with contemporary generators is not comparable with 1st-generation devices, neither for safety of therapy nor for comfort issues.
To the best of our knowledge, this is the first study analysing the influence of contemporary S-ICD on mental well-being and accomplishment of everyday life in comparison to TV-ICD, as prior data for example from the EFFORTLESS registry refer to 1st-generation devices only.17,18
As a major finding, we report that one-third of S-ICD patients experience a serious restriction in quality of life and expect an improvement of their general well-being by removal of the S-ICD. Of note, this impression was not driven by the fear of receiving shocks although a relevant higher rate of iAS in S-ICD compared to TV-ICD patients was documented in our study. In line with this, patients in both groups felt equally protected by their device.
The majority of all patients (S-ICD and TV-ICD) reported restrictions of almost any studied item of QoL without relevant between-group differences.
However, a gain in physical mobility which might have been allowed by the lack of transvenous leads in S-ICD patients could not be depicted in our population.
In fact, device-associated pain and discomfort are so important that many patients think their life will improve with explantation. Contradicting an observation from van der Stuijt et al that especially female S-ICD patients experience daily discomfort mainly caused by their bra, the distribution of men and women was similar in patients complaining about S-ICD-related pain in our cohort.11
Correct intraoperative placement and fixation of the generator in the intermuscular space between serratus and latissimus muscle deserves particular attention to satisfy requirements of durable defibrillation threshold as well as comfort issues. Moreover, our findings give a call for technical development enabling further reduction of device size which might increase general well-being of S-ICD patients.
Study Limitations
Our study has several important limitations. First, quality of life was assessed for one single time point only. Therefore, the course of impairment remains unclear. Nevertheless, earlier data indicate stable impairment of QoL a few months after implantation.17
Second, time since implantation differed significantly between S-ICD and TV-ICD. Even though a mean time since implantation of 3.0 years represents chronic ICD therapy this might have influenced our findings.
Third, our collective is younger and due to the high proportion of patients without cardiomyopathy, the mean EF is better than usual ICD collective. The absence of heart failure in about the half of our patients might have relevant impact on our QoL analysis.
Conclusion
In our cohort, the majority of S-ICD patients felt well protected by their defibrillator. Impairment of QoL was comparable between S-ICD and TV-ICD patients. Nevertheless, a relevant part expects an improvement of QoL by explantation of the S-ICD. This was not caused by the fear of receiving shocks but mainly by discomfort and pain caused by the pulse generator.
Data Sharing Statement
The data underlying this article will be shared anonymized on reasonable request to the corresponding author.
Ethical Approval and Consent to Participate
The analysis was conducted in accordance with the Declaration of Helsinki and was approved by the ethics committee of the Ludwig-Maximilian-University of Munich (accession number: 20-641). All participants gave written informed consent.
Consent for Publication
All authors gave consent for publication.
Acknowledgments
The abstract of this paper was presented at the “DGK Herztage 2021” as a poster presentation with interim findings.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Moss AJ, Hall WJ, Cannom DS, et al. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. Multicenter automatic defibrillator implantation trial investigators. N Engl J Med. 1996;335(26):1933–1940. doi:10.1056/nejm199612263352601
2. Moss AJ, Zareba W, Hall WJ, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346(12):877–883. doi:10.1056/NEJMoa013474
3. Kadish A, Dyer A, Daubert JP, et al. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med. 2004;350(21):2151–2158. doi:10.1056/NEJMoa033088
4. Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352(3):225–237. doi:10.1056/NEJMoa043399
5. Buxton AE, Lee KL, Fisher JD, Josephson ME, Prystowsky EN, Hafley G. A randomized study of the prevention of sudden death in patients with coronary artery disease. Multicenter unsustained tachycardia trial investigators. N Engl J Med. 1999;341(25):1882–1890. doi:10.1056/nejm199912163412503
6. Kirkfeldt RE, Johansen JB, Nohr EA, Jørgensen OD, Nielsen JC. Complications after cardiac implantable electronic device implantations: an analysis of a complete, nationwide cohort in Denmark. Eur Heart J. 2013;35(18):1186–1194. doi:10.1093/eurheartj/eht511
7. Providência R, Kramer DB, Pimenta D, et al. Transvenous Implantable Cardioverter-Defibrillator (ICD) lead performance: a meta-analysis of observational studies. J Am Heart Assoc. 2015;4(11). doi:10.1161/jaha.115.002418
8. Olsen T, Jørgensen OD, Nielsen JC, Thøgersen AM, Philbert BT, Johansen JB. Incidence of device-related infection in 97 750 patients: clinical data from the complete Danish device-cohort (1982–2018). Eur Heart J. 2019;40(23):1862–1869. doi:10.1093/eurheartj/ehz316
9. Knops RE, Olde Nordkamp LRA, Delnoy PHM, et al. Subcutaneous or transvenous defibrillator therapy. N Engl J Med. 2020;383(6):526–536. doi:10.1056/NEJMoa1915932
10. Gold MR, Lambiase PD, El-Chami MF, et al. Primary results from the understanding outcomes with the S-ICD in primary prevention patients with low ejection fraction (UNTOUCHED) Trial. Circulation. 2021;143(1):7–17. doi:10.1161/circulationaha.120.048728
11. van der Stuijt W, Quast ABE, Baalman SWE, Olde Nordkamp LRA, Wilde AAM, Knops RE. Improving the care for female subcutaneous ICD patients: a qualitative study of gender-specific issues. Int J Cardiol. 2020;317:91–95. doi:10.1016/j.ijcard.2020.05.091
12. Basu-Ray I, Liu J, Jia X, et al. Subcutaneous versus transvenous implantable defibrillator therapy: a meta-analysis of case-control studies. JACC. 2017;3(13):1475–1483. doi:10.1016/j.jacep.2017.07.017
13. Sears SF
14. Priori SG, Blomstrom-Lundqvist C, Mazzanti A, et al. 2015 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the task force for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death of the European Society of Cardiology (ESC). Endorsed by: association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J. 2015;36(41):2793–2867. doi:10.1093/eurheartj/ehv316
15. Brooks R. EuroQoL: the current state of play. Health Policy. 1996;37(1):53–72. doi:10.1016/0168-8510(96)00822-6
16. Lackermair K, Schuhmann CG, Kubieniec M, et al. Impairment of quality of life among patients with wearable cardioverter defibrillator therapy (LifeVest(R)): a preliminary study. Biomed Res Int. 2018;2018:6028494. doi:10.1155/2018/6028494
17. Pedersen SS, Carter N, Barr C, et al. Quality of life, depression, and anxiety in patients with a subcutaneous versus transvenous defibrillator system. Pacing Clin Electrophysiol. 2019;42(12):1541–1551. doi:10.1111/pace.13828
18. Kobe J, Hucklenbroich K, Geisendorfer N, et al. Posttraumatic stress and quality of life with the totally subcutaneous compared to conventional cardioverter-defibrillator systems. Clin Res Cardiol. 2017;106(5):317–321. doi:10.1007/s00392-016-1055-0
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

