Back to Journals » Drug Design, Development and Therapy » Volume 13
Impact of different emulsifiers on biocompatibility and inflammatory potential of Perfluorohexyloctane (F6H8) emulsions for new intravenous drug delivery systems
Authors Tsagogiorgas C , Anger F, Beck G, Breedijk A, Yard B, Hoeger S
Received 26 November 2018
Accepted for publication 15 May 2019
Published 27 June 2019 Volume 2019:13 Pages 2097—2110
DOI https://doi.org/10.2147/DDDT.S195954
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Sukesh Voruganti
Charalambos Tsagogiorgas,1 Friedrich Anger,1,2 Grietje Beck,1 Annette Breedijk,3 Benito Yard,3 Simone Hoeger3
1Department of Anaesthesiology and Critical Care Medicine, University Hospital Mannheim, Faculty of Medicine, University of Heidelberg, Mannheim, Germany; 2Department of General, Visceral, Vascular and Paediatric Surgery, Julius-Maximilians-Universität, University of Wuerzburg, Wurzburg, Germany; 3Department of Internal Medicine V, University Hospital Mannheim, Faculty of Medicine, University of Heidelberg, Mannheim, Germany
Background: Emulsions on the basis of Perfluorohexyloctane (F6H8), a semifluorinated alkane (SFA), have shown to dissolve and transport highly lipophilic compounds. It is unknown how F6H8-containing emulsions (F6H8-cEM) interact with compartment blood, the reticuloendothelial system (RES), or influence injured organs in vivo. The current study was conducted to investigate the in vitro biocompatibility of F6H8-cEM and their drug delivery properties. Afterward, an in vivo study was performed as a proof-of-concept study in a rat model of acute kidney injury (AKI), which focused on the potential influence of F6H8-cEM on inflammation in an injured organ.
Methods: Two different F6H8-cEM were stabilized by the emulsifying agents Poloxamer 188 (Pluronic® F68) or lecithin (S75). The two resulting emulsions F6H8-Pluronic or F6H8-lecithin were tested in vitro for the potential modulation of acute inflammation via whole blood assay, FACS, and ELISA. Antioxidant capacity and drug delivery properties were measured with an oxidation assay. Secondly, AKI was induced in the rats, which were treated with the F6H8-lecithin emulsion. Renal function and inflammation were assessed.
Results: Both F6H8-cEM were phagocytized by monocytes and both dose-dependently affected apoptosis (Annexin V binding) in monocytes. TNF-α expression increased dose-dependency for F6H8-Pluronic emulsion but not for F6H8-lecithin in a whole blood assay. Both F6H8-cEM were able to carry α-tocopherol as a model drug. Animals with AKI treated with the F6H8-lecithin emulsion showed a significantly better renal function and less infiltration of inflammatory cells in renal tissue compared to the control, while inflammatory markers in renal tissue, except HO-1, were not affected by F6H8-lecithin.
Conclusions: Pluronic® F68 does not seem suitable as a biocompatible surfactant for F6H8-cEM. The injured kidney was not negatively influenced by the F6H8-lecithin emulsion. Lecithin-stabilized F6H8-cEM could be tested for preclinical studies as a carrier system for lipophilic agents.
Keywords: Perfluorohexyloctane, emulsion, inflammation, drug delivery, acute kidney injury
Introduction
Lipophilic drugs are often difficult to transport to their site of action. Moreover, their carriers should be inert and have no side effects. Semifluorinated alkanes (SFAs) are potentially new excipients for formulations of poorly soluble, lipophilic drugs. In recent studies, two SFAs, Perfluorohexyloctane (F6H8) and Perfluorobutylpentane (F4H5), were investigated and have demonstrated a solubilizing capacity for selected lipophilic drugs, such as ibuprofen, propofol, cyclosporine A, and tacrolimus.1–4
As close chemical relatives of perfluorocarbons (PFCs), SFAs are considered to be chemically and biologically inert.5–7 In the past, liquid PFCs as well as PFC emulsions have been studied as oxygen carriers or drug carriers in various medical fields.8–10 As a further development, Krafft and Ries were able to show that SFAs stabilize PFC emulsions as co-surfactants in blood substitutes.6,11–13 Moreover, SFAs alone were able to form stable emulsions and could also be used as oxygen carriers instead of PFCs.7,14 In a recent study conducted by our group, emulsions based on the SFA F6H8 as the dispersed phase showed to be capable for intravenous drug delivery, eg the highly lipophilic compound propofol (2,6- diisopropylphenol).4
As shown by Flaim et al, the clearance of intravenously applied PFC emulsions from the blood is accomplished by phagocytosis of emulsion particles via reticuloendothelial macrophages (RES)15 which resulted in mild biological side effects. This mechanism is characterized by dose-related stimulation of macrophages and the subsequent release of pro-inflammatory intracellular products.15
Early PFC emulsions stabilized with emulsifiers like Pluronic had more clinical side effects16 compared to emulsions stabilized with lecithin – leading to the impression that emulsifiers might be of importance for the biocompatibility of PFC emulsions.
Nonetheless, a number of studies have demonstrated that the related PFCs per se are influencing inflammation17–20 by an unknown mechanism.
The SFA F6H8 examined as an excipient in emulsions for drug delivery seems to be safe since no casualties were observed in rats, with clinical chemistry and histology analysis revealing no major differences.4 Regarding the relationship with PFCs, it is not known whether F6H8-cEM interact with RES, and may cause inflammation or negatively affect already damaged organs.
We hypothesized that F6H8-cEM might show similar effects on RES as described for PFC emulsions and could potentially influence inflammation depending on the emulsifier used. We therefore evaluated the physicochemical properties of two different F6H8-cEM, emulsified with either Pluronic or lecithin, and tested their influence on monocytes in vitro regarding apoptosis and phagocytosis, as well as the dose-dependent cytokine production in native human whole blood. In addition, we tested the ability of the F6H8-cEM as a drug delivery system with α-tocopherol in vitro.
