Back to Journals » Journal of Asthma and Allergy » Volume 17
Impact of Exacerbation History on Dupilumab Efficacy in Children with Uncontrolled Moderate-to-Severe Asthma: LIBERTY ASTHMA VOYAGE Study
Authors Guilbert TW, Tolcachier A, Fiocchi AG, Katelaris CH, Phipatanakul W, Begin P, de Mir I, Altincatal A, Gall R, Ledanois O, Radwan A, Jacob-Nara JA, Deniz Y, Rowe PJ
Received 8 April 2023
Accepted for publication 6 February 2024
Published 5 March 2024 Volume 2024:17 Pages 143—159
DOI https://doi.org/10.2147/JAA.S416292
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Amrita Dosanjh
Video abstract presented by Guilbert.
Views: 77
Theresa W Guilbert,1 Alberto Tolcachier,2 Alessandro G Fiocchi,3 Constance H Katelaris,4,5 Wanda Phipatanakul,6,7 Philippe Begin,8 Inés de Mir,9 Arman Altincatal,10 Rebecca Gall,11 Olivier Ledanois,12 Amr Radwan,11 Juby A Jacob-Nara,13 Yamo Deniz,11 Paul J Rowe13
1Department of Pediatrics, Cincinnati Children’s Hospital and University of Cincinnati, Cincinnati, OH, USA; 2Center for Allergy and Respiratory Diseases, Buenos Aires, Argentina; 3Bambino Gesù Children’s Hospital IRCCS, Rome, Italy; 4Department of Medicine, Campbelltown Hospital, Campbelltown, NSW, Australia; 5Immunology & Allergy Unit, Western Sydney University, Sydney, NSW, Australia; 6Department of Allergy and Immunology, Boston Children’s Hospital, Boston, MA, USA; 7Department of Pediatrics, Harvard Medical School, Boston, MA, USA; 8Centre Hospitalier Universitaire (CHU) Sainte-Justine, Montreal, QC, Canada; 9Pediatric Pulmonary Unit, Hospital Universitari Vall d’Hebron, Barcelona, Spain; 10Sanofi, Cambridge, MA, USA; 11Regeneron Pharmaceuticals Inc., Tarrytown, NY, USA; 12Sanofi, Paris, France; 13Sanofi, Bridgewater, NJ, USA
Correspondence: Theresa W Guilbert, Cincinnati Children’s Hospital and University of Cincinnati, 3333 Burnet Ave, Cincinnati, OH, 45229, USA, Tel +1 513-636-6771, Email [email protected]
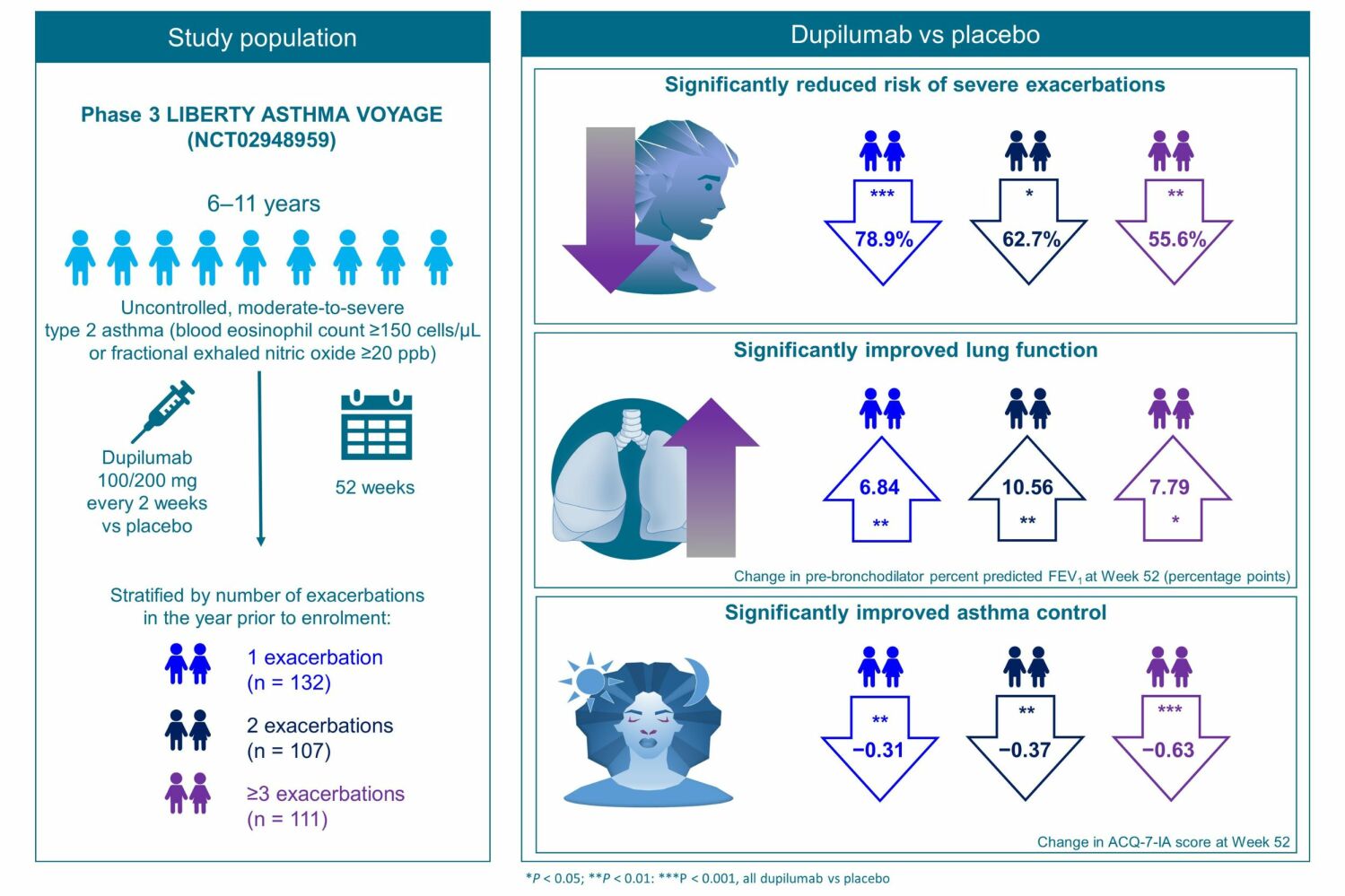
