Back to Journals » OncoTargets and Therapy » Volume 13
Impact of Programmed Death Ligand 1 Expression in Advanced Non-Small–Cell Lung Cancer Patients, Treated by Chemotherapy (GFPC 06-2015 Study)
Authors Auliac JB , Guisier F , Bizieux A, Assouline P, Bernardini M, Lamy R , Justeau G, François G, Damotte D, Chouaïd C
Received 30 October 2020
Accepted for publication 17 December 2020
Published 30 December 2020 Volume 2020:13 Pages 13299—13305
DOI https://doi.org/10.2147/OTT.S288825
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Alberto Bongiovanni
Jean-Bernard Auliac,1 Florian Guisier,2 Acya Bizieux,3 Pascal Assouline,4 Marie Bernardini,5 Régine Lamy,6 Grégoire Justeau,7 Geraldine François,8 Diane Damotte,9,10 Christos Chouaïd1,11
1Pneumology Department, Centre Hospitalier Intercommunal de Créteil, Créteil, France; 2Pulmonology, Thoracic Oncology and Respiratory Department, Rouen University Hospital, Rouen, France; 3Pneumology Department, Centre Hospitalier de Vendée, La Roche-sur-Yon, France; 4Pneumology Department, Centre Hospitalier de Bligny, Bligny, France; 5Pneumology Department, Centre Hospitalier d’Aix-En-Provence, Aix-en-Provence, France; 6Pneumology Department, Centre Hospitalier de Bretagne-Sud, Lorient, France; 7Pneumology Department, Centre Hospitalier Universitaire d’Angers, Angers, France; 8Pneumology Department, Centre Hospitalier Universitaire d’Amiens, Amiens, France; 9Department of Pathology, Hôpital Cochin, APHP, Paris, France; 10University Paris Descartes, Paris, France; 11Inserm U955, UPEC, IMRB, Équipe CEpiA, Créteil, France
Correspondence: Jean-Bernard Auliac
Service de Pneumologie, CHI Créteil, 40, Avenue de Verdun, Créteil Cedex 94010, France
Email [email protected]
Background: Few data have been published on the clinical and histopathological characteristics of advanced non-small–cell lung cancer (NSCLC) patients with high PD-L1 expression versus intermediate or none and the prognostic value of PD-L1 expression for patients treated with chemotherapy is unknown. This study was undertaken to prospectively assess the prognostic value of tumor-cell (TC) and immune-cell (IC) PD-L1 expressions for advanced NSCLC patients.
Methods: It was a prospective, multicenter study on advanced NSCLC patients, with performance status 0/1, scheduled, consecutively, to receive first-line platin-based chemotherapy. PD-L1 expression was determined immunochemically (Dako Autostainer and monoclonal antibody 22C3) and its impact on progression-free survival (PFS) and overall survival (OS) assessed.
Results: Among 198 patients screened in 19 centers, 140 were included median age: 66.5 ± 10 years; 76.4% men; 79.3% Caucasians; 10.7% nonsmokers; 63.6% adenocarcinomas; < 1%, 1– 50% and ≥ 50% TC PD-L1–expression rates were 47.1%, 25.7% and 27.2% of patients, respectively; respective null, intermediate and high rates on ICs were 35.7%, 38.6% and 25.7%. Second- and third-line chemotherapies were administered to 58.6% and 26.4% of the patients, respectively. None received immunotherapy. First-, second- and third-line median (95% CI) PFS lasted 4.6 (3.6– 5.2), 3.7 (2.3– 4.7) and 2.2 (1.5– 4.3) months, respectively; median OS was 16.9 (11.4– 19.9) months. No significant PFS and OS differences were observed according to TC or IC PD-L1 expression.
Conclusion: According to the results of this prospective, multicenter study, neither TC nor IC PD-L1 expression appears to be prognostic for chemotherapy-managed advanced NSCLC patients.