Finally, to ensure that F6H8-cEM pose no threat in situations with already damaged organs, an in vivo study was performed as a proof-of-concept study using a rat AKI model.
Materials and methods
Materials
For the dispersed phase emulsions, F6H8 was purchased from Novaliq GmbH (Heidelberg, Germany). Glycerin, 2-[4-(2-Hydroxyethyl)-1piperazinyl]-ethanesulfonic acid (HEPES), and sodium hydroxide (NaOH) were purchased from Merck (Darmstadt, Germany). As surfactants, Poloxamer 188 (Pluronic F68 from Sigma-Aldrich, Steinheim, Germany) or lecithin (S75 from Lipoid, Ludwigshafen, Germany) were used for the in vitro studies. The lipophilic dye 1,1ʹ-dioctadecyl-3,3,3ʹ,3ʹ-tetramethylindocarbocyanine perchlorate (DiI) was purchased from Sigma-Aldrich (Steinheim, Germany). All other chemicals were purchased from Sigma-Aldrich (Steinheim, Germany), if not stated otherwise.
For the in vivo experiments, F6H8-lecithin emulsion was used to avoid Pluronic-based side-effects in the animals.
Preparation of F6H8-cEM
Pluronic or lecithin (4 wt.% in relation to the organic phase) was added to a continuous phase containing 10 mMol HEPES, 2 wt.% Glycerin, and deionized water and stirred for 10 mins to achieve a clear solution. F6H8 (equal to 40 wt.% of the final emulsion) was added under vigorous stirring to the surfactant solution. This mixture was stirred for 1 hr at 2000 rpm. The emulsion was then prepared by a high-pressure homogenization process using an Avestin EmulsiFlex® apparatus (Avestin Inc., Ottawa, Canada) at a pressure of 1100 bar in continuous process mode for 1 hr. Afterward, the pH was adjusted to 7.3–7.5 by adding NaOH. The final F6H8-cEM (F6H8-Pluronic or F6H8-lecithin) as well as the pure surfactant stock solutions (Pluronic or lecithin, 4 wt.%) were filled into vials, closed, and sealed after blanketing with nitrogen. Subsequently, the vials were sterilized at 121°C for 10 mins. For the phagocytosis experiments, F6H8 was fully saturated with the lipophilic dye DiI and displayed high solubility in F6H8 (data not shown). This solution was processed as described above and resulted in the emulsions DiI-F6H8-Pluronic or DiI-F6H8-lecithin. For the drug delivery experiments, F6H8 was fully saturated with α-tocopherol (solubility 31 mg/mL; data not shown). This solution was processed as described above and resulted in the emulsions F6H8-Pluronic+α-toc and F6H8-lecithin+α-toc. The formulation optimization was carried out on the basis of data from preliminary studies but also from already published work of our group.4,14
Physicochemical properties of the different F6H8-cEM
Droplet size measurement
The average droplet size of F6H8-Pluronic or F6H8-lecithin was measured by dynamic light scattering (DLS) using a PSS Nicomp 380 (Santa Barbara, California, USA) after sterilization on the day of production (t=0d) and on day 84 (t=84d) after storage at room temperature (21°C) in a dark and dry place. The F6H8 emulsions were used within 60 days from the initial preparation date.
Osmolarity and pH
A pH meter (Eutech instrument, Landsmeer, Netherlands) was used to measure the pH of F6H8-cEM. Osmolarity of both F6H8-cEM was determined using the freezing-point method via a micro-osmometer autocal type 13 (Roebling, Berlin, Germany). For this, 100 μL of each native nanoemulsion were filled into a microtube and measurements were performed, as described previously.21
Biocompatibility of different F6H8-cEM
Isolation of peripheral blood mononuclear cells
Purified peripheral blood mononuclear cells (PBMC) were isolated from heparinized human blood samples as described previously.22 Briefly, whole blood was diluted in phosphate-buffered saline (PBS) and centrifuged with Ficoll Paque (GE Healthcare Europe GmbH, Germany).22 The mononuclear layer (interphase) was then transferred and renewed by repeated centrifugations in PBS buffer. Lastly, the cells were re-suspended and cultured in nutrient medium.
FACS analysis
FACS analysis was used to determine the influence of F6H8-cEM on apoptosis (Annexin V).
For this analysis, PBMC were incubated in PBS with either F6H8-Pluronic, F6H8-lecithin, or the pure surfactant stock solutions (Pluronic or lecithin alone) at various concentrations. The original F6H8-emulsions (containing 40 wt.% F6H8, as shown in Table 1) were diluted 1:10, 1:100, 1:1,000, and 1:10,000 in order to yield the organic phase end concentrations used in the experiments of 4%, 0.4%, 0.04%, and 0.004%, respectively. Therefore, the volume to be pipetted for the final concentration in the PBMC cell culture was first calculated and added to the cell cultures. Since the concentrations of the pure surfactants Pluronic and lecithin in the emulsions are 10 times lower (4 wt.%), the surfactant stock solutions were also diluted 1:10, 1:100, 1:1,000, 1:10,000. To increase legibility, dilutions are shown as 4%, 0.4%, 0.04%, and 0.004% in all figures and tables.
 |
Table 1 Composition of the emulsions (wt.% = weight percent). F6H8: Perfluorohexyloctane |
After 24 hrs of incubation, the monocytes were transferred into FACS analysis tubes.
FACS analysis was then performed with 2×106 cells using the FITC-conjugated monoclonal antibody anti-human Annexin-V (R&D, Minneapolis, MN). The antibody was added for 30 mins at 4ºC, followed by extensive washing with PBS. Flow cytometry was performed on a FACScalibur equipped with the CELLQuest software (Becton Dickinson, Heidelberg, Germany). The data were analyzed by Windows Multiple Document Interface (WinMDI) software (Version 2.8).
Phagocytosis of F6H8-cEM PBMC (106 cells/mL) were isolated from human blood as described above, transferred to cell culture dishes, and then incubated with DiI-F6H8-Pluronic or DiI-F6H8-lecithin (4%, w/v) for 24 hrs. DiI reaches maximum absorption and emission at 549 nm (green) and 565 nm (yellow-red), respectively.
