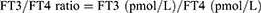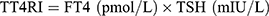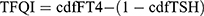Back to Journals » Clinical Interventions in Aging » Volume 18
Impaired Sensitivity to Thyroid Hormones is Associated with Mild Cognitive Impairment in Euthyroid Patients with Type 2 Diabetes
Authors Yu ZW, Pu SD, Sun XT, Wang XC, Gao XY , Shan ZY
Received 22 March 2023
Accepted for publication 28 July 2023
Published 3 August 2023 Volume 2023:18 Pages 1263—1274
DOI https://doi.org/10.2147/CIA.S413584
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Maddalena Illario
Zi-Wei Yu,1,* Sheng-Dan Pu,2,* Xiao-Tong Sun,2 Xi-Chang Wang,1 Xin-Yuan Gao,2 Zhong-Yan Shan1
1Department of Endocrinology and Metabolism and the Institute of Endocrinology, The NHC Key Laboratory of Diagnosis and Treatment of Thyroid Diseases, First Hospital of China Medical University, Shenyang, 110001, People’s Republic of China; 2Department of Endocrinology, First Affiliated Hospital of Harbin Medical University, Harbin, 150001, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Zhong-Yan Shan; Xin-Yuan Gao, Tel +8613804905018 ; +8613904517001, Email [email protected]; [email protected]
Purpose: The prevalence of mild cognitive impairment (MCI) in patients with type 2 diabetes (T2D) is rapidly increasing. Thyroid hormones are key regulators of cognitive function in adults. The purpose of this study was to investigate the relationship between thyroid hormone sensitivity and MCI in euthyroid T2D patients.
Patients and Methods: A total of 400 euthyroid T2D patients were enrolled in this cross-sectional study, including 218 patients with normal cognition and 182 MCI patients. The Montreal Cognitive Assessment (MoCA) was used to evaluate cognitive function. The free triiodothyronine to free thyroxine (FT3/FT4) ratio was calculated as a measure of peripheral sensitivity to thyroid hormones; the thyroid-stimulating hormone index (TSHI), thyrotrophic thyroxine resistance index (TT4RI) and thyroid feedback quantile-based index (TFQI) were calculated as measures of central sensitivity to thyroid hormones. Linear regression analysis and logistic regression analysis were performed to explore the relationships between these indices of thyroid hormone sensitivity and the MoCA score and MCI, respectively.
Results: Compared with the normal cognitive function group, patients in the MCI group had higher TSHI, TT4RI and TFQI but a lower FT3/FT4 ratio (P< 0.05). The MoCA score was positively correlated with the FT3/FT4 ratio but negatively correlated with TSHI, TT4RI and TFQI (P< 0.05). Multivariate logistic regression analysis showed that a low FT3/FT4 ratio and high TSHI, TT4RI and TFQI were independently associated with MCI (P< 0.05). After adjustment for confounding factors, the odds ratio (OR) for the association between MCI and the highest tertile of the FT3/FT4 was 0.455 (95% CI: 0.264– 0.785), for the highest tertile of TSHI, the OR was 2.380 (95% CI: 1.376– 4.119), for the highest tertile of TT4RI, the OR was 2.342 (95% CI:1.353– 4.054), and for the highest tertile of TFQI, the OR was 2.536 (95% CI: 1.466– 4.387) (P< 0.05).
Conclusion: Impaired sensitivity to thyroid hormones is associated with MCI in euthyroid T2D patients.
Keywords: type 2 diabetes, mild cognitive impairment, thyroid hormones, sensitivity to thyroid hormones
A Letter to the Editor has been published for this article.
A Response to Letter by Dr Zhai has been published for this article.
Introduction
The type 2 diabetes (T2D) epidemic is a global public health problem. The burden of T2D and its associated complications is rapidly increasing.1 Aside from the traditional macrovascular and microvascular complications, mild cognitive impairment (MCI; an intermediate stage between healthy aging and dementia) and dementia are also closely associated with T2D.2 A cohort study revealed that T2D can increase the risk of MCI by 1.39 times.3 More concerning is that T2D can also accelerate the conversion of MCI to dementia, thus affecting the quality of life of patients and aggravating the burden on families and society.4 Therefore, it is of great clinical significance to identify and manage MCI in T2D patients as soon as possible.