Keywords: chemotherapy, immunotherapy, non-small–cell lung cancer, PD-L1, prognostic
Introduction
Lung cancer is the most common cause of cancer-related deaths worldwide. Non-small–cell lung cancer (NSCLC) constitutes the predominant histological type, representing more than 85% of all lung tumors.1 A high percentage of the patients are diagnosed with locally advanced-stage or metastatic disease. Recent advances in molecular-targeted therapy have led to markedly improved prognoses for patients with alterations in oncogenes, such as the epidermal growth factor receptor (EGFR) and anaplastic lymphoma-kinase (ALK) genes.2 Recent clinical trials of immunotherapy’s targeting programmed cell death-1 (PD-1) or its ligand-1 (PD-L1) have shown the safety and remarkable antitumor activities of these agents against various cancers, including NSCLCs.3 Notably, for NSCLC patients, PD-1 inhibitors, like nivolumab and pembrolizumab, and PD-L1 inhibitors, such as atezolizumab, have obtained survival benefits comparable with that of conventional standard therapy, and the subset analyses of those studies revealed close associations between PD-L1 expression and efficacy. PD-1 is expressed on the surface of activated T cells and regulates their activity through interaction with its ligands PD-L1 and PD-L2 expressed on the surface of NSCLC cells. Those interactions attenuate T-cell activity, resulting in the down regulation of the immune response against cancer cells. Inhibiting those interactions can enhance T-cell functions.3,4 However, immunotherapy is only effective for a small percentage of cancer patients and the complexity of the tumor immune microenvironment may account for this phenomenon.5
Limited data on European populations have been published on the clinical and histopathological characteristics of high PD-L1 expression compared to intermediate or no expression. A recent systematic literature review, including 35 eligible studies did not support an association between PD-L1 expression and gender, age, smoking history, tumor histology, performance status, pathologic tumor grade or EGFR/KRAS/ALK mutational status. Moreover, the impact of high PD-L1 expression in NSCLCs is not definitive: some studies indicated it was a positive factor,6 while others showed it to be a negative prognostic factor for chemotherapy efficacy.7–9
The primary objective of this analysis was to evaluate clinical and histopathological characteristics of high PD-L1 expression in advanced NSCLC patients compared to those with weak or no expression. The secondary objective was to compare clinical outcomes as a function of PD-L1–expression levels in these immunotherapy-naïve patients and to assess the prognostic impact of PD-L1 expression.
Methods
This multicenter, prospective, observational study included adult patients diagnosed with stage IIIB–IV NSCLCs, scheduled to receive first-line standard-dose platin-based chemotherapy (cisplatin or carboplatin with gemcitabine, vinorelbine, docetaxel, paclitaxel, pemetrexed or bevacizumab, with the latter two being restricted to nonsquamous histology). Neo- or adjuvant therapies were allowed if they had been administered at least 1 year before inclusion. The other main inclusion criteria were: Eastern Cooperative Oncology Group performance status (ECOG PS) 0/1, stage IIIB–IV NSCLC, histological diagnosis, no known EGFR mutation or ALK or receptor tyrosine kinase (ROS) translocations, at least two slides of the initial tumor sample available and a Response Evaluation Criteria In Solid Tumors 1.1 (RECIST) target lesion.
Exclusion criteria were pregnancy, known immune deficiency, PS > 2, inclusion in a first-line clinical therapeutic trial, patient who received first-line PD-1/PD-L1 immunotherapy.
The following information was collected: age; sex; smoking status; histology; stage; metastasis number(s) and site(s); EGFR and ALK/ROS, if tested; PD-L1 status; first-, second-, and third-line treatments; RECIST 1.1 responses to each treatment line.
Local response evaluations, blinded to PD-L1 expression, were done every 6 weeks during platin-doublet chemotherapy, then every 8 weeks for the following lines and for patients no longer on treatment but who had not yet progressed.
To assure that PD-L1 expression was assessed with the same methodology for all patients, immunohistochemical PD-L1 expression was determined centrally for tumor-tissue slides with ≥100 cells. For each patient’s specimen, immune-labeling followed the procedure recommended by Dako, with the monoclonal antibody 22C3 in the Dako Autostainer. Each labeling run included a negative control (slide without antibody) and pathologists considered that at least one immune cell on each cell had to be positive for PD-L1 for the tumor to IC PD-L1+ in each slide. When all cells were negative, a new immune-labeling test was done on another slide. Two totally negative immune-labeling experiments were considered a true negative. The percentage of PD-L1–expressing tumor cells was evaluated (0 à 100%); the semi-quantitative immune-cell score for the lymphocyte infiltrate in the lung parenchyma was graded as follows: null, negative; intermediate, several positive cells; high, numerous positive cells.
