Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 13
Impact of using the new GOLD classification on the distribution of COPD severity in clinical practice
Authors Hernández M, García G, Falco J, García AR, Martín V, Ibarrola M, Quadrelli S
Received 11 May 2016
Accepted for publication 1 July 2017
Published 17 January 2018 Volume 2018:13 Pages 351—356
DOI https://doi.org/10.2147/COPD.S112551
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Richard Russell
Marcos Hernández, Gabriel García, Jimena Falco, Agustín R García, Vanina Martín, Manuel Ibarrola, Silvia Quadrelli
Department of Respiratory Medicine, Güemes Foundation, Buenos Aires, Argentina
Objective: The objective of this study was to examine how COPD patients were classified by the Global Initiative for Chronic Obstructive Lung Disease (GOLD) spirometry-based severity system and the distribution of COPD severity using the new GOLD 2011 assessment framework.
Materials and methods: This was an observational, retrospective cohort study conducted in a single tertiary center on a prospective database, which aimed to evaluate the prevalence, incidence, severity, and comorbidities of COPD. Inclusion criteria were age ≥40 years and COPD diagnosis according to GOLD 2007 classification. Clinical factors were compared between the categories in GOLD 2007 and 2011 groups by using the χ2 test for categorical data and the analysis of variance for continuous data.
Results: In total, 420 COPD patients were included in the analysis. The distribution of patients into GOLD 2007 categories was as follows: 6.4% (n=27) of them were classified into subgroup I, 42.1% (n=177) into subgroup II, 37.9% (n=159) into subgroup III, and 13.6% (n=57) into subgroup IV. The distribution of patients into GOLD 2011 categories was as follows: 16.4% (n=69) of them were classified into subgroup A (low risk and fewer symptoms), 32.1% (n=135) into subgroup B (low risk and more symptoms), 21.6% (n=91) into subgroup C (high risk and fewer symptoms), and 29.7% (n=125) into subgroup D (high risk and more symptoms). After the application of the new GOLD 2011 (modified Medical Research Council [mMRC] system), 22% (n=94) of patients were upgraded to a higher level than their spirometry level, and 16.2% (n=68) of them were downgraded in their severity category, meaning that almost 40% of patients changed their severity assessment category. In total, 22% of patients in stage I were allocated to group B, and 35% of patients in stage IV were allocated to group C. Patients in stage III were the most frequently upgraded to a higher risk group (D), taking into account mMRC and exacerbation history.
Conclusion: Classifying patients using the new GOLD 2011 criteria reallocated a relevant proportion of patients to a different risk category and identified larger proportions of patients in the mildest and more severe groups compared with GOLD 2007 classification.
Keywords: incidence, severity, comorbidities, spirometry level, exacerbation
Introduction
COPD is a progressive respiratory disease characterized by persistent airflow obstruction. While conventional COPD classification was mainly based on airflow limitation, it is now accepted that forced expiratory volume in 1 second (FEV1) is an insufficient marker of the severity of the disease. The Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2011 document has proposed a new, multidimensional approach and now recommends considering symptoms and exacerbation risk to grade disease severity into risk groups A–D.1
Since the introduction of the new GOLD assessment system, few studies have evaluated how it compares to the traditional spirometry-based staging system. Most studies have been conducted with COPD patients recruited from research cohorts enrolled in longitudinal studies or from large epidemiological databases.2,3 It has been reported that the correlations between FEV1 and clinical outcomes are weak when changes in lung function are compared to symptoms scores or multidimensional measures.4–6 In spite of the limitations of the new GOLD classification, it is clear that the multidimensional assessment of COPD patients has important clinical and research implications.
The primary objective of this study was to examine, in a university hospital-based cohort, how COPD patients were staged by the traditional GOLD spirometry-based severity system and the distribution of COPD severity using the new GOLD 2011 assessment framework.
Materials and methods
This was an observational, retrospective cohort study conducted in a single tertiary center on a prospective database, which aimed to evaluate the prevalence, incidence, severity, comorbidities, and burden of disease in patients with COPD. Patients were recruited at outpatient clinics from the Department of Respiratory Medicine. This study was approved by a formally constituted ethics committee (Sanatorio Guemes Ethics Committee), and written informed consent was obtained from the patients.
The patients were included if they were aged ≥40 years and had been diagnosed with COPD according to GOLD 2007 criteria. There should be clinical stability in their diagnoses in the last month. In this present study, we included only patients in the database who met the American Thoracic Society (ATS)/European Respiratory Society (ERS) quality standards and who provided all information needed for GOLD staging and appropriate self-assessment and documented care for at least 1 year at outpatient clinic. Patients were excluded if they had a current asthma diagnosis, had a primary pulmonary vascular disease presenting at the time of performing the study, had any serious physical and/or mental impediment that would impede the respiratory function tests possible, were unable to complete study procedures, or had participated in a clinical trial within the prior 12 months. We included only patients who were current or ex-smokers.