Thyroid hormones are known to play an important role in maintaining cognitive function during both the prenatal developmental stage and adulthood.5,6 Thyroid dysfunction is considered a risk factor for cognitive impairment.7,8 Previous studies have confirmed that both overt hypothyroidism and overt hyperthyroidism are closely related to cognitive dysfunction.9,10 Similarly, subclinical hypothyroidism and subclinical hyperthyroidism may also increase the risk of cognitive impairment.10,11 Even in the euthyroid population, changes in serum thyroid-stimulating hormone (TSH) and thyroid hormones may affect cognitive function. A prospective cohort study found that a lower serum free triiodothyronine (FT3) level, but still within the normal range, was associated with an increased risk of Alzheimer’s disease (AD).12 Meta-analyses and observational studies have confirmed that high-normal serum free thyroxine (FT4) levels are associated with dementia.13,14 Many epidemiological studies have reported that a lower serum TSH level, within the normal range, is an independent risk factor for MCI or AD.15–17 Conversely, some studies have found that higher serum TSH, in the normal range, is associated with poor cognitive performance.18,19 However, a recent large study did not find thyroid dysfunction to be associated with cognitive function or incident dementia.20 Similarly, another study reported that subtle changes in serum thyroid hormones and TSH do not affect cognitive function in older adults with normal thyroid function.21 The majority of available studies have evaluated thyroid function by serum FT3, FT4, and TSH measures. However, thyroid hormone sensitivity more comprehensively reflects thyroid homeostasis than serum hormone levels.22 Therefore, changes in thyroid hormone sensitivity may be a potential explanation for these contradictory findings.
Researchers have recently developed a series of indices to assess thyroid hormone sensitivity. The thyroid feedback quantile index (TFQI), proposed by Laclaustra et al, is a novel index for evaluating central sensitivity to thyroid hormones.23 Other indices reflecting central sensitivity to thyroid hormones include the TSH index (TSHI) and thyrotrophic thyroxine resistance index (TT4RI).24,25 The FT3/FT4 ratio reflects peripheral sensitivity to thyroid hormones. Several studies have shown that impaired sensitivity to thyroid hormones is associated with metabolic diseases such as hypertension,26 diabetes,23 dyslipidemia,22 fatty liver,27 hyperhomocysteinemia,28 hyperuricemia,29 etc. However, no study has yet demonstrated the relationship between thyroid hormone sensitivity and MCI. Therefore, the purpose of this study was to investigate the relationship between central and peripheral sensitivity to thyroid hormones and MCI in euthyroid T2D patients based on the above indices.
Materials and Methods
Subjects
A total of 400 patients with T2D were enrolled in this study. Patients were recruited from the First Affiliated Hospital of Harbin Medical University between January 2022 and December 2022 (Figure 1). The diagnosis of T2DM was based on the diagnostic criteria of the American Diabetes Association.30 The exclusion criteria were as follows: 1) Type 1 diabetes or specific types of diabetes, acute complications of diabetes, or history of severe hypoglycaemia; 2) Thyroid dysfunction (ie, overt or subclinical hyper/hypothyroidism), history of thyroid disease, history of thyroid surgery, or history of medication that could influence thyroid function; 3) Neurological diseases that affect cognitive function, mental illness, or a history of head trauma or surgery; 4) Auditory or visual impairment; 5) Acute and chronic infections, severe liver or renal dysfunction, cancer and pregnancy; 6) incomplete data. This study complied with the Declaration of Helsinki and was approved by the Ethics Committee of the First Affiliated Hospital of Harbin Medical University. All patients provided written informed consent.
 |
Figure 1 Study flowchart. |
Data Collection
Demographic and clinical data, including smoking and drinking history, education level, history of hypertension, duration of diabetes, diabetes complications and diabetes medication, were collected by trained clinical coordinators using pre-designed questionnaires. Height, weight and blood pressure were measured by skilled nurses in a quiet environment. The body mass index (BMI) was calculated as follows: weight (kg) /height 2 (m2). Blood samples were taken after a 12-hour overnight fast. The samples were tested for glycosylated haemoglobin A1c (HbA1c), fasting C-peptide, triglyceride (TG), total cholesterol (TC), low-density lipoprotein (LDL), high-density lipoprotein (HDL), uric acid (UA), creatinine (Cr), FT3, FT4, TSH, thyroglobulin antibody (TgAb) and thyroid peroxidase antibody (TPOAb). HbA1c was measured by high-performance liquid chromatography. Fasting C-peptide was determined using radioimmunoassay. TG, TC, LDL, HDL, UA and Cr were measured utilizing an automatic biochemical analyser. The TC/HDL, TG/HDL and LDL/HDL ratios were calculated. FT3, FT4, TSH, TgAb and TPOAb were measured using electrochemiluminescence immunoassays. The normal reference ranges for TSH, FT4, FT3, TgAb and TPOAb were 0.35–4.94 mIU/L, 9.01–19.05 pmol/L, 2.63–5.70 pmol/L, <4.11 IU/mL and<5.61 IU/mL, respectively.