Statistical methods
First-line progression-free survival (PFS1) was defined as the time between starting first-line chemotherapy and RECIST progression or death; second progression-free survival (PFS2) and third progression-free survival (PFS3) were defined as the time between starting second and third-line chemotherapy and RECIST progression or death; overall survival (OS) was defined as the time between first-line–treatment onset and death.
Different subpopulations were subjected to descriptive and comparative analyses of qualitative and quantitative variables. Categorical variables were compared with the Chi2 test; comparisons of continuous variables, whose distributions were close to normal used non-significant Shapiro–Wilk test, the Student’s t-test or analysis of variance. When distributions were not normal non-parametric Wilcoxon, Kruskal–Wallis tests were used. All tests were two-sided, with alpha fixed at 5%.
Ethical Considerations
The study was conducted in accordance with the Declaration of Helsinki. It was recorded to ClinicalTrials.gov: NCT02785562 and was approved by the Personal Protection Committee (comite de protection des personnes n° 15041MS6) for all participating centers. Written informed consent was obtained from each patient.
All participating physicians were trained in good clinical practices.
Role of the Funding Source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had the final responsibility for the decision to submit for publication.
Results
Between 22 July 2016 and 31 May 2017, 19 centers screened 198 consecutive, immunotherapy-naïve, advanced-NSCLC patients and included 140 of them: median age ± standard deviation (SD) 66.5 ± 10 years, 76.4% men, 79.3% Caucasian and 10.7% nonsmokers. The main reason for non-inclusion was insufficient biopsy-tissue available (n = 32), cytology-based diagnosis (n = 11), not eligible for first-line platin-doublet chemotherapy (n = 15). Almost two-thirds of the tumors were adenocarcinomas, 81.5% of the patients were in good general condition with ECOG PS = 0/1, 81.4% were symptomatic at diagnosis, and 22.1% had >5% weight loss (Table 1). Eight (2.9%) patients had tumors harboring an oncogenic mutation: EGFR (n = 4), BRAF (v-RAF murine sarcoma viral oncogene homolog B; n = 1) or HER2 (human epidermal growth factor receptor-2; n = 1); or a ROS1 (n = 1) or ALK translocation (n = 1).
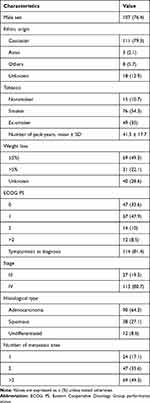 |
Table 1 Characteristics of the 140 NSCLC Patients at Diagnosis |
The percentages of patients whose tumor cells expressed <1%, <50% or ≥50% PD-L1, respectively, were 47.1%, 25.7% or 27.2%. The respective null, moderate or high immune-cell PD-L1–expression scores were 35.7%, 38.6% or 25.7%. Patient characteristics according to PD-L1 expression on tumor or immune cells did not differ significantly (Table 2). Too few patients had an oncogenic mutation to make analyses pertinent.
 |
Table 2 Characteristics of the 140 NSCLC Patients According to Tumor-Cell or Immune-Cell PD-L1 Expressions |
All patients received first-line platin-doublet chemotherapy, 82 (58.6%) were given second-line therapy, mainly docetaxel monotherapy and gemcitabine and 37 (26.4%) a third line (gemcitabine or vinorelbine). None received immunotherapy during administration of those lines.