Purpose: Dupilumab, a fully human monoclonal antibody, blocks the shared receptor component for interleukins-4/-13, key and central drivers of type 2 inflammation in multiple diseases. This post hoc analysis of the Phase 3 LIBERTY ASTHMA VOYAGE study (NCT02948959) evaluated the efficacy of dupilumab in children aged 6 to 11 years with moderate-to-severe asthma with a type 2 inflammatory phenotype (blood eosinophil count ≥ 150 cells/μL or fractional exhaled nitric oxide [FeNO] ≥ 20 ppb) and a history of 1, 2, or ≥ 3 prior exacerbations. The impact of baseline type 2 biomarker levels on the efficacy of dupilumab in this population was also investigated.
Patients and Methods: Patients were stratified by the number of exacerbations in the prior year (1, 2, or ≥ 3) and level of FeNO or blood eosinophil count at baseline. Endpoints included rate of severe exacerbations, percentage of non-exacerbators, and change from baseline in both lung function parameters (pre- and post-bronchodilator [BD] percent predicted forced expiratory volume in 1 s (ppFEV1) and ppFEV1/forced vital capacity [FVC] ratio) and Asthma Control Questionnaire 7 Interviewer-Administered (ACQ-7-IA) score.
Results: A total of 350 patients were included in this analysis. Across patients with 1, 2, or ≥ 3 prior exacerbations and different levels of type 2 biomarkers, dupilumab reduced the risk of severe asthma exacerbations vs placebo by 53.0– 96.0% and improved both pre-BD ppFEV1 and pre-BD FEV1/FVC ratio at Week 52. Dupilumab led to significant reductions in ACQ-7-IA scores in all groups of patients by Week 52.
Conclusion: In children with uncontrolled, moderate-to-severe asthma with a type 2 phenotype, dupilumab consistently reduced the risk of asthma exacerbations, improved lung function, and reduced ACQ-7-IA scores, regardless of exacerbation history.
Keywords: pediatric asthma, type 2 asthma, lung function, asthma control, biologics, anti-interleukin-4 and -13
Graphical Abstract:
Introduction
Asthma, the most prevalent childhood chronic disease, affects 1 in 12 children in the US,1 with approximately 5–10% presenting with poorly controlled or uncontrolled severe disease despite receiving standard-of-care treatments at maximally tolerated doses.2,3 Over half of children with asthma are estimated to have at least one potentially life-threatening severe exacerbation per year.1 Asthma exacerbations carry significant mortality and economic burden and can predict risk of future exacerbations.4–6 Thus, there is a considerable need for novel treatments that can reduce asthma exacerbations and improve lung function and asthma control.