Thereafter, cytospins were prepared and the cells were analyzed by fluorescence microscopy.
Briefly, mononuclear cells were removed from the dishes, washed in PBS, centrifuged at 1,500 rpm, and re-suspended in PBS. 8 μL of the recovered cell suspension was then diluted (1: 1,000), followed by electronic cell counting (Casy1, Schärfe System, Reutlingen, Germany). The dilution of the monocyte suspensions was carried out for a final cell number of 2×105/mL and the preparations displaying the best cell dispersion were used. Cell nuclei were stained with the fluorescent dye DAPI (4ʹ,6-diamidin-2-phenylindole; maximum absorption and emission of 358 nm (ultraviolet) and 461 nm (blue), respectively). The images were generated and processed under the fluorescence microscope (Olympus BX51, Olympus, Hamburg, Germany) using the analySIS Image Processing Software (Soft Imaging System, Olympus, Hamburg, Germany).
Inflammatory response
Influence of F6H8-cEM on TNF-α production in whole blood assay
Freshly drawn blood from 5 different healthy donors was diluted (1:4) in Dulbecco’s Modified Eagle Medium (DMEM) to test the impact of inflammatory response; the non-stimulated whole blood served as control.
Equal volumes of DMEM medium, F6H8-Pluronic or F6H8-lecithin emulsions in different concentrations (F6H8: 4, 0.4, or 0.04 wt.%, respectively) or the pure surfactant stock solutions (Pluronic or lecithin 0.4, 0.04, or 0.004 wt.%, respectively) were added to the whole blood assay.
After 24 hrs of stimulation, the supernatants were harvested and assessed for TNF-α production by ELISA (R&D, Wiesbaden, Germany). Each experimental condition was performed in triplicate and each experiment was confirmed at least 5 times using different blood donors.
Antioxidant capacity and drug delivery properties of F6H8-cEM (in vitro)
To test the antioxidant capacity of both F6H8-Pluronic and F6H8-lecithin emulsions alone and with addition of the antioxidant α-tocopherol, the luminol oxidation assay was used. To this end, serial dilutions of either F6H8-Pluronic, F6H8-lecithin, F6H8-Pluronic+α-toc, or F6H8-lecithin+α-toc (1%, w/v; concentration of α-tocopherol: 775 µg/mL) were added to 200 µL of reaction mixture containing 0.005 U of horseradish peroxidase (HRP) and 20 µM of luminol. The reaction was immediately recorded in a luminometer (Lumat LB9507, Berthold) after addition of 0.01% of hydroxide peroxide (H2O2). The influence of F6H8-cEM on luminol oxidation mediated by N-Formylmethionyl-leucyl-phenylalanine (fMLP) or phorbol myristate acetate (PMA) activated neutrophils was also assessed. Freshly drawn blood was diluted 1:1 with PBS and layered with Ficoll-paqueTM PLUS (GE Healthcare Bio-Sciences AB, Uppsala, Sweden). Neutrophils were isolated from the sediment by lysis of erythrocytes using ammonium chloride (15 mM). Hereafter, neutrophils were thoroughly washed with PBS and dissolved in RPMI1640 containing 20 µM of luminol and either F6H8-Pluronic, F6H8-lecithin, F6H8-Pluronic+α-toc, or F6H8-lecithin+α-toc (1%, w/v; concentration of α-tocopherol: 775 µg/m:). The cells were adjusted to a concentration of 5.106 cells/mL and incubated at 37°C. Neutrophil activation was started by addition of fMLP (10 µM) or PMA (1 µg/mL). Chemoluminesence was measured every 2 mins in the luminometer.
Animals
Inbred male Lewis rats (n=6 per group) weighing 200–250 g were obtained from Charles River (Sulzfeld, Germany). All procedures were performed according to the Guide for the Care and Use of Laboratory Animals published by the National Academy of Sciences and were approved by the local authorities (Regional Council Karlsruhe, Germany (reference number 35-9185.81/G -64/09)).
Acute kidney injury animal model
Animals were anesthetized with xylazine (Rompun 2%®, Bayer Vital GmbH, Leverkusen, Germany) and ketamine (Ketamin 10%®, Intervet GmbH, Unterschleißheim, Germany), heparinized (100 IE, Heparin-Natrium ratiopharm®, Ratiopharm GmbH, Ulm, Germany) and subsequently injected intravenously with the F6H8-lecithin emulsion or NaCl (NaCl 0.9%, Fresenius Kabi GmbH, Bad Homburg, Germany) directly before the renal artery was clamped for 45 mins (0.5 mL). The contra-lateral kidney was nephrectomized. For animal welfare reasons (reduction principle) and as the lecithin-stabilized emulsion showed better results in vitro, especially no major inflammatory properties, the decision was made to test the F6H8-lecithin emulsion only. Renal function was assessed in all animals by serum creatinine measurements on days 0, 1, 3, and 5 with an enzymatic method using a Hitachi 917 Autoanalyzer (Roche, Mannheim, Germany).
Immunohistochemistry (AKI)
Kidneys were investigated 5 days after induction of AKI. Paraffin embedding of renal tissue was performed using routine procedures. For immunohistochemical analysis, the sections were incubated with ED-1 or MHCII+ monoclonal antibody for detection of monocytes and macrophages (Linaris, Wertheim, Germany). Sections incubated with murine or rabbit IgG were used as negative controls. Standard avidin-biotin complex staining was performed according to the manufacturer’s instructions (Vector, Burlingame, CA, USA). ED-1 positive cells were counted by two blinded investigators, and 20 microscopic fields per kidney (n=6 per group) were evaluated.
Quantitative PCR (AKI)
Snap-frozen renal tissue samples harvested 5 days after induction of AKI were homogenized. A total of 1 µg of RNA was reverse transcribed into cDNA. Quantitative PCR was performed on a real-time PCR platform (AB 7900HT) using TaqMan® probes for VCAM-1 (Rn00563627_m1), E-selectin (Rn00594072_m1), TNFα (Rn99999017_m1), IL10 (Rn00563409_m1), and HO1 (Rn01536932_m1) (Applied Biosystems, Germany). Amplification was performed as proposed by the manufacturer. All samples were normalized for an equal expression of β-Actin.