After median follow-up of 16 (95% confidence interval (CI): 8.2–22.2) months, PFS1, PFS2 and PFS3 were, respectively, 4.6 (95% CI, 3.6–5.2), 3.7 (95% CI, 2.3–4.7) and 2.2 (95% CI, 1.5–4.3) months (Figure 1A–C, respectively). PFS did not differ significantly according to PD-L1 expression on tumor or immune cells (Table 3). Median OS lasted 16.9 (95% CI, 11.4–19.9) months (Figure 2), with no significant difference according to tumor or immune cell PD-L1 expression.
 |
Table 3 PFS and OS According to Tumor-Cell PD-L1 Expression and Immune-Cell Score |
 |
Figure 1 Progression-free survival probability after (A) first-line, (B) second-line or (C) third-line treatment. |
 |
Figure 2 Median overall survival. |
Discussion
The results of this multicenter, prospective observational study based on 140 immunotherapy-naïve advanced-NSCLC patients not given anti-PD-1/PD-L1 agents indicated no PFS or OS differences as a function of PD-L1 expression on tumor or immune cells. In this context, PD-L1 expression does not seem to be a prognostic factor.
Reported data in this setting have been contradictory but most studies were retrospective and few concerned metastatic diseases.6–20 According to a retrospective analysis of 120 NSCLC-tissue specimens,8 PD-L1 expression was not associated with patient age, sex or histo-pathological type, but was significantly associated with the degree of tumor-cell differentiation, tumor-node-metastasis (TNM) stage and OS. Poor tumor-cell differentiation and advanced TNM stage were associated with higher PD-L1 expression. Five-year OS was longer for patients with PD-L1-negative NSCLCs than those with tumors expressing PD-L1 (P < 0.0001). A meta-analysis19 based on 1550 NSCLC patients from 9 studies showed that sex, smoking status, histological type, invasive depth of tumor, lymph-node metastatic status and TNM stage were not associated with PD-L1 expression. High immune-cell PD-L1 expression was associated with poor tumor differentiation (odds ratio: 0.53, 95% CI: 0.39–0.72, p < 0.0001) and shorter OS (hazards ratio: 1.47; 95% CI: 1.19–1.83, p = 0.0004). In contrast, the results of another analysis13 that evaluated 204 advanced-NSCLC patients found no statistically significant association between PD-L1 expression and OS. Those findings were unchanged when PD-L1 levels were stratified by median or tertiles.
Our analysis was conducted on patients with metastatic NSCLCs. It is possible that PD-L1 expression might be a better prognostic factor for earlier disease stages, knowing that one of the limitations of these studies is the representativity of the patients analyzed. According to an analysis of patients with inoperable locally advanced NSCLCs (concurrent or sequential radio-chemotherapy), only 43 of the 107 screened patients had sufficient tissue for IHC.15 As herein, their patients’ characteristics (eg, age, smoking status and sex) did not differ significantly according to PD-L1 expression. After median follow-up of 103.6 months, PFS lasted 19.9 months for patients with PD-L1–negative tumors and 10.1 months for those whose NSCLCs expressed PD-L1 (P = 0.006), with respective median OS of 28.4 and 12.1 months (P = 0.012). In that study, PD-L1 expression was negative prognostic factor for PFS and OS after concurrent chemo-radiotherapy. However, because too few patients had sufficient tumor samples for immune-histochemistry analysis, no definitive conclusions could be drawn.15
Several studies16–22 included patients with early-stage NSCLCs. PD-L1 analysis of a series of 108 patients’ surgically resected primary NSCLCs showed that median OS was significantly longer for those with PD-L1–negative tumors that those whose tumors expressed the ligand (96 vs 33 months, respectively; P < 0.001). According to the analysis of the non-squamous-cell carcinoma subgroup, median OS was longer for patients with PD-L1–expressing tumors than without (113 vs 37 months, respectively; P < 0.001).