Type 2 inflammation has been identified as the most common driver of asthma in children,7 and type 2 inflammatory cytokines such as interleukin (IL)-4 and IL-13 are key in the pathogenesis of asthma.8 Dupilumab is a fully human monoclonal antibody that blocks the shared receptor component for signaling by IL-4 and IL-13, key and central drivers of type 2 diseases such as atopic dermatitis (AD), asthma, allergic rhinitis, and food allergies, which are often associated comorbidities.9–11
In VOYAGE (NCT02948959), a 52-week, randomized, double-blind, placebo-controlled study, dupilumab showed clinical efficacy with an acceptable safety profile in children aged 6 to 11 years with uncontrolled, moderate-to-severe asthma who received medium- or high-dose inhaled corticosteroids (ICS).12
The aim of this post hoc analysis was to evaluate the efficacy of dupilumab in pediatric patients with uncontrolled, moderate-to-severe asthma with a type 2 inflammatory phenotype and a history of 1, 2, or ≥3 exacerbations in the year before VOYAGE enrollment. We also assessed whether type 2 inflammation biomarker levels had any effect on the efficacy of dupilumab in this population.
Materials and Methods
Study Design and Patients
Full details of the VOYAGE (NCT02948959) study have been described previously.12 In brief, VOYAGE was a 52-week, phase 3, randomized, double-blind, placebo-controlled, parallel-group, multinational study that assessed the efficacy and safety of dupilumab in children aged 6 to 11 years with uncontrolled moderate-to-severe asthma. Children were eligible to participate if they had physician-diagnosed moderate-to-severe asthma according to Global Initiative for Asthma (GINA) 2020 guidelines.4 Complete inclusion and exclusion criteria have been previously reported.12 Eligible patients were randomized to receive add-on subcutaneous dupilumab 100 mg/200 mg (according to baseline bodyweight ≤30 kg/>30 kg, respectively) every 2 weeks for 52 weeks or volume-matched placebo. Full details of the study design and methodology have been previously reported.12
The study was conducted in accordance with the Declaration of Helsinki, the International Conference on Harmonisation Good Clinical Practice guidelines, and applicable regulatory requirements. An independent data and safety monitoring committee conducted blinded monitoring of patient safety data. The Institutional Review Board of the study was the Washington University School of Medicine, which oversaw trial conduct and documentation according to the local ethics committee at each study center (Supplementary Sections 1 and 2). Parents or guardians of patients provided written informed consent before trial participation began. The children provided assent according to standard practice for pediatric patients that had been approved by the ethics committee at each participating center.
Populations Analyzed
This post hoc analysis included patients who participated in VOYAGE and had a type 2 inflammatory asthma phenotype (defined as blood eosinophil count ≥150 cells/µL or FeNO ≥20 ppb) at study baseline. Patients were categorized into a total of 15 subgroups: 3 groups according to the number of exacerbations they experienced in the year prior to VOYAGE (1, 2, or ≥3 prior exacerbations), and this grouping was applied to each of 5 populations divided according to their levels of type 2 biomarkers, taken by the physician at the first visit (VOYAGE baseline): blood eosinophil count ≥150 cells/µL or FeNO ≥20 ppb; blood eosinophil count ≥150 cells/µL; FeNO ≥20 ppb; blood eosinophil count ≥300 cells/µL; and blood eosinophil count ≥500 cells/µL.
Endpoints
The efficacy endpoints assessed in this analysis were the annualized rate of severe exacerbations at the end of the 52-week treatment period; the proportion of children without exacerbations (non-exacerbators); and the change from baseline in pre-BD ppFEV1, post-BD ppFEV1, pre-BD FEV1/forced vital capacity [FVC] ratio, and Asthma Control Questionnaire 7 Interviewer-Administered (ACQ-7-IA) score at Week 52. ACQ-7-IA scores range from 0 (totally controlled asthma) to 6 (severely uncontrolled asthma), with a change in ACQ-7-IA score >0.5 indicating a minimal clinically important change (MCID) in asthma control.13
Safety endpoints included frequency of patients reporting treatment-emergent adverse events (TEAEs) and treatment-emergent serious adverse events (SAEs) during the 52-week treatment period.
Statistical Analyses
Efficacy analyses were performed in all groups of patients by number of prior exacerbations and baseline levels of type 2 biomarkers. Safety analysis was performed in the group of patients with ≥1 prior exacerbation and blood eosinophil count ≥500 cells/µL at baseline.