Data collection included the following: sex, age, height, weight, body mass index (BMI), duration of COPD, smoking history, and comorbidities (cardiovascular disease, cerebrovascular disease, cancer, and diabetes mellitus) defined as an ever-recorded diagnosis. Symptoms were quantified with the modified Medical Research Council (mMRC) scale.7,8
The most recently recorded spirometries at the moment of the first visit to the pulmonary clinic were documented. Patients were classified into their traditional obstruction severity stage based on their percentage of predicted FEV1 using GOLD guidelines: I (mild): FEV1 >80% predicted; II (moderate): FEV1 =50%–79% predicted; III (severe): FEV1 =30%–49% predicted; and IV (very severe): FEV1 <30% predicted.9
The number of COPD exacerbations in the previous 12 months was determined by asking the patients and consulting the hospital medical file. An exacerbation was defined as 1) worsening of the subject’s condition beyond normal day-to-day variations that required additional treatment with oral or intravenous corticosteroids or antibiotics; 2) attendance at an emergency center for worsening of symptoms; or 3) a hospital admission with a primary diagnosis of COPD.10 Data were entered into SPSS (version 15) for analyses. Summary statistics included the mean ± SD or median and interquartile range for continuous data and the number (percentage) for categorical data. Clinical factors were compared between the different categories in GOLD 2007 and 2011 groups by using the χ2 test for categorical data and the analysis of variance for continuous data. All the analyses used a two-sided P of 0.05 for significance.
Results
Table 1 presents the demographic characteristics of the 420 COPD patients included in the analysis. The average age at the time of the first consultation in our clinic was 63.7 (SD =7.3) years, 72.2% of them were male, 22.1% (n=93) were current smokers, 74.5% (n=306) were former smokers, and only 2.9% (n=12) were never smokers (missing data for 9 patients). Approximately 67.8% (n=285) of patients had one or more comorbidities (Table 1). The median time of the previous diagnosis of COPD was 3.9±4.1 years (range 0.2–20).
  | Table 1 Demographic characteristics |
The proportion of patients receiving any COPD medication at the first visit was 87.1%. The distribution of patients into the GOLD 2007 categories showed that 6.4% (n=27) were classified into subgroup I, 42.1% (n=177) were classified into subgroup II, 37.9% (n=159) were classified into subgroup III, and 13.6% (n=57) were classified into subgroup IV (Table 2).
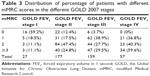
The incidence of exacerbations in the last year increased with the severity of airflow obstruction (GOLD FEV1 stages I–IV; Table 2) but without reaching the statistical significance (P=0.448) and, as expected, also the magnitude of symptoms assessed by mMRC (P>0.001; Table 3).
By GOLD 2007 classification, patients in the subgroups with greatest airflow limitation (III and IV) were older (65.4±7.1 vs 61.8±7.2 years old; P>0.001) and had a shorter time of having received a previous diagnosis of COPD compared with patients in GOLD stages I and II. Patients in GOLD stages III and IV had higher levels of respiratory comorbidities (19.9% vs 12.7%; P=0.049) but no cardiovascular comorbidities (38.4% vs 35.8%; P=0.641) compared with those in stages I and II.
The distribution of patients into the GOLD 2011 categories showed that 16.4% (n=69) of them were classified into subgroup A (low risk and fewer symptoms), 32.1% (n=135) were classified into subgroup B (low risk and more symptoms), 21.6% (n=91) were classified into subgroup C (high risk and fewer symptoms), and 29.7% (n=125) were classified into subgroup D (high risk and more symptoms; Table 4; Figure 1).
  | Table 4 Distribution of GOLD 2007 stages among each GOLD 2011 category |
  | Figure 1 Distribution of GOLD 2007 and GOLD 2011 categories. |
When classified by the GOLD 2011 criteria, patients in the higher symptom subgroups (B and D) were older and had higher prevalence of cardiovascular comorbidities compared with the low-symptom subgroups (A and C; 38.5 vs 21.9; P=<0.001).
After the application of the new GOLD 2011 (mMRC system), 22% (n=94) of patients were upgraded to a higher level than their spirometry level, and 16.2% (n=68) of them were downgraded in their severity category, meaning that almost 40% of patients changed their severity assessment category (Table 5). Among the patients with the mildest airflow obstruction (stage I), 22% were allocated to group B, and among the patients with the most severe airflow obstruction (stage IV), 35% were allocated to a lower risk group (GOLD C). Patients with severe airflow obstruction (stage III) were the most frequently upgraded to a higher risk group (GOLD D) when taking into account mMRC and exacerbation history (Figure 2).