Cognitive Function Assessment
MCI was determined according to the diagnostic guidelines for MCI developed by the National Institute on Aging and the Alzheimer’s Association (NIA-AA) workgroups, as follows:31 1) subjective memory complaint from the patient or their family; 2) objective evidence of impairment in one or more cognitive domains, as assessed in this study using the Beijing version of the Montreal Cognitive Assessment (MoCA-BJ); 3) preserved daily functional abilities; 4) no dementia. The MoCA-BJ is a brief and sensitive screening tool for MCI. The maximum total MoCA score is 30. MCI is defined as a MoCA score greater than or equal to 19 and less than 26; one point is added if the subject has less than 12 years of formal education.32
Indices of Thyroid Hormone Sensitivity
The FT3/FT4 ratio was used as a measure of peripheral thyroid hormone sensitivity and was calculated as follows:
A higher FT3/FT4 ratio indicates higher peripheral sensitivity to thyroid hormones.
Indices reflecting central thyroid hormone sensitivity, including TSHI, TT4RI, and TFQI, were calculated as follows:
Higher TSHI and TT4RI indicate lower central sensitivity to thyroid hormones. TFQI values range from −1 to 1, with negative values indicating higher central sensitivity and positive values indicating lower central sensitivity.
Statistical Analyses
A required sample size of 192 participants was estimated using G*Power version 3.1 with the power set at 0.8, alpha at 0.05, and a small effect size (OR=1.68). A total of 400 patients were included in this study, which was an adequate sample size. The normality of continuous variables was tested using the Kolmogorov–Smirnov method. Continuous variables with normal distributions were expressed as the mean ± standard deviation and compared using two-sample independent t-tests. Continuous variables with skewed distributions were expressed as medians (interquartile ranges) and compared using the Mann–Whitney U-test. Categorical variables were expressed as percentages and compared using the Chi-square test. Linear regression analysis was used to investigate the relationships between thyroid parameters and the MoCA score. Univariate and multivariate logistic regression analyses were used to explore the relationships between indices of thyroid hormone sensitivity and MCI. In addition, stratified analysis by age was performed to explore the potential effect of age on the relationships between indices of thyroid hormone sensitivity and MCI. The statistical analyses were performed using SPSS version 25.0, and p-values <0.05 were considered statistically significant.
Results
Baseline Characteristics
The baseline characteristics of the subjects are presented in Table 1. Of the 400 T2D subjects, 218 had normal cognitive function (MoCA ≥26; 126 men and 92 women) and 182 had MCI (19<MoCA<26; 91 men and 91 women). Patients in the MCI group were older, had a longer diabetes duration, and had higher FT4, TSH, TSHI, TT4RI and TFQI values compared with patients in the normal cognitive function group (P<0.05). However, the education level, MoCA score, fasting C-peptide, UA, FT3 and FT3/FT4 values in the MCI group were lower than those in the normal cognitive function group (P<0.05). No statistically significant differences were observed between the two groups in terms of sex, BMI, blood pressure, smoking and drinking history, hypertension history, HbA1C, TC, TG, HDL, LDL, TC/HDL ratio, TG/HDL ratio, LDL/HDL ratio, Cr, TgAb, TPOAb, diabetic retinopathy (DR), diabetic nephropathy (DN), diabetic peripheral neuropathy (DPN), lower-limb atherosclerosis, carotid atherosclerosis, insulin use, metformin use, sodium-glucose cotransporter-2 (SGLT-2) inhibitor use, dipeptidyl peptidase-4 (DPP-4) inhibitor use and statins use (P>0.05).
 |
Table 1 Baseline Characteristics Between the Normal Cognitive Function Group and the MCI Group |
Relationships Between Thyroid Parameters and the MoCA Score
Univariate linear regression analyses showed that FT3 (β=0.179, P<0.001) and FT3/ FT4 (β=0.273, P<0.001) were positively associated with the MoCA score. FT4 (β=−0.205, P<0.001), TSH (β=−0.138, P=0.006), TSHI (β=−0.169, P=0.001), TT4RI (β=−0.181, P<0.001) and TFQI (β=−0.127, P=0.011) were negatively associated with the MoCA score.