The adjusted annualized rate of severe exacerbations was derived using a negative binomial model, with the total number of events occurring from randomization to Week 52 after treatment start or last contact date (whichever came earlier) as the response variable and with pooled treatment groups, age, baseline weight group (≤30 kg, >30 kg), baseline eosinophil stratum (<300 cells/µL, ≥300 cells/µL), baseline FeNO level (<20 ppb, ≥20 ppb), baseline ICS dose level, region, and number of severe exacerbation events in the previous year as covariates. Log-transformed standardized observation duration was the offset variable. All severe exacerbations occurring up to Week 52 were included in the analysis, regardless of whether the patient was receiving treatment. The relative risk ratio of severe exacerbations (dupilumab vs placebo) was calculated, with P values derived using the delta method. Overall P values for interactions between subgroups were derived using a negative binomial model as above with subgroup (if different from the aforementioned covariates) and subgroup-by-treatment interactions as additional covariates. The covariates baseline eosinophil stratum (<300 cells/µL, ≥300 cells/µL), baseline FeNO level (<20 ppb, ≥20 ppb), and number of severe exacerbation events within 1 year prior to the study may have been included/excluded based on the subgroup under consideration.
The number needed to treat (NNT) to prevent 1 exacerbation was calculated as: 1/([annualized rate of severe exacerbations]placebo – [annualized rate of severe exacerbations]dupilumab).
Changes from baseline in pre-BD ppFEV1, post-BD ppFEV1, pre-BD FEV1/FVC ratio, and ACQ-7-IA score were derived using mixed-effects models with repeated measures. The models included change from baseline value to Week 52 after treatment start as the response variable, and treatment, age, baseline weight group (≤30 kg, >30 kg), region, baseline eosinophil stratum (<300 cells/µL, ≥300 cells/µL), baseline FeNO level (<20 ppb, ≥20 ppb), baseline ICS dose level, visit, treatment-by-visit interaction, baseline-by-visit interaction, and baseline value of pre-BD ppFEV1 (for pre-BD ppFEV1) or post-BD ppFEV1 (for post-BD ppFEV1) or pre-BD FEV1/FVC ratio (for pre-BD FEV1/FVC ratio) or ACQ-7-IA score (for ACQ-7-IA score) as covariates. Additional covariates in the model were ethnicity for pre-BD ppFEV1 and post-BD ppFEV1 and sex and baseline height for pre-BD FEV1/FVC ratio. Overall P values for interactions between subgroups were derived using mixed-effects models with repeated measures as above, with subgroup (if different from the aforementioned covariates), subgroup-by-treatment interactions, and subgroup-by-treatment-by-visit interactions as additional covariates. The covariates baseline eosinophil stratum (<300 cells/µL, ≥300 cells/µL) and baseline FeNO level (<20 ppb, ≥20 ppb) may have been included/excluded based on the subgroup under consideration.
In all the models mentioned above, low-dose baseline ICS was considered under the medium-dose baseline ICS level. A P value of <0.05 for the comparison between dupilumab and placebo (within each group) was considered statistically significant. All analyses were conducted using SAS software, version 9.4.
Results
Study Patients
Of the 408 patients who underwent randomization in VOYAGE, 350 patients with a type 2 inflammatory asthma phenotype at baseline (defined as blood eosinophil count ≥150 cells/µL or FeNO ≥20 ppb) and ≥1 prior exacerbations (per VOYAGE inclusion criterion) were included in this analysis: 236 were randomized to dupilumab and 114 to placebo. Of these, 132 experienced 1 prior exacerbation (per VOYAGE inclusion criterion, dupilumab: 85; placebo: 47), 107 patients experienced 2 prior exacerbations (dupilumab: 75; placebo: 32) and 111 experienced ≥3 prior exacerbations (dupilumab: 76; placebo: 35).
There were no major differences in baseline demographic or disease characteristics between treatment arms across exacerbation history groups, except that the mean concentration of total IgE tended to be lower in the placebo vs dupilumab arm in patients with 1 or 2 prior exacerbations and tended to be higher in those with ≥3 prior exacerbations in all biomarker subgroups analyzed (Tables 1–5). Lung function and asthma control parameters were comparable across exacerbation history groups.