Statistical analysis
Data are presented as mean ± SD for the indicated number of separate experiments. All in vitro analyses were based on more than three separate experiments. Differences between groups were determined by Student’s T-test. For the in vivo data (renal function and PCR-analysis) statistical analysis was performed using two-way ANOVA and the Kruskal–Wallis test with an option for multiple comparisons. A P-value of less than 0.05 was considered statistically significant.
Results
Physicochemical properties of F6H8-cEM
Composition of the emulsions is shown in Table 1. Except for the different surfactants used (Pluronic or lecithin), there were no differences in concentration of the organic phase (F6H8), the surfactants, or in the aqueous phase.
Droplet size and osmolarity
Mean droplet size of F6H8-Pluronic and F6H8-lecithin emulsions was measured on the day of preparation (t=0 d; 157±4 and 136±5, respectively) and after 84 days (t=84 d; 225±7 and 142±8, respectively), as shown in Table 2.
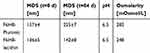 |
Table 2 Physicochemical properties of F6H8 emulsions (MDS – mean droplet size (mean± SD); pH; osmolarity) |
F6H8-Pluronic showed a tendency to higher mean droplet sizes after the observation period of 84 days (p<0.001; 157±4 vs 225±7), whereas F6H8-lecithin mean droplet size did not change. Osmolarity revealed no physiological differences (F6H8-Pluronic vs F6H8-lecithin: 282 vs 248 mOsmol/L, respectively).
These data indicate that the formulations were physically stable for nearly three months (United States Pharmacopeia Chapter 729). The emulsion did not have a tendency to coalescence nor to creaming or cracking in this period of time.
Influence of F6H8-cEM on apoptosis
The FACS-based Annexin V binding assay of early apoptosis was applied to measure cell-mediated cytotoxicity. As shown in Figure 1 (upper panels), Annexin V expression at the highest dose (4%) led to increased levels of apoptosis (visible by the right shift on the x-axis) for all emulsions, with no gross differences seen between the F6H8-cEM. In the experiments with only the surfactant solutions of Pluronic or lecithin (Figure 1, lower panels), the different concentrations showed no differences between Pluronic and lecithin regarding the Annexin V binding, although for the Pluronic solution at a lower concentration fewer events were detectable. Summarizing the data above, no notable impact on apoptosis, except a trend in high doses, could be observed for the F6H8-cEM. Similarly, no impact on apoptosis was seen for the surfactant solutions alone.
Phagocytosis
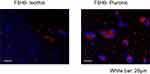
After incubating PBMC with DiI-F6H8-Pluronic or DiI-F6H8-lecithin, cellular uptake was analyzed by fluorescence microscopy. As seen in Figure 2, F6H8-Pluronic emulsion (right panel) seems to be more phagocytized in comparison to the F6H8-lecithin emulsion (left panel).
Inflammatory response
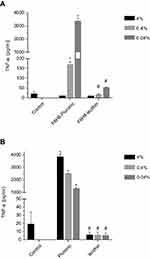
After incubating healthy human blood with the different F6H8-cEM at varying concentrations or with just the plain surfactants, we assessed the TNF-α expression via ELISA. Interestingly, the highest concentrations of TFN-α were seen in the lowest concentration of F6H8-Pluronic (0.04%; TNF-α: 3335±277.9 pg/mL; p<0.001), while the highest concentration (4%) showed no remarkable differences between the two F6H8-cEM (Figure 3A; 8.67±2.5 vs 8.34±2.51; F6H8-Pluronic vs F6H8-lecithin, respectively).
The F6H8-lecithin emulsion exhibited no difference to control in TNF-α expression, at all concentrations.
Regarding surfactants only, Pluronic seems to stimulate TNF-α expression in healthy human whole blood in comparison to control at all concentrations (4%, 0.4%, 0.04%; p<0.001, respectively), while lecithin alone does not (Figure 3B).
Antioxidative capacity and drug delivery properties of F6H8-cEM
To test if antioxidative properties were the underlying cause of the inhibitory effect of F6H8-cEM, we next assessed if the different emulsions were able to inhibit horseradish peroxidase-mediated luminol oxidation. Additionally, the drug delivery capacity of F6H8-cEM was tested by adding α-tocopherol, as described above. While the F6H8-cEM alone did not influence luminol-mediated chemoluminescence, this was dose-dependently inhibited by α-tocopherol containing F6H8 emulsions (Figure 4A). There was no apparent difference between the surfactants, ie, Pluronic or lecithin, in this regard. In addition, only emulsions containing α-tocopherol were able to diminish ROS generated by PMA or fMLP-activated neutrophils (Figure 4B).
Animal model
Impact on renal function after induction of AKI
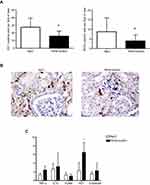
We first investigated if treatment with the F6H8-lecithin emulsion influences renal function after ischemia-induced AKI in vivo. By assessing s-creatinine levels, we could determine that the F6H8-lecithin emulsion significantly improved renal function on days 1, 3, and 5 after the onset of AKI (F6H8-lecithin vs NaCl, P<0.01; Figure 5).
Inflammatory response after AKI
Immune histology, which was performed five days after the onset of AKI, revealed a significant reduction of infiltrated ED-1 (p<0.01) and MHCII (p<0.01) positive monocytes/macrophages in the rats treated with F6H8-lecithin (Figure 6A–B). However, expression of the adhesion molecules VCAM-1 and E-selectin was not affected by F6H8-lecithin treatment, as demonstrated by qPCR analysis. Similarly, no significant difference was seen between the F6H8-lecithin emulsion compared to NaCl treatment with regard to the expression of TNFα and IL10. Moreover, compared to native renal tissue, the investigated inflammatory markers had no significant elevation in either the F6H8-lecithin or the NaCl group (Figure 6C). However, HO1 expression revealed a significant increase in the F6H8-lecithin group (F6H8-lecithin vs NaCl; *p<0.05; Figure 6C).