In multivariate analyses, PD-L1 expression was significantly associated with shorter OS.19 However, analysis of 224 resected NSCLC specimens showed that OS as a function of PD-L1 expression or not was comparable for squamous-cell histology. PD-L1 expression on nonsquamous-cell NSCLCs was associated with significantly shorter OS (P = 0.01).22
Finally, based on 205 surgically resected NSCLCs, ≥1% PD-L1–positivity was not associated with any clinical–histopathological characteristic and prognoses were poorer for patients with PD-L1–positive tumors than those PD-L1-negative, defined as <1%.12
In addition to PD-L1 expression on tumor cells, the tumor’s immune environment is of importance. Based on a series of stage I squamous-cell NSCLCs,15 PD-L1 expression was significantly associated with higher percentages of tumor epithelial CD8+ T cells and stromal CD4+ T cells; PD-L1–positive tumor cells were negatively associated with PD-L1–positive immune cells within the tumor stroma and tumor PD-L1 expression; and more extensive CD4+ T-cell infiltrations were independent predictors of longer OS.17 In addition, analysis of 323 surgically resected lung adenocarcinomas6 showed that former smokers had higher (≥50%) PD-L1 expression than current smokers (P = 0.026) and was associated with more pack-years (P = 0.016). PD-L1–positivity was significantly associated with adenocarcinoma histology (P < 0.001), abnormal p53 expression (P < 0.001) and immune-cell PD-L1 expression (P < 0.001). In light of the poorer relapse-free survival (P < 0.001) and OS (P < 0.001) for patients with stage I–III NSCLCs harboring PD-L1–positive tumor cells, the authors concluded that tumor and immune cells expressing PD-L1, adenocarcinoma histology and abnormal p53 expression are significantly associated with survival and should be considered when analyzing these patients’ clinical outcomes.
Our study, despite its prospective and multicenter design, has several limitations. A selection bias might be inherent because only patients with sufficient tumor material were included, ie, among the 198 patients screened, only 140 could be retained for analysis. Because it was an observational study, some data could be missing. Nonetheless, because none of the patients had received anti-PD-1/PD-L1 immunotherapy, interaction with the disease’s natural history can be excluded.
In conclusion, based on this prospective, multicenter study on consecutive series of advanced-NSCLC patients managed with chemotherapy, PD-L1 expression does not seem to be a prognostic factor.
Acknowledgments
The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Funding
Funding for this research was provided by MSD. The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Disclosure
Dr Jean-Bernard Auliac reports grants, personal fees, non-financial support from Astra Zeneca and MSD; grants from BMS and Roche; personal fees from Amgen and Boehringer Ingelheim, during the conduct of the study. Dr Florian Guisier reports personal fees, non-financial support from BMS, MSD, Roche, Boehringer Ingelheim, and Astra Zeneca, outside the submitted work. Professor Christos Chouaïd reports grants, personal fees, non-financial support from Roche, MSD, AZ, BMS, Amgen, Lilly, BI, GSK, Janssen, Pfizer, Takeda, and Novartis, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Wang Y, Pang Z, Chen X, et al. Survival nomogram for patients with initially diagnosed metastatic non-small-cell lung cancer: a SEER-based study. Future Oncol. 2019;15(29):3395–3409. doi:10.2217/fon-2019-0007
2. Huang J, Reckamp KL. Immunotherapy in advanced non-small cell lung cancer. Semin Respir Crit Care Med. 2020;41(03):400–408. doi:10.1055/s-0040-1710077
3. Chen R, Manochakian R, James L, et al. Emerging therapeutic agents for advanced non-small cell lung cancer. J Hematol Oncol. 2020;13(1):58. doi:10.1186/s13045-020-00881-7
4. Petrelli F, Maltese M, Tomasello G, et al. Clinical and molecular predictors of PD-L1 expression in non-small-cell lung cancer: systematic review and meta-analysis. Clin Lung Cancer. 2018;19(4):315–322. doi:10.1016/j.cllc.2018.02.006