 |
Table 2 Baseline Demographic and Clinical Characteristics of LIBERTY ASTHMA VOYAGE Patients Included in This Analysis with Blood Eosinophil Count ≥150 Cells/µL at Baseline by Exacerbation History |
 |
Table 3 Baseline Demographic and Clinical Characteristics of LIBERTY ASTHMA VOYAGE Patients Included in This Analysis with FeNO ≥20 Ppb at Baseline by Exacerbation History |
 |
Table 4 Baseline Demographic and Clinical Characteristics of LIBERTY ASTHMA VOYAGE Patients Included in This Analysis with Blood Eosinophil Count ≥ 300 Cells/µL at Baseline by Exacerbation History |
 |
Table 5 Baseline Demographic and Clinical Characteristics of LIBERTY ASTHMA VOYAGE Patients Included in This Analysis with Blood Eosinophil Count ≥ 500 Cells/µL at Baseline by Exacerbation History |
In each group defined by baseline type 2 biomarker levels and exacerbation history, baseline characteristics were generally balanced between dupilumab and placebo arms. Baseline values of lung function and asthma control parameters were comparable in all groups regardless of baseline type 2 biomarker levels or number of prior exacerbations.
Severe Exacerbations
Risk of Severe Exacerbations
In children with type 2 asthma, dupilumab vs placebo significantly reduced the adjusted annualized severe exacerbation rate in the majority of subgroups with 1, 2, or ≥3 prior exacerbations regardless of baseline type 2 biomarker levels (P < 0.05) (Figure 1), with no significant difference between subgroups (all interaction P > 0.05).
In patients with type 2 asthma, exacerbation risk was reduced by 78.9%, 62.7%, and 55.6% in patients with 1, 2, and ≥3 prior exacerbations, respectively, with corresponding NNT to decrease the AER by 1 of 2.24, 2.53, and 1.72. Overall, in groups of patients with 1 and 2 prior exacerbations, reductions in exacerbation risk ranged from 78.9% to 87.1% (1 exacerbation, NNT ranging from 1.98 to 3.75) and from 53.0% to 96.0% (2 exacerbations, NNT ranging from 0.81 to 3.37) across groups by baseline type 2 biomarker levels, with greater reductions generally detected with increasing blood eosinophil count. In the group of patients with ≥3 prior exacerbations, the reductions in exacerbation risk ranged from 54.5% to 61.1%, with NNT values ranging from 1.41 to 3.31.
Proportion of Non-Exacerbators During the Study
In the majority of all exacerbation history subgroups, dupilumab vs placebo increased the percentage of patients who did not experience any severe asthma exacerbation during the 52-week treatment period (non-exacerbators) (Figure 2).
The difference in the percentages of non-exacerbators for dupilumab vs placebo trended numerically smaller with increasing number of exacerbations in the previous year (Figure 2).
Lung Function
Pre-BD and Post-BD ppFEV1
At Week 52, dupilumab vs placebo significantly improved pre-BD ppFEV1 in patients with 1, 2, or ≥3 prior exacerbations across the majority of groups by baseline type 2 biomarker levels (P < 0.05), with no significant difference between groups (all interaction P > 0.05) (Figure 3). Numerical improvements in post-BD ppFEV1 were seen at Week 52 in all patients with 1, 2, or ≥3 prior exacerbations across all groups by baseline type 2 biomarker levels, reaching significance for patients with FeNO ≥20 ppb and 1 prior exacerbation (LS mean difference vs placebo 7.59% [95% CI 1.38, 13.79]; P = 0.0173) (Figure 4).
Pre-BD FEV1/FVC Ratio
Dupilumab vs placebo improved the pre-BD FEV1/FVC ratio at Week 52 across the majority of groups by number of prior exacerbations and baseline type 2 biomarker levels (Figure 5). The benefits were statistically significant in all groups, except in patients with blood eosinophil count ≥500 cells/µL and either 1 or 2 prior exacerbations (LS mean difference vs placebo: 3.54% [95% CI −0.63, 7.71]; P = 0.0944 and 3.64% [95% CI −2.37, 9.66]; P = 0.2260, respectively) (Figure 5).
Considering all groups by number of prior exacerbations and baseline type 2 biomarker levels, LS mean differences vs placebo in change from baseline in pre-BD FEV1/FVC ratio ranged from 2.59% to 6.42%. Values tended to be numerically greater with increasing levels of type 2 biomarkers at baseline and in those groups with ≥3 prior exacerbations (except in patients with FeNO ≥20 ppb) (Figure 5).