Discussion
In the present study, we examined two different F6H8-cEM, stabilized by emulsifying agents with either block copolymer Poloxamer 188 (Pluronic F68) or lecithin (S75), their impact on the mononuclear phagocytic system (MPS) (also known as the reticuloendothelial system (RES)), and their inflammatory potential in human blood. Moreover, we showed that F6H8-cEM are capable of delivering α-tocopherol as a highly lipophilic drug in vitro. We further examined the influence of a F6H8-lecithin emulsion on organ injury in a rat model of acute kidney injury (AKI).
In brief, we found that the biocompatibility of F6H8-cEM in vitro is negatively influenced by the emulsifier Pluronic. Therefore, lecithin as an emulsifier for F6H8-cEM was used in the animal experiments. The F6H8-lecithin emulsion showed no additional harmful effects on the damaged kidney in vivo. Therefore, it seems to be more suitable for a new drug delivery system, even in diseased organisms. Interestingly, the F6H8-lecithin emulsion improved renal function compared to the control (NaCl).
Physicochemical properties revealed no gross differences between the two emulsions; neither for mean droplet size, which were initially both beneath 200 nm, nor for osmolarity. The emulsion did not tend to coalescence or to creaming or cracking in this period of time.
Droplet size was stable for 84 days – although F6H8-Pluronic showed a tendency for higher droplet sizes after this time. This might point to both coalescence of the F6H8-Pluronic emulsion droplets and a potentially lower stability.
Human plasma has an osmolarity of about 300 mOsm/l; therefore, solutions with a greater (or lower) osmolarity than 300 mOsm/l are hypertonic (or hypotonic). The lower tolerability limit for osmolarity with no risk of hemolysis during intravenous administration in healthy people is around 200–220 mOsm/l. For peripheral intravenous administration, the recommended upper limit should be generally controlled under 1000 mOsm/l for small volume injections (<100 mL) and 500 mOsm/l for large volume injections (>100 mL).23 Both F6H8-cEM fall within this range. Therefore, it seems unlikely that differences seen between the two emulsions could be attributed to osmolarity.
Particle size plays a key role in the final biodistribution and blood clearance of particles; the exact reason for size dependencies has still not yet been fully elucidated, however.24 RES uptake has been reported to be size-dependent, while clearance increases with increasing particle size.25 Interestingly, the same authors postulate that low RES uptake and lower RES impairment are expected for lecithin emulsions,25 which is in line with our findings showing fewer effects for the F6H8-lecithin emulsion in comparison to the F6H8-Pluronic emulsion.
As stated above, phagocytosis by the RES is a normal host-defense mechanism that leads to characteristic, predictable, and reversible biological effects. This mechanism is characterized by dose-related stimulation of macrophages and subsequent release of intracellular products, which can result in significant cytotoxicity and can range from fibrosis to necrosis. Unreactive particles, including PFC emulsion droplets, result in temporary stimulation and a mild, reversible response such as flushing, lower back pain, or flu-like symptoms.15
In the past, intravenous injections of Pluronic have shown to stimulate the phagocytic activity of liver and spleen macrophages in relation to clearance and phagocytosis of particles from the blood.26,27 Phagocytic activation of human neutrophils by Pluronic has also been reported.28 Moreover, activated monocytes and macrophages release cytokines such as IL-1, IL-6, TNF-α, and INF-α/β.29
This is in line with our findings that F6H8-Pluronic, in comparison to F6H8-lecithin, shows increased phagocytosis, dose-dependent apoptosis, and TNF-α expression.
Dinkelmann et al compared emulsions of the PFC Perfluorodecalin (PFD) emulsified with either Pluronic or lecithin.30 However, this study revealed contrasting findings. Pluronic-based PFD emulsion showed no significant increase in toxicity compared to PFD alone.30 The influence of the PFD-Pluronic emulsion on cell proliferation was low, whereas the PFD-lecithin emulsion showed considerable toxicity for phagocytic cells, especially monocytes. The cell proliferation of endothelial and monocytic cells was also nearly abolished.30 The authors postulated that the potential toxicity cannot be attributed to PFD but rather to the emulsifier or the interactive effects of the entire PFD emulsion. Interestingly, Edwards et al studied a PFD-based emulsion with lecithin which showed a dose-dependent decrease in PMA-induced neutrophil chemoluminescence. In contrast to the Dinkelmann experiments, this effect could not be attributed to lecithin.31 It must be stated that there is a major difference between Perfluorodecalin compared to SFAs concerning hydro- and lipophobicity.32 SFAs, such as F6H8 or F4H5, have been revealed to be inert and biocompatible.7,13 Moreover, in comparison to PFCs, SFAs are more lipophilic and have the ability to dissolve selected hydrophobic drugs.1,4,33 This might explain the difference in biocompatibility of related emulsions, but this should be elucidated in further studies.
Annexin V as marker of apoptosis is used as a non-quantitative probe to detect cells that have expressed phosphatidylserine (PS) on the cell surface, an event found in apoptosis as well as other forms of cell death.34–36 In our study, discrete apoptosis was detected in the high concentrations of F6H8-Pluronic or F6H8-lecithin (4%). Assuming that the blood volume of a healthy adult is 5,000 mL, the intravenous injection volume would be 200 mL to reach a dilution of 4% F6H8-lecithin emulsion in the circulatory system (regardless of elimination kinetics). Nonetheless, in vivo toxicity studies should be performed in the future to elucidate potential side effects regarding the injection volume.