5. Yuan M, Huang LL, Chen JH, et al. The emerging treatment landscape of targeted therapy in non-small-cell lung cancer. Signal Transduct Target Ther. 2019;4(1):61. doi:10.1038/s41392-019-0099-9
6. Adam J, Boris A, Lacas B, et al. Prognostic value of PDL1 expression in stage III non-small cell lung cancer (NSCLC) treated by chemoradiotherapy (CRT). Eur J Cancer. 2015;51:S604. doi:10.1016/S0959-8049(16)31668-9
7. Cha YJ, Kim HR, Lee CY, et al. Clinicopathological and prognostic significance of programmed cell death ligand-1 expression in lung adenocarcinoma and its relationship with p53 status. Lung Cancer. 2016;97:73–80. doi:10.1016/j.lungcan.2016.05.001
8. Chen YB, Mu CY, Huang JA. Clinical significance of programmed death ligand-1 expression in patients with non-small cell lung cancer: a 5-year follow-up study. Tumori. 2012;98(6):751–755. doi:10.1177/030089161209800612
9. Brody R, Zhang Y, Ballas M, et al. PD-L1 expression in advanced NSCLC: insights into risk stratification and treatment selection from a systematic literature review. Lung Cancer. 2017;112:200–215. doi:10.1016/j.lungcan.2017.08.005
10. Cooper WA, Tran T, Vilain RE, et al. PD-L1 expression is a favorable prognostic factor in early stage non-small cell carcinoma. Lung Cancer. 2015;89(2):181–188. doi:10.1016/j.lungcan.2015.05.007
11. Mu CY, Huang JA, Chen Y, et al. High expression of PD-L1 in lung cancer may contribute to poor prognosis and tumor cells immune escape through suppressing tumor infiltrating dendritic cells maturation. Med Oncol. 2011;28(3):682–688. doi:10.1007/s12032-010-9515-2
12. Schmidt LH, Kummel A, Gorlich D, et al. PD-1 and PD-L1 expression in NSCLC indicate a favorable prognosis in defined subgroups. PLoS One. 2015;10(8):e0136023. doi:10.1371/journal.pone.0136023
13. Sorensen S, Zhou W, Dolled-Filhart M, et al. PD-L1 expression and survival among patients with advanced non-small cell lung cancer treated with chemotherapy. Transl Oncol. 2016;9(1):64–69. doi:10.1016/j.tranon.2016.01.003
14. Takada K, Okamoto T, Toyokawa G, et al. The expression of PD-L1 protein as a prognostic factor in lung squamous cell carcinoma. Lung Cancer. 2017;104:7–15. doi:10.1016/j.lungcan.2016.12.006
15. Vrankar M, Zwitter M, Kern I, Stanic K. PD-L1 expression can be regarded as prognostic factor for survival of non-small cell lung cancer patients after chemoradiotherapy. Neoplasma. 2018;65(01):140–146. doi:10.4149/neo_2018_170206N77
16. Wang A, Wang HY, Liu Y, et al. The prognostic value of PD-L1 expression for non-small cell lung cancer patients: a meta-analysis. Eur J Surg Oncol. 2015;41(4):450–456. doi:10.1016/j.ejso.2015.01.020
17. Yang CY, Lin MW, Chang YL, et al. Programmed cell death-ligand expression is associated with a favourable immune microenvironment and better overall survival in stage I pulmonary squamous cell carcinoma. Eur J Cancer. 2016;57:91–103. doi:10.1016/j.ejca.2015.12.033
18. Zhang Y, Wang L, Li Y, et al. Protein expression of programmed death 1 ligand 1 and ligand 2 independently predict poor prognosis in surgically resected lung adenocarcinoma. Onco Targets Ther. 2014;7:567–573. doi:10.2147/OTT.S59959
19. Zhou ZJ, Zhan P, Song Y. PD-L1 over-expression and survival in patients with non-small cell lung cancer: a meta-analysis. Transl Lung Cancer Res. 2015;89(2):203–208. doi:10.1016/j.lungcan.2015.06.005
20. Zhou C, Tang J, Sun H, et al. PD-L1 expression as poor prognostic factor in patients with non-squamous non-small cell lung cancer. Oncotarget. 2017;8(35):58457–58468. doi:10.18632/oncotarget.17022
21. Pan ZK, Ye F, Wu X, et al. Clinicopathological and prognostic significance of programmed cell death ligand1 (PD-L1) expression in patients with non-small cell lung cancer: a meta-analysis. J Thorac Dis. 2015;7(3):462–470. doi:10.3978/j.issn.2072-1439.2015.02.13
22. Shimoji M, Shimizu S, Sato K, et al. Clinical and pathologic features of lung cancer expressing programmed cell death ligand 1 (PD-L1). Lung Cancer. 2016;98:69–75. doi:10.1016/j.lungcan.2016.04.021
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