Asthma Control
In patients with type 2 asthma, dupilumab vs placebo produced statistically significant improvements in ACQ-7-IA score at Week 52, regardless of the number of prior exacerbations (Figure 6). Similar significant improvements in ACQ-7-IA score were also observed in patients with blood eosinophil count ≥150 cells/μL, regardless of the number of prior exacerbations. For patients with FeNO ≥20 ppb, improvements in ACQ-7-IA score were significant for groups of patients with 2 or ≥3 prior exacerbations but did not reach significance for those with 1 prior exacerbation (LS mean square difference vs placebo: −0.17 [95% CI −0.48, 0.14]; P = 0.2786) (Figure 6). Significant improvements were also seen in patients with blood eosinophil count ≥300 cells/μL, regardless of the number of prior exacerbations (except for the subgroup with only 2 prior-year exacerbations); however, for patients with blood eosinophil count ≥500 cells/μL, improvements reached significance in only the group with ≥3 exacerbations (Figure 6).
Numerically greater benefits were observed with increasing number of prior exacerbations, and significant improvements in asthma control were found in the groups of patients with ≥3 prior exacerbations, regardless of baseline type 2 biomarker levels (Figure 6).
Safety
Safety was assessed in the group of patients with ≥1 exacerbation in the prior year and blood eosinophil count ≥500 cells/µL who had received at least 1 dose of the treatment (124 patients in the dupilumab arm and 47 in the placebo arm). A total of 106 (85.5%) patients in the dupilumab arm and 35 (74.5%) patients in the placebo arm reported at least 1 TEAE. Treatment-emergent SAEs were reported in 10 (8.1%) patients in the dupilumab arm and 2 (4.3%) patients in the placebo arm. One patient included in the safety analysis (dupilumab arm) reported a TEAE leading to permanent treatment discontinuation. Dupilumab has demonstrated an acceptable safety profile.12
Discussion
In this post hoc analysis, treatment with dupilumab for 52 weeks vs placebo significantly reduced the risk of severe asthma exacerbations, regardless of whether patients had experienced 1, 2, or ≥3 exacerbations in the previous year, with mean reductions in exacerbation risk ranging from 53.0% to 96.0%. As higher numbers of prior exacerbations are associated with a greater risk of future exacerbations,14 our findings suggest that dupilumab is efficacious in reducing risk of exacerbations even among patients with the highest number of prior exacerbations analyzed, who tend to be at the greatest risk of future exacerbations. At Week 52, the majority of groups of patients achieved statistically significant reductions in ACQ-7-IA scores, ppFEV1, and pre-BD FEV1/FVC ratio with dupilumab treatment, regardless of number of prior exacerbations or baseline levels of type 2 biomarkers.
The categorization of patients into subgroups by exacerbation history and levels of type 2 biomarkers (baseline blood eosinophil count and FeNO) was based on the fact that recent asthma exacerbation history has been associated with higher risk of future exacerbations and a decline in lung function,4,15 and patients with elevated type 2 biomarkers normally present with more severe disease.16
Dupilumab’s effect in reducing exacerbations was consistent across groups with different levels of type 2 biomarkers; however, this reduction did not reach statistical significance in the group of patients with ≥3 prior exacerbations and blood eosinophil count ≥500 cells/µL (or in the group of patients with ≥2 prior-year exacerbations and blood eosinophil count ≥300 cells/µL), possibly due to the high severity of disease that would be expected in this population,4,16 or due to the low number of patients. In line with that, the group with ≥3 prior exacerbations and blood eosinophil count ≥500 cells/µL was the only group where dupilumab failed to increase the proportion of patients without exacerbations during the study, compared with placebo. In patients with 1 or 2 prior exacerbations, reductions in exacerbations tended to be greater with increasing eosinophil counts.
Dupilumab also provided significant improvement in lung function parameters (pre-BD ppFEV1 and pre-BD FEV1/FVC ratio) in the majority of patients, irrespective of number of prior exacerbations or baseline type 2 biomarkers. The main exception to this was patients with blood eosinophil count ≥500 cells/µL, in whom numerical improvements in pre-BD FEV1 reached significance in only patients with 1 prior exacerbation. Improvements in pre-BD FEV1/FVC ratio reached statistical significance in the ≥3 prior exacerbation group only – again, this could be explained by the high disease severity expected in this population or by the low number of patients in the group.4,16 Dupilumab also provided some benefits in terms of post-BD ppFEV1, especially in the population of patients with 1 prior exacerbation and with FeNO ≥20 ppb.
Significant improvements in ACQ-7-IA scores >0.5 points (MCID) were observed in patients with ≥3 prior exacerbations across baseline type 2 biomarker levels, indicating clinically important improvements in asthma control in these patient groups. Most subgroups – except for patients with 2 exacerbation events in the year prior and blood eosinophil count ≥300 cells/µL or ≥500 cells/µL, or with 1 prior exacerbation and blood eosinophil count ≥500 cells/µL or FeNO ≥20 ppb – also showed significant improvements vs placebo even though they did not reach the MCID threshold, which could also be due to the low patient numbers.