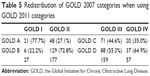  | Table 5 Redistribution of GOLD 2007 categories when using GOLD 2011 categories |
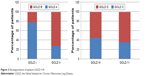  | Figure 2 Recategorization of patients GOLD I–IV. |
Discussion
The 2011 revision of the global strategy for the diagnosis, management, and prevention of COPD (GOLD) has defined two methods of assessing exacerbation risk. One is using the GOLD spirometric classification with GOLD III or GOLD IV categories indicating a high risk, and the other one is based on the individual patient’s history of exacerbations,1 with two or more exacerbations in the preceding year indicating a high risk. The assessment system that includes chronic respiratory symptoms information and recent exacerbation history allows reclassifying patients into a two-dimensional model and should be a more accurate assessment and a more proper guide for therapy.
Our study has shown that, when using the GOLD 2011 evaluation system in a COPD population of specialty clinic, almost 40% of patients changed their severity assessment category. We have also shown that according to the GOLD 2011 classification, a greater proportion of patients were identified as being at a high risk of adverse health outcomes, and patients with severe airflow obstruction (stage III) were the most frequently upgraded to a higher risk group.
The proportion of patients with mild airflow obstruction is lower in our population than in the study of Haughney et al3 (6.4% vs 17.1%), which is expected as our study is based on a speciality clinic population that usually includes more severe patients. In spite of these differences, our findings have identified that compared with the GOLD 2007 classification, the GOLD 2011 categories had more patients in both extremes of the spectrum of severity: the mildest (group A) and most severe groups (group D). Similarly, Lange et al reported a higher proportion of patients in the GOLD 2011 group D (4.5%) when compared with GOLD IV 2007 (0.7%), which was shown by Nadeau et al in a mixed COPD population although skewed toward specialist care rather than toward primary care.2,11
We have also shown that the history of exacerbations increases steadily by mMRC level, which was also reported by Mapel et al in a primary care cohort of 445 patients. As the evaluation system intends to define the risk of adverse health care events, these findings help to validate that the system is working as expected.12
More than 8% of patients had experienced two or more exacerbations in the previous year despite having “low-risk” airflow limitation (GOLD I or II), and more than 70% of patients with “high-risk” airflow limitation (GOLD III or IV) had no or one exacerbation in the previous year. A systematic literature review by Hogendoorn et al including 37 relevant studies showed that annual event-based exacerbation frequencies per GOLD stage were estimated at 0.82 for mild, 1.17 for moderate, 1.61 for severe, and 2.10 for very severe COPD, strongly suggesting an increased risk of exacerbations with increased levels of airflow limitation.13 However, our findings, similar to those reported by the ECLIPSE study and in agreement with the study of Haughney et al, provide enough evidence to support that in daily practice lung function alone does not predict the likelihood of having an exacerbation.3,14
Beyond looking for a better system to predict exacerbations, the poor correlation between the degree of airflow obstruction and other clinical outcomes suggested that GOLD 2007 did not provide strong evidence to suggest treatment recommendations. It has previously been reported that the traditional COPD severity system based solely on spirometry did not correlate well with either patient or physician perception of severity.12
Our findings have shown that a relevant proportion of patients should change their treatment modality when turning their assessment of severity into the GOLD 2011 system. Interestingly, although less than 40% of our patients had a history of two or more exacerbations during the previous year, more than 65% were receiving inhaled corticosteroids (ICS). In our study, we found ICS overuse in COPD patients, a situation that is frequently described. It was previously reviewed the association between the overuse and increased rates of exacerbations. Additionally, comorbidities can affect the overall impact on the COPD patient. In our study, hypertension was highly prevalent (61.7%) and also other cardiovascular diseases (32.1%). In view of BMI, as an important factor not only for prognosis but also for exacerbations of COPD, we found a high prevalence of obese patients in these groups (BMI ≥30 kg/m2) in 26.2%, that is, a common comorbidity in Latin American COPD patient population (the PLATINO study17). We also found that our patients with mMRC score ≥2, which is higher among stage II than among stage III (GOLD 2007), may have cardiac comorbidities or obesity. Interestingly, we could not confirm a higher prevalence of cardiovascular comorbidities in groups with a higher degree of airflow obstruction (GOLD stages III and IV), similar to the findings reported by Echave-Sustaeta et al although that was the case when severity was classified by the GOLD 2011 criteria.15 Patients in the higher symptom subgroups (B and D) had a higher prevalence of cardiovascular comorbidities compared with the low-symptom subgroups (A and C). These findings stress the importance of assessing patients with COPD for other respiratory comorbidities, regardless of airflow obstruction severity but mainly when they show a higher level of symptoms.