In our study, we used an established in vitro model using isolated human monocytes24,37,38 to investigate inflammation. TNF-α expression was shown to be dose-dependently higher for F6H8-Pluronic, while no difference was observed for F6H8-lecithin to control. The surfactant Pluronic alone was found to dose-dependently enhance the cytokine release. These results support the hypothesis that Pluronic was responsible for the immunogenic effects. At lower concentrations of F6H8-Pluronic, the TNF-α expression was higher than expected. Pluronic applied to cells is rapidly internalized, achieving access to intracellular structures, including alteration of cell signaling, activation of transcription, and other processes.39 The interaction of surfactants with biological systems can be associated with their ability to penetrate into the biological membranes, resulting in toxic effects and limiting their usage in pharmaceuticals,40 and might be a possible explanation for our findings. Future developments could therefore include surfactant-free emulsions to reduce severe side effects and cytotoxicity.40
Interestingly, and unexpectedly, lower TNF-α concentration was seen with the higher F6H8-Pluronic emulsion concentration. Many observations in the past suggest that part of the mechanism for the anti-inflammatory effects of PFCs is that they serve as a physical barrier.41 The anti-inflammatory effects of PFCs, however, cannot be solely explained by a barrier mechanism. In the past, it was shown that exposure of alveolar macrophages to the PFC perfluorooctylbromide (PFOB) in vitro decreased the responsiveness of macrophages to potent stimuli (like LPS or TNF-α).42 In addition, PFOB occurred in the vacuoles of macrophages, suggesting that intracellular processes could be altered by PFCs.42 The process of pinocytosis involves packaging of the ingested material in cell membrane-derived phagocytic vacuoles and may lead to an inability to perform other cell-membrane-dependent functions.42 Since our study found that phagocytosis of the F6H8-Pluronic emulsion was higher, it could potentially disturb intracellular processes which also can lead to decreased responsiveness to TNF-α. However, this explanation remains speculative and must be clarified in further studies.
Since the F6H8-lecithin emulsion showed more promising results in vitro, specifically due to its lower inflammatory effects, and taking animal welfare into consideration, the decision was made to test F6H8-lecithin in vivo only.
F6H8-lecithin was examined in vivo using a rat model of AKI to detect any additional effects of F6H8-cEM in this injurious condition. During AKI, F6H8-lecithin-treated animals showed a faster recovery in renal function and a decreased infiltration of monocytes in the injured renal tissue. At first glance, it was surprising that F6H8-lecithin led to an improvement in creatinine levels compared to NaCl. Like PFCs, SFAs have the capability to dissolve gases such as oxygen or carbon dioxide.7,10 Since the AKI model is an ischemia/reperfusion model, it is possible that F6H8-lecithin increased oxygen delivery.7 In an animal study of ischemia/reperfusion of the brain, Seiffge et al demonstrated that the administration of a F6H8-cEM in conjunction with normobaric hyperoxygenation increased the oxygen content in the plasma compartment, thus improving oxygen supply to the endangered tissue.14 This might help explain our findings concerning faster recovery of serum creatinine levels in this AKI model, although this must be elucidated in further studies.
An influence of F6H8-lecithin on gene expression of the immune response could not be detected; only HO-1 expression was significantly up-regulated in animals treated with F6H8-lecithin. The cytoprotective properties of HO-1 were first recognized in a model of heme protein-induced AKI and were subsequently shown to be a protectant in AKI-induced ischemia-reperfusion.43,44 The major actions of HO-1 include the degradation of heme, procurement of cytoprotectants, and engagement of iron handling proteins. These processes lead to vasorelaxant, antioxidant, anti-inflammatory, and anti-apoptotic effects that could be protective in AKI.45,46 Accordingly, in the past, it was shown that PFC administration in inflammatory conditions might lead to overexpression of HO-1 in lungs47 and kidney tissue after storage in an oxygenated PFC-University of Wisconsin (UW) solution for transplantation.48
Inflammation seems to play a critical role in the pathophysiology of AKI.49,50 Renal inflammation is associated with endothelial cell activation, vascular disintegration, and increased vascular permeability. This, in turn, facilitates leucocyte recruitment in the renal parenchyma through endothelial expression of adhesion molecules and chemokines.49–52 Due to the fact a number of studies have already shown that PFCs have anti-inflammatory properties,17–20 AKI seems to be a good model to test F6H8-lecithin emulsions in an inflammatory environment.
Indeed, we found significantly fewer infiltrated monocytes in the injured renal tissue and, very likely as a result of reduced injury, a significantly better recovery of renal function after F6H8-lecithin treatment. It is not surprising that no elevated TNF-α levels could be found in our AKI data, as Fontana et al demonstrated in a recent study that cytokines such as TNF-α returned to physiological levels within 96 hrs after AKI induction in rats.53 Similar results were seen for VCAM-1, IL10, and E-selectin expression. Administration of F6H8-lecithin caused no additional inflammation in this AKI model.
SFAs are not only oxygen carriers, but in comparison to PFCs, are also potential excipients for the development of new drug delivery systems. These could be emulsions, buccal drug delivery formulations, or topical eye drops.6,33,54 For example, SFA as an eye drop drug delivery system are the first on market without preservatives – which in this case is advantageous.54 Moreover, there might be situations where both are needed – oxygen and drug delivery – but this has to be elucidated in other studies.
Based on our data, it seems that the surfactants used for F6H8-cEM influence phagocytosis and immunogenic modulation; this is in line with findings evaluated for PFC.15 Moreover, in vivo data revealed both reduced infiltration of inflammatory cells and better renal function, indicating that the F6H8-lecithin emulsion did not additionally harm the diseased kidney and might be used as a drug delivery system for highly hydrophobic drugs for the treatment of AKI or other inflammatory events.
Conclusion
Pluronic seems to not be suitable as a bio-compatible surfactant for F6H8-cEM. This study provides a scientific rationale for extended preclinical studies testing the use of lecithin-stabilized F6H8 emulsions as a carrier system for lipophilic agents during inflammatory processes without relevantly disturbing normal immune responses.
Ethics approval
The healthy blood donors consisted of 5 adults (3 females and 2 males) recruited from our laboratory who voluntarily decided to participate. Healthy blood donors had no history of diabetes, cardiovascular, or kidney disease. All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1984 Helsinki Declaration and its later amendments or comparable ethical standards.