Multivariate linear regression analysis showed that after adjusting for age, sex, diabetes duration, education level, BMI, drinking history, hypertension history, HbA1C, fasting C-peptide, UA and DPN, the MoCA score remained positively associated with FT3 (β=0.130, P=0.003) and FT3/ FT4 (β=0.196, P<0.001) but negatively associated with FT4 (β =−0.171, P<0.001), TSH (β =−0.122, P =0.005), TSHI (β=−0.145, P=0.001), TT4RI (β=−0.157, P<0.001) and TFQI (β=−0.111, P =0.011) (Table 2).
 |
Table 2 Relationships Between Thyroid Parameters and the MoCA Score |
Associations Between Indices of Thyroid Hormone Sensitivity and MCI
After adjustment for age, sex, diabetes duration, education level, BMI, drinking history, hypertension history, HbA1c, fasting c-peptide, UA and DPN, higher TSHI (OR=1.493, 95% CI=1.018–2.190, P=0.040), TT4RI (OR=1.025, 95% CI=1.006–1.044, P=0.009), and TFQI (OR=1.483, 95% CI=1.013–2.170, P=0.043) scores were associated with an increased risk of MCI, while a lower FT3/FT4 ratio (OR=0.001, 95% CI=0–0.050, P=0.001) was associated with an increased risk of MCI. Similar results were observed in the unadjusted model and the age-sex-adjusted model.
The indices for evaluating thyroid hormone sensitivity were divided into tertiles. In the multivariate-adjusted model, compared with the first tertile, the OR values for the highest tertiles of the FT3/FT4 ratio, TSHI, TT4RI and TFQI were 0.455 (95% CI=0.264–0.785) (P for trend=0.005), 2.380 (95% CI=1.376–4.119) (P for trend=0.002), 2.342 (95% CI=1.353–4.054) (P for trend =0.002) and 2.536 (95% CI=1.466–4.387) (P for trend=0.001), respectively. This is similar to the results of the unadjusted model and the age-sex-adjusted model (Table 3).
 |
Table 3 Associations Between Indices of Thyroid Hormone Sensitivity and MCI |
The associations between the indices of thyroid hormone sensitivity and MCI were then explored after stratifying the population by age, and the interaction effects were further investigated. After adjusting for confounding factors, there were no interaction effects between indices of thyroid hormone sensitivity and age on MCI (all P values for interactions>0.05) (Figure 2).
Discussion
To our knowledge, this study is the first to explore the relationship between sensitivity to thyroid hormones and MCI in T2D patients with normal thyroid function. The main findings are summarized as follows: 1) Compared with the normal cognitive function group, patients in the MCI group had higher TSHI, TT4RI and TFQI, but a lower FT3/FT4 ratio; 2) The MoCA score was negatively correlated with TSHI, TT4RI, and TFQI but positively correlated with the FT3/FT4 ratio; 3) High TSHI, TT4RI and TFQI were independent risk factors for MCI. A low FT3/FT4 ratio was an independent risk factor for MCI.
Thyroid hormones are essential for the development and function of the central nervous system (CNS).6,33 Thyroid hormones are involved in many CNS activities, including neurogenesis, neuronal plasticity processes, regulation of growth factors and neurotransmitters, and regulation of cytoskeleton dynamics.5,7,33 The relationship between thyroid function and cognitive impairment has been a hot topic for researchers and clinicians. Previous epidemiological investigations have shown that both hypothyroidism and hyperthyroidism increase the risk of cognitive impairment.7,8 Animal experiments have indicated that hypothyroidism may disrupt cognitive function by reducing hippocampal synaptic plasticity and altering the expression of growth factors and neurotransmitters.34 In addition, hypothyroidism promotes tau hyperphosphorylation, which is a key pathological feature of AD.35 Imaging evidence has suggested that hypothyroidism reduces the hippocampus volume and cerebral blood flow, leading to cognitive decline.36 Hyperthyroidism may induce oxidative stress and damage neurons, affecting cognitive function.33 Subtle changes in thyroid hormone levels have also been reported to be associated with cognitive impairment in the euthyroid population. However, to date, the findings are conflicting. The study by Quinlan et al found that low serum FT3 was strongly associated with AD in people with normal thyroid function, while neither serum TSH nor FT4 was associated with AD risk.12 The Rotterdam study found that elevated serum FT4 was associated with a higher risk of dementia, while elevated serum TSH was associated with a reduced risk of dementia in euthyroid individuals.16 Hogervorst et al found that high serum FT4 was associated with poor cognitive performance in people with normal thyroid function, but this study found no relationship between serum TSH and cognitive impairment.14 Johansson et al identified elevated serum TSH in euthyroid AD patients compared to healthy controls. However, there were no significant differences in serum FT3 and FT4 between the two groups.18 A Mendelian randomization study also found that serum FT4 level was not associated with AD. However, in contradiction to the Johansson et al study, a causal relationship between reduced serum TSH, in the normal range, and an increased risk of AD was observed.15 In short, the relationships between cognitive function and serum FT3, FT4 and TSH levels in the general euthyroid population are unclear.