Our study has some important limitations. Although we used standardized questionnaires that were administered by the respiratory physicians, most of the information relies on nonchecked answers from the patients themselves. Additionally, this study was carried out in a single institution and we cannot assure that the data set generated was representative of the primary or specialist care throughout the country; we understand that these findings cannot be extrapolated to other countries due to differences in health care systems, mainly accessibility to primary and specialist care. Another limitation is that we included only mMRC in our symptom assessment because COPD assessment test (CAT) data were collected in an insufficient number of patients. It has been reported that the classifications of COPD produced by the mMRC or CAT score are not identical and researchers or practitioners using different tools may have different findings.16
However, in spite of these limitations, this population is quite representative of a specialty clinical-based practice.
Conclusion
We have showed that classifying patients using the new GOLD 2011 criteria reallocated a relevant proportion of patients to a different risk category and identified larger proportions of patients in the mildest and more severe groups compared with the GOLD 2007 classification. The GOLD 2011 classification should help to identify patients in higher risk of exacerbations and comorbidities and those who require more frequent monitoring in order to achieve a really personalized targeted care.
Disclosure
The authors report no conflicts of interest in this work.
References
Global Initiative for Chronic Obstructive Lung Disease (GOLD); 2011. Available from: http://www.goldcopd.org/. Accessed January 25, 2016. | ||
Lange P, Marott JL, Vestbo J, et al. Prediction of the clinical course of chronic obstructive pulmonary disease, using the new GOLD classification: a study of the general population. Am J Respir Crit Care Med. 2012;186(10):975–981. | ||
Haughney J, Gruffydd-Jones K, Roberts J, Lee AJ, Hardwell A, McGarvey L. The distribution of COPD in UK general practice using the new GOLD classification. Eur Respir J. 2014;43(4):993–1002. | ||
Jones P, Miravitlles M, van der Molen T, Kulich K. Beyond FEV1 in COPD: a review of patient-reported outcomes and their measurement. Int J Chron Obstruct Pulmon Dis. 2012;7:697–709. | ||
Westwood M, Bourbeau J, Jones PW, Cerulli A, Capkun-Niggli G, Worthy G. Relationship between FEV1 change and patient-reported outcomes in randomised trials of inhaled bronchodilators for stable COPD: a systematic review. Respir Res. 2011;12:40. | ||
Tsiligianni I, Kocks J, Tzanakis N, Siafakas N, van der Molen T. Factors that influence disease-specific quality of life or health status in patients with COPD: a review and meta-analysis of Pearson correlations. Prim Care Respir J. 2011;20(3):257–268. | ||
Bestall JC, Paul EA, Garrod R, Garnham R, Jones PW, Wedzicha JA. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999;54(7):581–586. | ||
Fletcher CM, Elmes PC, Fairbairn AS, Wood CH. The significance of respiratory symptoms and the diagnosis of chronic bronchitis in a working population. Br Med J. 1959;2(5147):257–266. | ||
Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176:532–555. | ||
Vestbo J, Hurd SS, Agusti AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347–365. | ||
Nadeau G, Adamek L, Small M. Distribution of COPD patients in the GOLD assessment framework by exacerbations. Eur Respir J. 2012;40(Suppl 56):p980. | ||
Mapel DW, Dalal AA, Johnson PT, Becker LK, Hunter AG. Application of the new GOLD COPD staging system to a US primary care cohort, with comparison to physician and patient impressions of severity. Int J Chron Obstruct Pulmon Dis. 2015;10:1477–1486. | ||
Hogendoorn M, Feenstra TL, Hoogenveen RT, Al M, Mölken MR. Association between lung function and exacerbation frequency in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2010;5:435–444. | ||
Agusti A, Calverley PMA, Celli B, et al. Characterisation of COPD heterogeneity in the ECLIPSE cohort. Respir Res. 2010;11:122. | ||
Echave-Sustaeta JM, Comeche Casanova L, Cosio BG, Soler-Cataluña JJ, Garcia-Lujan R, Ribera X. Comorbidity in chronic obstructive pulmonary disease. Related to disease severity? Int J Chron Obstruct Pulmon Dis. 2014;9:1307–1314. | ||
Kim S, Oh J, Kim YI, et al. Differences in classification of COPD group using COPD assessment test (CAT) or modified Medical Research Council (mMRC) dyspnea scores: a cross-sectional analyses. BMC Pulm Med. 2013;13:35. | ||
Menezes AMB, Perez-Padilla R, Jardim JRB, et al. Chronic obstructive pulmonary disease in five Latin American cities (the PLATINO study): a prevalence study. Lancet. 2005;366(9500):1875–1881. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.