Acknowledgments
The authors want to thank Sophie Gärtner for her excellent technical support.
This study was partially supported by a grant from Novaliq GmbH, Heidelberg, Germany.
Disclosure
Dr Tsagogiorgas and Dr Hoeger report grants and nonfinancial support from Novaliq GmbH, during the conduct of the study. The authors report no other conflicts of interest in this work.
References
1. Agarwal P, Scherer D, Gunther B, Rupenthal ID. Semifluorinated alkane based systems for enhanced corneal penetration of poorly soluble drugs. Int J Pharm. 2018;538(1–2):119–129. doi:10.1016/j.ijpharm.2018.01.019
2. De Majumdar S, Subinya M, Korward J, Pettigrew A, Scherer D, Xu H. A low concentration of tacrolimus/semifluorinated alkane (SFA) eyedrop suppresses intraocular inflammation in experimental models of uveitis. Curr Mol Med. 2017;17(3):211–220.
3. Tsagogiorgas C, Jung T, Krebs J, et al. Aerosolized semifluorinated alkanes as excipients are suitable for inhalative drug delivery – a pilot study. Int J Pharm. 2012;422(1–2):194–201.
4. Tsagogiorgas C, Theisinger S, Heesch E, et al. Evaluation of pharmacokinetic properties and anaesthetic effects of propofol in a new perfluorohexyloctane (F6H8) emulsion in rats – a comparative study. Int J Pharm. 2015;486(1–2):69–76.
5. Krafft MP, Riess JG. Highly fluorinated amphiphiles and colloidal systems, and their applications in the biomedical field. A contribution. Biochimie. 1998;80(5–6):489–514.
6. Krafft MP, Riess JG. Chemistry, physical chemistry, and uses of molecular fluorocarbon–hydrocarbon diblocks, triblocks, and related compounds – unique “apolar” components for self-assembled colloid and interface engineering. Chem Rev. 2009;109(5):1714–1792.
7. Meinert H, Knoblich A. The use of semifluorinated alkanes in blood-substitutes. Biomater Artif Cells Immobilization Biotechnol. 1993;21(5):583–595.
8. Lehmler HJ. Perfluorocarbon compounds as vehicles for pulmonary drug delivery. Expert Opin Drug Deliv. 2007;4(3):247–262.
9. Li X, Sui Z, Li X, et al. Perfluorooctylbromide nanoparticles for ultrasound imaging and drug delivery. Int J Nanomedicine. 2018;13:3053–3067.
10. Riess JG. Perfluorocarbon-based oxygen delivery. Artif Cells Blood Substit Immobil Biotechnol. 2006;34(6):567–580. doi:10.1080/10731190600973824
11. Krafft MP, Chittofrati A, Riess JG. Emulsions and microemulsions with a fluorocarbon phase. Curr Opin Colloid Interface Sci. 2003;8(3):251–258. doi:10.1016/S1359-0294(03)00045-1
12. Riess JG. Oxygen carriers (“blood substitutes”) – raison d’etre, chemistry, and some physiology. Chem Rev. 2001;101(9):2797–2920.
13. Meinert H, Roy T. Semifluorinated alkanes – a new class of compounds with outstanding properties for use in ophthalmology. Eur J Ophthalmol. 2000;10(3):189–197. doi:10.1177/112067210001000301
14. Seiffge DJ, Lapina NE, Tsagogiorgas C, Theisinger B, Henning RH, Schilling L. Improvement of oxygen supply by an artificial carrier in combination with normobaric oxygenation decreases the volume of tissue hypoxia and tissue damage from transient focal cerebral ischemia. Exp Neurol. 2012;237(1):18–25. doi:10.1016/j.expneurol.2012.06.007
15. Flaim SF. Pharmacokinetics and side effects of perfluorocarbon-based blood substitutes. Artif Cells Blood Substit Immobil Biotechnol. 1994;22(4):1043–1054. doi:10.3109/10731199409138801
16. Ingram DA, Forman MB, Murray JJ. Activation of complement by Fluosol attributable to the pluronic detergent micelle structure. J Cardiovasc Pharmacol. 1993;22(3):456–461.
17. Schoof E, von der Hardt K, Kandler MA, et al. Aerosolized perfluorocarbon reduces adhesion molecule gene expression and neutrophil sequestration in acute respiratory distress. Eur J Pharmacol. 2002;457(2–3):195–200.
18. Gale SC, Gorman GD, Copeland JG, McDonagh PF. Perflubron emulsion prevents PMN activation and improves myocardial functional recovery after cold ischemia and reperfusion. J Surg Res. 2007;138(1):135–140.
19. Haeberle HA, Nesti F, Dieterich HJ, Gatalica Z, Garofalo RP. Perflubron reduces lung inflammation in respiratory syncytial virus infection by inhibiting chemokine expression and nuclear factor-kappa B activation. Am J Respir Crit Care Med. 2002;165(10):1433–1438.
20. Burkhardt W, Koehne P, Wissel H, et al. Intratracheal perfluorocarbons diminish LPS-induced increase in systemic TNF-alpha. Am J Physiol Lung Cell Mol Physiol. 2008;294(6):L1043–L1048.
21. Gue E, Since M, Ropars S, Herbinet R, Le Pluart L, Malzert-Freon A. Evaluation of the versatile character of a nanoemulsion formulation. Int J Pharm. 2016;498(1–2):49–65.
22. Fuss IJ, Kanof ME, Smith PD, Zola H. Isolation of whole mononuclear cells from peripheral blood and cord blood. Curr Protoc Immunol. 2009;Chapter 7:Unit7.1.
23. Wang W. Tolerability of hypertonic injectables. Int J Pharm. 2015;490(1–2):308–315.
24. Owens DE
25. Carrstensen H, Muller RH, Muller BW. Particle size, surface hydrophobicity and interaction with serum of parenteral fat emulsions and model drug carriers as parameters related to RES uptake. Clin Nutr. 1992;11(5):289–297.
26. Moghimi SM, Murray JC. Poloxamer-188 revisited: a potentially valuable immune modulator. J Natl Cancer Inst. 1996;88(11):766–768.