At present, studies on the relationships between thyroid hormones and MCI in euthyroid T2D patients are very limited. Zhang et al found that low serum FT3 may be related to MCI in T2D patients without diagnosed thyroid disease, while there seemed to be no significant associations between MCI and serum FT4 and TSH levels.37 Our results are not entirely consistent with previous studies. In the current study, low serum FT3 and high serum FT4 and TSH were positively associated with MCI. These results are contradictory to the above studies, suggesting that thyroid hormones or TSH alone may not be sufficient to explain the relationship between the thyroid system and cognitive impairment. Recently, Laclaustra et al proposed that there may be a mild acquired resistance to thyroid hormones in the general population.23 Physiologically, thyroid hormones are negatively correlated with TSH due to the negative feedback loop regulation of the hypothalamic-pituitary-thyroid (HPT) axis.38 However, individuals with mild resistance to thyroid hormones have coexisting high FT4 and high TSH.39 Resistance to thyroid hormones can be classified as either central resistance to thyroid hormones, which is characterised by a decrease in the pituitary response to thyroid hormones, and peripheral resistance to thyroid hormones, which is characterised by a decrease in the peripheral bioavailability of thyroid hormones.23 Central resistance to thyroid hormones is often accompanied by peripheral resistance to thyroid hormones.22 Previous studies based on human and animal models have confirmed that thyroid hormone resistance is related to learning disabilities and attention deficit.40,41 However, to date, no study has investigated the relationship between thyroid hormone sensitivity and MCI in euthyroid T2D patients. Given the complex interactions between serum thyroid hormones and TSH, an increasing number of studies are beginning to investigate the thyroid status of subjects using composite indices rather than single parameters.22,26–29 Indices used to reflect central resistance to thyroid hormones include TT4RI, TSHI and TFQI. The FT3/FT4 ratio is used as a measure of peripheral resistance to thyroid hormones. This study attempted to evaluate thyroid hormone sensitivity through these comprehensive indicators and to analyse the relationship between thyroid hormone sensitivity and MCI in order to unravel these contradictory relationships between thyroid hormones and cognitive impairment.
An important finding of this study was that serum FT4 and TSH levels in the MCI group were higher than those in the normal cognitive function group. This indicates that there is relative central resistance to thyroid hormones in this group. Furthermore, high TT4RI, TSHI and TFQI were associated with an increased risk of MCI. These results further suggest that impaired central thyroid hormone sensitivity is associated with MCI. The specific mechanism underlying the cognitive impairment caused by impaired central thyroid sensitivity remains unclear. It is possible that under thyroid hormone resistance, impaired T3 receptor binding may lead to abnormal axonal routing and neuron proliferation and migration, thus damaging brain structures and causing cognitive damage.40,41 More in vivo and in vitro experiments are needed to explore the underlying pathophysiological mechanisms of this relationship. In this study, a decrease in the FT3/FT4 ratio was associated with MCI. This indicates that impaired peripheral thyroid hormone sensitivity is related to MCI. This result is consistent with previous research. Quinlan et al showed that compared with the control group, the FT3/FT4 ratio in the AD group was lower.42 A decrease in the FT3/FT4 ratio indicates that the peripheral transformation from FT4 to FT3 is reduced; this may be caused by a decrease in type 2-deiodinase (D2) activity. Thr92AlaD2, a single nucleotide polymorphism commonly found in the DIO2 gene encoding D2, downregulates D2 activity, thereby mediating a reduction in FT4 to FT3 conversion. Thr92AlaD2 has been reported to alter transcription in processes associated with neurodegenerative diseases, such as the accumulation of the amyloid-β (Aβ) peptide.43,44 Importantly, the Thr92AlaD2 polymorphism has been found to be associated with AD risk in the African American population.43 Furthermore, Luo et al found a potential link between the Thr92AlaD2 polymorphism and MCI in a male Chinese Uyghur population.45 Since age is an important risk factor for MCI, we further carried out stratification and sensitivity analyses. The results showed that age did not affect the relationship between thyroid hormone sensitivity and MCI. The prevailing perspective suggests that the majority of patients with thyroid hormone resistance do not require treatment, as impaired thyroid hormone sensitivity can be compensated by elevated levels of thyroid hormones.39 However, considering that impaired sensitivity to thyroid hormones may adversely affect the cognitive function of patients, thyroid hormone agonists should be actively developed as a promising treatment for impaired thyroid hormone sensitivity, thus mitigating the adverse effects on cognitive impairment.