The efficacy profile of dupilumab reported here across exacerbation history and baseline type 2 biomarker populations in pediatric patients is in line with similar findings in adult patients, where dupilumab provided significant reductions in severe exacerbation rates and improvements in asthma control and lung function in patients with elevated type 2 inflammation biomarkers, irrespective of exacerbation history.17 The safety findings in this post hoc analysis of patients with ≥1 prior exacerbations in the past year and baseline blood eosinophil count ≥500 µ/L were consistent with the known dupilumab safety profile in patients aged 6 to 11 years with uncontrolled, moderate-to-severe asthma.12
This analysis is limited by its post hoc nature, since the VOYAGE study was not statistically powered to detect differences in the groups presented here. The sample size of some groups in this analysis is low, and the results are to be thus interpreted considering these circumstances. Finally, the etiology of the asthma exacerbations considered in this analysis (before and during the study treatment period) was unknown.
Conclusion
In pediatric patients with uncontrolled, moderate-to-severe asthma with a type 2 phenotype, dupilumab consistently reduced the risk of asthma exacerbations, improved lung function (pre-BD ppFEV1 and pre-BD FEV1/FVC ratio), and reduced ACQ-7-IA scores, regardless of exacerbation history.
Data Sharing Statement
Qualified researchers may request access to patient-level data and related study documents, including the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan, and dataset specifications. Patient-level data will be anonymized, and study documents will be redacted to protect the privacy of our trial participants. Further details on Sanofi’s data-sharing criteria, eligible studies, and process for requesting access can be found athttp://www.vivli.org/.
Acknowledgments
Research sponsored by Sanofi and Regeneron Pharmaceuticals Inc. ClinicalTrials.gov Identifier: NCT02948959. Medical writing/editorial assistance was provided by Natalia Crespo, PhD, of Excerpta Medica, and was funded by Sanofi and Regeneron Pharmaceuticals Inc., according to the Good Publication Practice guidelines: 2022 update.
Some data from this paper have been presented at the 2023 Annual Meeting of the American Academy of Allergy Asthma & Immunology (AAAAI; poster abstract published in “Poster Abstracts” in The Journal of Allergy and Clinical Immunology: https://doi.org/10.1016/j.jaci.2022.12.064), the 2023 Congress of the European Academy of Paediatrics (EAP), and the 22nd International Conference on Pediatric Pulmonology (CIPP 2023) as abstract presentations with interim findings.
Author Contributions
All authors made significant contributions to the work reported, whether to the study conception, design, and execution; data acquisition, analysis, and interpretation; or all of these areas. All authors contributed to drafting, revising, or critically reviewing the article; gave final approval of the version to be published; agreed on the journal to which the article has been submitted; and agreed to be accountable for all aspects of the work.
Disclosure
Guilbert TW has received personal fees and/or grants from AiCME, Amgen, AstraZeneca, Best Pharmaceuticals for Children Act (BPCA), Genentech, GSK, NIH, Novartis, OM Pharma, Polarean, Polaris, Regeneron Pharmaceuticals Inc., and Sanofi, and royalties from UpToDate. Tolcachier A has conducted clinical trials for AstraZeneca, GSK, Novartis, and Sanofi, and received fees as a speaker for Novartis, Merck, and Sanofi. Fiocchi AG has acted as an advisor for Abbott, Danone, DBV Technologies, HiPP Organic, Novartis, and Stallergenes Greer, and received research sponsorship from Danone, Ferrero, HiPP Organic, and Sanofi. Katelaris CH was Principal Investigator of the dupilumab asthma phase 2b (NCT01854047) and phase 3 (NCT02414854) studies for Regeneron Pharmaceuticals Inc. and Sanofi. Phipatanakul W has acted as a consultant for and received clinical trial support/medication support from AstraZeneca, Genentech, GSK for Asthma Therapeutics, Merck, Regeneron Pharmaceuticals Inc., and Sanofi. Begin P has acted as Principal Investigator for DBV Technologies, Novartis, Regeneron Pharmaceuticals Inc., and Sanofi, and has received speaker/consulting fees from ALK, AstraZeneca, Aralez Bio, Bausch Health, DBV Technologies, Novartis, Pfizer, Sanofi, and Valeo Pharma. de Mir I has received fees for lectures, boards, and conferences from Aldo-Unión, GSK, and Novartis. Altincatal A is a Sanofi employee and may hold stock and/or stock options in the company. Gall R is a Regeneron Pharmaceuticals Inc. employee and shareholder. Ledanois O is a Sanofi employee and may hold stock and/or stock options in the company. Radwan A is a Regeneron Pharmaceuticals Inc. employee and shareholder. Jacob-Nara JA is a Sanofi employee and may hold stock and/or stock options in the company. Deniz Y is a Regeneron Pharmaceuticals Inc. employee and shareholder. Rowe PJ is a Sanofi employee and may hold stock and/or stock options in the company. The authors report no other conflicts of interest in this work.