27. Moghimi SM, Hedeman H, Christy NM, Illum L, Davis SS. Enhanced hepatic clearance of intravenously administered sterically stabilized microspheres in zymosan-stimulated rats. J Leukoc Biol. 1993;54(6):513–517.
28. Ingram DA, Forman MB, Murray JJ. Phagocytic activation of human neutrophils by the detergent component of fluosol. Am J Pathol. 1992;140(5):1081–1087.
29. Dale DC, Boxer L, Liles WC. The phagocytes: neutrophils and monocytes. Blood. 2008;112(4):935–945.
30. Dinkelmann S, Rohlke W, Meinert H, Northoff H. A system establishment compatibility profiles for artificial oxygen carriers and other substances. Artif Cells Blood Substit Immobil Biotechnol. 2001;29(1):57–70.
31. Edwards CM, Lowe KC, Rohlke W, Geister U, Reuter P, Meinert H. Effects of a novel perfluorocarbon emulsion on neutrophil chemiluminescence in human whole blood in vitro. Artif Cells Blood Substit Immobil Biotechnol. 1997;25(3):255–260.
32. Riess JG. Understanding the fundamentals of perfluorocarbons and perfluorocarbon emulsions relevant to in vivo oxygen delivery. Artif Cells Blood Substit Immobil Biotechnol. 2005;33(1):47–63.
33. Tsagogiorgas C, Theisinger S, Holm P, Thiel M, Quintel M, Holm R. Buccal absorption of propofol when dosed in 1-perfluorobutylpentane to anaesthetised and conscious Wistar rats and Gottingen mini-pigs. Eur J Pharm Biopharm. 2013;85(3Pt B):1310–1316.
34. Koopman G, Reutelingsperger CP, Kuijten GA, Keehnen RM, Pals ST, van Oers MH. Annexin V for flow cytometric detection of phosphatidylserine expression on B cells undergoing apoptosis. Blood. 1994;84(5):1415–1420.
35. Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immunol Methods. 1995;184(1):39–51.
36. Crowley LC, Marfell BJ, Scott AP, Waterhouse NJ. Quantitation of apoptosis and necrosis by Annexin V binding, propidium iodide uptake, and flow cytometry. Cold Spring Harb Protoc. 2016;2016:11.
37. De Jaeghere F, Allemann E, Feijen J, Kissel T, Doelker E, Gurny R. Cellular uptake of PEO surface-modified nanoparticles: evaluation of nanoparticles made of PLA:PEO diblock and triblock copolymers. J Drug Target. 2000;8(3):143–153.
38. Jaulin N, Appel M, Passirani C, Barratt G, Labarre D. Reduction of the uptake by a macrophagic cell line of nanoparticles bearing heparin or dextran covalently bound to poly(methyl methacrylate). J Drug Target. 2000;8(3):165–172.
39. Sahay G, Batrakova EV, Kabanov AV. Different internalization pathways of polymeric micelles and unimers and their effects on vesicular transport. Bioconjug Chem. 2008;19(10):2023–2029.
40. Setti C, Suarato G, Perotto G, Athanassiou A, Bayer IS. Investigation of in vitro hydrophilic and hydrophobic dual drug release from polymeric films produced by sodium alginate-MaterBi(R) drying emulsions. Eur J Pharm Biopharm. 2018;130:71–82.
41. Heard SO, Puyana JC. The anti-inflammatory effects of perfluorocarbons: let’s get physical. Crit Care Med. 2000;28(4):1241–1242.
42. Smith TM, Steinhorn DM, Thusu K, Fuhrman BP, Dandona P. A liquid perfluorochemical decreases the in vitro production of reactive oxygen species by alveolar macrophages. Crit Care Med. 1995;23(9):1533–1539.
43. Nath KA, Balla G, Vercellotti GM, et al. Induction of heme oxygenase is a rapid, protective response in rhabdomyolysis in the rat. J Clin Invest. 1992;90(1):267–270.
44. Shimizu H, Takahashi T, Suzuki T, et al. Protective effect of heme oxygenase induction in ischemic acute renal failure. Crit Care Med. 2000;28(3):809–817.
45. Agarwal A, Bolisetty S. Adaptive responses to tissue injury: role of heme oxygenase-1. Trans Am Clin Climatol Assoc. 2013;124:111–122.
46. Nath KA. Heme oxygenase-1: a provenance for cytoprotective pathways in the kidney and other tissues. Kidney Int. 2006;70(3):432–443.
47. Ge ZJ, Jiang GJ, Zhao YP, Wang GX, Tan YF. Systemic perfluorohexane attenuates lung injury induced by lipopolysaccharide in rats: the role of heme oxygenase-1. Pharmacol Rep. 2010;62(1):170–177.
48. Maluf DG, Mas VR, Yanek K, et al. Molecular markers in stored kidneys using perfluorocarbon-based preservation solution: preliminary results. Transplant Proc. 2006;38(5):1243–1246.
49. Bonventre JV, Zuk A. Ischemic acute renal failure: an inflammatory disease? Kidney Int. 2004;66(2):480–485.
50. Friedewald JJ, Rabb H. Inflammatory cells in ischemic acute renal failure. Kidney Int. 2004;66(2):486–491.
51. Akcay A, Nguyen Q, Edelstein CL. Mediators of inflammation in acute kidney injury. Mediators Inflamm. 2009;2009:137072.
52. Burne-Taney MJ, Rabb H. The role of adhesion molecules and T cells in ischemic renal injury. Curr Opin Nephrol Hypertens. 2003;12(1):85–90.
53. Fontana J, Vogt A, Hohenstein A, et al. Impact of steroids on the inflammatory response after ischemic acute kidney injury in rats. Indian J Nephrol. 2017;27(5):365–371.
54. Steven P, Augustin AJ, Geerling G, et al. Semifluorinated alkane eye drops for treatment of dry eye disease due to meibomian gland disease. J Ocul Pharmacol Ther. 2017;33(9):678–685.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.