The current findings support the guideline recommendations for screening thyroid function in patients with cognitive decline.46 Moreover, this study introduces a novel perspective by demonstrating that the investigation of thyroid function should not be confined to single hormone alterations but should instead encompass an evaluation of sensitivity to thyroid hormones. In short, these findings may contribute to new strategies for clinically predicting the onset or progression of cognitive impairment and this, in turn, could contribute to the effective management of human health and extend human life expectancy. Nonetheless, it is essential to acknowledge the limitations of the current study. Firstly, as this was a cross-sectional study, no causal inferences can be made. Secondly, the small sample size obtained from a single centre may limit the generalization of the results. Future multicentre cohort studies are warranted to enhance our understanding of this association and obtain more comprehensive and reliable results. Additionally, serum total T3, serum total T4, cerebrospinal fluid thyroid hormones and serum D2 activity were not measured in this study.
Conclusion
In summary, the present study found that impaired thyroid hormone sensitivity may be associated with an increased risk of MCI in euthyroid T2D patients. Although this research provides new insights for the diagnosis and management of MCI in patients with T2D, large multicentre prospective studies are needed to verify these findings.
Acknowledgments
This study was supported by the Chinese National Natural Science Foundation (No.81970682).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14(2):88–98. doi:10.1038/nrendo.2017.151
2. Biessels GJ, Despa F. Cognitive decline and dementia in diabetes mellitus: mechanisms and clinical implications. Nat Rev Endocrinol. 2018;14(10):591–604. doi:10.1038/s41574-018-0048-7
3. Roberts RO, Knopman DS, Geda YE, et al. Association of diabetes with amnestic and nonamnestic mild cognitive impairment. Alzheimers Dement. 2014;10(1):18–26. doi:10.1016/j.jalz.2013.01.001
4. Biessels GJ, Strachan MW, Visseren FL, Kappelle LJ, Whitmer RA. Dementia and cognitive decline in type 2 diabetes and prediabetic stages: towards targeted interventions. Lancet Diabetes Endocrinol. 2014;2(3):246–255. doi:10.1016/S2213-8587(13)70088-3
5. Sawicka-Gutaj N, Zawalna N, Gut P, Ruchala M. Relationship between thyroid hormones and central nervous system metabolism in physiological and pathological conditions. Pharmacol Rep. 2022;74(5):847–858. doi:10.1007/s43440-022-00377-w
6. Accorroni A, Chiellini G, Origlia N. Effects of thyroid hormones and their metabolites on learning and memory in normal and pathological conditions. Curr Drug Metab. 2017;18(3):225–236. doi:10.2174/1389200218666170116112407
7. Khaleghzadeh-Ahangar H, Talebi A, Mohseni-Moghaddam P. Thyroid disorders and development of cognitive impairment: a Review Study. Neuroendocrinology. 2022;112(9):835–844. doi:10.1159/000521650
8. Eslami-Amirabadi M, Sajjadi SA. The relation between thyroid dysregulation and impaired cognition/behaviour: an integrative review. J Neuroendocrinol. 2021;33(3):e12948. doi:10.1111/jne.12948
9. Folkestad L, Brandt F, Lillevang-Johansen M, Brix TH, Hegedus L. Graves’ disease and toxic nodular goiter, aggravated by duration of hyperthyroidism, are associated with Alzheimer’s and vascular dementia: a registry-based long-term follow-up of two large cohorts. Thyroid. 2020;30(5):672–680. doi:10.1089/thy.2019.0672
10. Wieland DR, Wieland JR, Wang H, et al. Thyroid disorders and dementia risk: a Nationwide Population-Based Case-Control Study. Neurology. 2022;99(7):e679–e687. doi:10.1212/WNL.0000000000200740
11. Kalmijn S, Mehta KM, Pols HA, Hofman A, Drexhage HA, Breteler MM. Subclinical hyperthyroidism and the risk of dementia. The Rotterdam study. Clin Endocrinol. 2000;53(6):733–737. doi:10.1046/j.1365-2265.2000.01146.x