References
1. Zahran HS, Bailey CM, Damon SA, et al. Vital signs: asthma in children — United States, 2001–2016. MMWR Morb Mortal Wkly Rep. 2018;67:149–155. doi:10.15585/mmwr.mm6705e1
2. Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43(2):343–373 doi:10.1183/09031936.00202013.
3. Ramratnam SK, Bacharier LB, Guilbert TW. Severe asthma in children. J Allergy Clin Immunol Pract. 2017;5(4):889–898. doi:10.1016/j.jaip.2017.04.031
4. Global Initiative for Asthma. Global strategy for asthma management and prevention; 2020. Available from: https://ginasthma.org/wp-content/uploads/2020/06/GINA-2020-report_20_06_04-1-wms.pdf.
5. Krishnan V, Diette GB, Rand CS, et al. Mortality in patients hospitalized for asthma exacerbations in the United States. Am J Respir Crit Care Med. 2006;174(6):633–638. doi:10.1164/rccm.200601-007OC
6. Lane S, Molina J, Plusa T. An international observational prospective study to determine the cost of asthma exacerbations (COAX). Respir Med. 2006;100(3):434–450 doi:10.1016/j.rmed.2005.06.012
7. Maspero J, Adir Y, Al-Ahmad M, et al. Type 2 inflammation in asthma and other airway diseases. ERJ Open Res. 2022;8(3):00576–2021. doi:10.1183/23120541.00576-2021
8. Gandhi NA, Pirozzi G, Graham NMH. Commonality of the IL-4/IL-13 pathway in atopic diseases. Expert Rev Clin Immunol. 2017;13(5):425–437. doi:10.1080/1744666X.2017.1298443
9. Macdonald LE, Karow M, Stevens S, et al. Precise and in situ genetic humanization of 6 Mb of mouse immunoglobulin genes. Proc Natl Acad Sci U S A. 2014;111(14):5147–5152 doi:10.1073/pnas.1323896111
10. Murphy AJ, Macdonald LE, Stevens S, et al. Mice with megabase humanization of their immunoglobulin genes generate antibodies as efficiently as normal mice. Proc Natl Acad Sci U S A. 2014;111(14):5153–5158. doi:10.1073/pnas.1324022111
11. Gandhi NA, Bennett BL, Graham NM, Pirozzi G, Stahl N, Yancopoulos GD. Targeting key proximal drivers of type 2 inflammation in disease. Nat Rev Drug Discov. 2016;15(1):35–50. doi:10.1038/nrd4624
12. Bacharier LB, Maspero JF, Katelaris CH, et al. Dupilumab in children with uncontrolled moderate-to-severe asthma. N Engl J Med. 2021;385(24):2230–2240. doi:10.1056/NEJMoa2106567
13. Juniper EF, Svensson K, Mörk AC, Ståhl E. Measurement properties and interpretation of three shortened versions of the asthma control questionnaire. Respir Med. 2005;99:553–558. doi:10.1016/j.rmed.2004.10.008
14. Tanaka A, Uno T, Sato H, et al. Predicting future risk of exacerbations in Japanese patients with adult asthma: a prospective 1-year follow up study. Allergol Int. 2017;66(4):568–573 doi:10.1016/j.alit.2017.02.013
15. Sears MR. Lung function decline in asthma. Eur Respir J. 2007;30:411–413. doi:10.1183/09031936.00080007
16. Fahy JV. Type 2 inflammation in asthma--present in most, absent in many. Nat Rev Immunol. 2015;15(1):57–65. doi:10.1038/nri3786
17. Corren J, Katelaris CH, Castro M, et al. Effect of exacerbation history on clinical response to dupilumab in moderate-to-severe uncontrolled asthma. Eur Respir J. 2021;58(4):2004498. doi:10.1183/13993003.04498-2020
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.