12. Quinlan P, Horvath A, Wallin A, Svensson J. Low serum concentration of free triiodothyronine (FT3) is associated with increased risk of Alzheimer’s disease. Psychoneuroendocrinology. 2019;99:112–119. doi:10.1016/j.psyneuen.2018.09.002
13. Wu Y, Pei Y, Wang F, Xu D, Cui W. Higher FT4 or TSH below the normal range are associated with increased risk of dementia: a meta-analysis of 11 studies. Sci Rep. 2016;6:31975. doi:10.1038/srep31975
14. Hogervorst E, Huppert F, Matthews FE, Brayne C. Thyroid function and cognitive decline in the MRC Cognitive Function and Ageing Study. Psychoneuroendocrinology. 2008;33(7):1013–1022. doi:10.1016/j.psyneuen.2008.05.008
15. Marouli E, Yusuf L, Kjaergaard AD, et al. Thyroid function and the risk of Alzheimer’s disease: a Mendelian Randomization Study. Thyroid. 2021;31(12):1794–1799. doi:10.1089/thy.2021.0321
16. Chaker L, Wolters FJ, Bos D, et al. Thyroid function and the risk of dementia: the Rotterdam Study. Neurology. 2016;87(16):1688–1695. doi:10.1212/WNL.0000000000003227
17. van Osch LA, Hogervorst E, Combrinck M, Smith AD. Low thyroid-stimulating hormone as an independent risk factor for Alzheimer disease. Neurology. 2004;62(11):1967–1971. doi:10.1212/01.wnl.0000128134.84230.9f
18. Johansson P, Almqvist EG, Johansson JO, et al. Reduced cerebrospinal fluid level of thyroxine in patients with Alzheimer’s disease. Psychoneuroendocrinology. 2013;38(7):1058–1066. doi:10.1016/j.psyneuen.2012.10.012
19. van Boxtel MP, Menheere PP, Bekers O, Hogervorst E, Jolles J, van Boxtel MPJ. Thyroid function, depressed mood, and cognitive performance in older individuals: the Maastricht Aging Study. Psychoneuroendocrinology. 2004;29(7):891–898. doi:10.1016/j.psyneuen.2003.08.002
20. van Vliet NA, van Heemst D, Almeida OP, et al. Association of thyroid dysfunction with cognitive function: an individual participant data analysis. JAMA Intern Med. 2021;181(11):1440–1450. doi:10.1001/jamainternmed.2021.5078
21. Stern RA, Davis JD, Rogers BL, et al. Preliminary study of the relationship between thyroid status and cognitive and neuropsychiatric functioning in euthyroid patients with Alzheimer dementia. Cogn Behav Neurol. 2004;17(4):219–223.
22. Sun H, Zhu W, Liu J, An Y, Wang Y, Wang G. Reduced sensitivity to thyroid hormones is associated with high remnant cholesterol levels in Chinese euthyroid adults. J Clin Endocrinol Metab. 2022;108(1):166–174. doi:10.1210/clinem/dgac523
23. Laclaustra M, Moreno-Franco B, Lou-Bonafonte JM, et al. Impaired sensitivity to thyroid hormones is associated with diabetes and metabolic syndrome. Diabetes Care. 2019;42(2):303–310. doi:10.2337/dc18-1410
24. Jostel A, Ryder WD, Shalet SM. The use of thyroid function tests in the diagnosis of hypopituitarism: definition and evaluation of the TSH index. Clin Endocrinol. 2009;71(4):529–534. doi:10.1111/j.1365-2265.2009.03534.x
25. Yagi H, Pohlenz J, Hayashi Y, Sakurai A, Refetoff S. Resistance to thyroid hormone caused by two mutant thyroid hormone receptors beta, R243Q and R243W, with marked impairment of function that cannot be explained by altered in vitro 3,5,3’-triiodothyroinine binding affinity. J Clin Endocrinol Metab. 1997;82(5):1608–1614. doi:10.1210/jcem.82.5.3945
26. Mehran L, Delbari N, Amouzegar A, Hasheminia M, Tohidi M, Azizi F. Reduced sensitivity to thyroid hormone is associated with diabetes and hypertension. J Clin Endocrinol Metab. 2022;107(1):167–176. doi:10.1210/clinem/dgab646
27. Lai S, Li J, Wang Z, Wang W, Guan H. Sensitivity to thyroid hormone indices are closely associated with NAFLD. Front Endocrinol. 2021;12:766419. doi:10.3389/fendo.2021.766419
28. Ding X, Wang Y, Liu J, Wang G. Impaired sensitivity to thyroid hormones is associated with elevated homocysteine levels in the euthyroid population. J Clin Endocrinol Metab. 2022;107(9):e3731–e3737. doi:10.1210/clinem/dgac371
29. Sun Y, Teng D, Zhao L, et al. Impaired sensitivity to thyroid hormones is associated with hyperuricemia, obesity, and cardiovascular disease risk in subjects with subclinical hypothyroidism. Thyroid. 2022;32(4):376–384. doi:10.1089/thy.2021.0500
30. American Diabetes A. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2018. Diabetes Care. 2018;41(Suppl 1):S13–S27. doi:10.2337/dc18-S002
31. Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):270–279. doi:10.1016/j.jalz.2011.03.008
32. Yu J, Li J, Huang X. The Beijing version of the Montreal cognitive assessment as a brief screening tool for mild cognitive impairment: a community-based study. BMC Psychiatry. 2012;12(1):156. doi:10.1186/1471-244X-12-156
33. Tan ZS, Vasan RS. Thyroid function and Alzheimer’s disease. J Alzheimers Dis. 2009;16(3):503–507. doi:10.3233/JAD-2009-0991
34. Koromilas C, Liapi C, Schulpis KH, Kalafatakis K, Zarros A, Tsakiris S. Structural and functional alterations in the hippocampus due to hypothyroidism. Metab Brain Dis. 2010;25(3):339–354. doi:10.1007/s11011-010-9208-8
35. Chaalal A, Poirier R, Blum D, Laroche S, Enderlin V. Thyroid hormone supplementation restores spatial memory, hippocampal markers of neuroinflammation, plasticity-related signaling molecules, and beta-amyloid peptide load in hypothyroid Rats. Mol Neurobiol. 2019;56(1):722–735. doi:10.1007/s12035-018-1111-z
36. Cooke GE, Mullally S, Correia N, O’Mara SM, Gibney J. Hippocampal volume is decreased in adults with hypothyroidism. Thyroid. 2014;24(3):433–440. doi:10.1089/thy.2013.0058
37. Zhang H, Yang S, Zhu W, et al. Free triiodothyronine levels are related to executive function and scene memory in type 2 diabetes mellitus patients without diagnosed thyroid diseases. Diabetes Metab Syndr Obes. 2022;15:1041–1050. doi:10.2147/DMSO.S355656
38. Ortiga-Carvalho TM, Chiamolera MI, Pazos-Moura CC, Wondisford FE. Hypothalamus-pituitary-thyroid axis. Compr Physiol. 2016;6(3):1387–1428. doi:10.1002/cphy.c150027
39. Visser WE, van Mullem AA, Visser TJ, Peeters RP. Different causes of reduced sensitivity to thyroid hormone: diagnosis and clinical management. Clin Endocrinol. 2013;79(5):595–605. doi:10.1111/cen.12281
40. McDonald MP, Wong R, Goldstein G, Weintraub B, Cheng SY, Crawley JN. Hyperactivity and learning deficits in transgenic mice bearing a human mutant thyroid hormone beta1 receptor gene. Learn Mem. 1998;5(4–5):289–301. doi:10.1101/lm.5.4.289
41. Hauser P, Zametkin AJ, Martinez P, et al. Attention deficit-hyperactivity disorder in people with generalized resistance to thyroid hormone. N Engl J Med. 1993;328(14):997–1001. doi:10.1056/NEJM199304083281403
42. Quinlan P, Horvath A, Eckerstrom C, Wallin A, Svensson J. Altered thyroid hormone profile in patients with Alzheimer’s disease. Psychoneuroendocrinology. 2020;121:104844. doi:10.1016/j.psyneuen.2020.104844
43. McAninch EA, Rajan KB, Evans DA, et al. A common DIO2 polymorphism and Alzheimer disease dementia in African and European Americans. J Clin Endocrinol Metab. 2018;103(5):1818–1826. doi:10.1210/jc.2017-01196
44. Castagna MG, Dentice M, Cantara S, et al. DIO2 Thr92Ala reduces deiodinase-2 activity and serum-T3 levels in thyroid-deficient patients. J Clin Endocrinol Metab. 2017;102(5):1623–1630. doi:10.1210/jc.2016-2587
45. Luo M, Zhou XH, Zou T, Keyim K, Dong LM. Type II deiodinase polymorphisms and serum thyroid hormone levels in patients with mild cognitive impairment. Genet Mol Res. 2015;14(2):5407–5416. doi:10.4238/2015.May.22.10
46. Knopman DS, DeKosky ST, Cummings JL, et al. Practice parameter: diagnosis of dementia (an evidence-based review). report of the quality standards subcommittee of the American Academy of Neurology. Neurology. 2001;56(9):1143–1153. doi:10.1212/wnl.56.9.1143
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.