Back to Journals » Clinical Ophthalmology » Volume 15
Impact of Different Clinical Baseline Characteristics on Intravitreal Dexamethasone Implant Ozurdex® Outcomes
Authors Udaondo P , Hervas-Ontiveros A, Rosemblatt A, Garcia-Delpech S
Received 30 August 2021
Accepted for publication 6 October 2021
Published 14 October 2021 Volume 2021:15 Pages 4153—4162
DOI https://doi.org/10.2147/OPTH.S336865
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Patricia Udaondo,1,2 Ana Hervas-Ontiveros,1,2 Amir Rosemblatt,3,4 Salvador Garcia-Delpech1,2
1Ophthalmology Department, Hospital Universitario y Politécnico La Fe, Valencia, Spain; 2Aiken Prevencción y Cirugía Ocular, Valencia, Spain; 3Department of Ophthalmology, Tel-Aviv Sourasky Medical Center, Tel-Aviv, Israel; 4Sackler Faculty of Medicine, Tel-Aviv University, Tel-Aviv, Israel
Correspondence: Patricia Udaondo
Aiken Prevención y Cirugía Ocular, Pizarro, 15 Bajo, Valencia, 46004, Spain
Tel +34 647869228
Email [email protected]
Purpose: To determine the impact of different baseline clinical characteristics on the improvement in best corrected visual acuity (BCVA) in patients with diabetic macular edema (DME) who underwent the intravitreal dexamethasone implant (DEX) Ozurdex®.
Methods: This was a single center retrospective study conducted on patients with DME, either naïve or previously treated, who were treated with one or more DEX and had a follow-up of at least 6 months. The main outcome measure was the proportion of DEX achieving an improvement ≥ 15 letters in BCVA.
Results: The study analyzed 192 DEX implants administered to 97 eyes (65 patients). Among the 192 DEX analyzed, 57 (29.7%) implants achieved a BCVA improvement ≥ 15 letters (ETDRS) from baseline, with a mean time for achieving such improvement of 89.2 (39.7) days. Eyes who received an additional DEX and those with a duration of DME < 6 months had a greater probability of achieving a BCVA improvement ≥ 15 letters (odds-ratio: 2.55, p = 0.0028 and odds-ratio: 1.93, p = 0.0434). The mean (standard deviation) change in BCVA from baseline was 7.5 (14.5) letters, p < 0.0001. The mean change in central macular thickness (CMT) from baseline was − 128.0 (151.0) μm, p < 0.0001. The mean number of DEX implanted was 1.9 (0.8). Four (2.1%) DEX experienced an intraocular pressure increased ≥ 10 mm Hg; all the cases were successfully managed with topical antiglaucoma medication.
Conclusion: The results of this study confirmed previous evidence suggesting that DEX is effective for improving BCVA and CMT in patients with DME.
Keywords: diabetic macular edema, dexamethasone intravitreal implant, Ozurdex, best corrected visual acuity, central macular thickness
Introduction
Among the different currently available options for treating diabetic macular edema (DME), intravitreal corticosteroids and vascular endothelial growth factor inhibitors (anti-VEGF) have emerged as first-line treatment for this pathology.1
DME presents complex and multifactorial pathogenic mechanisms, with different factors including chronic hyperglycemia, hypercholesterolemia, free oxygen radicals, advanced glycation end-products and protein kinase C involved in its pathophysiologic process.2
Because inflammation has been identified as an important pathophysiological mechanism in DME, corticosteroids have taken an active role in the treatment of DME.3 Corticosteroids inhibit many of the processes involved in the pathophysiology of the DME, through anti-inflammatory properties4 and VEGF inhibition.5
Over the past several years, slow-release intravitreal corticosteroid implants, including dexamethasone posterior segment delivery system (Ozurdex, Allergan, Irvine, CA, United States),6 and fluocinolone (Iluvien, Alimera Sciences Limited, Aldershot, UK)7 have been commercially available.
According to the European Society of Retina Specialists (EURETINA) Guidelines, in DME patients, dexamethasone should be used first, while fluocinolone is recommended in patients with chronic DME who did not respond to other treatments.1
Many different studies have demonstrated that the intravitreal dexamethasone implant (DEX) Ozurdex® significantly improved the functional (visual acuity) and anatomic (central macular thickness) outcomes in patients with DME.8–16 However, the impact of different baseline clinical characteristics on DEX outcomes has not been fully elucidated.
Because not all patients respond similarly to DME treatment, determining potential predictive factors is crucial for customizing treatment and optimizing clinical outcomes.
Different optical coherence tomography (OCT) biomarkers, including presence of submacular fluid, absence/number of hyperreflective foci, integrity of the inner segment-outer segment, outer nuclear layer, disorganization of the retinal inner layers, outer retinal layer, and presence of cysts have been identified as prognostic and predictive factors in patients with DME.17–20
The purpose of this study was to determine the impact of different baseline clinical characteristics on the improvement in best corrected visual acuity (BCVA) in patients with DME who underwent the DEX Ozurdex®.
Methods
Design
Single center retrospective study conducted on patients with diabetic macular edema, either naïve or previously treated, referred or recruited in a third-level hospital.
Study protocol was approved by the Institutional Review Board of the La Fe University Hospital with the number (2014/00373/EO) and the study was conducted in accordance with the rules of the Declaration of Helsinki. The board waived the need for informed consent for study participation, since it was a retrospective study.
The data was anonymized for preserving the confidentiality of patients and will only be used for purposes related to this research.
Patient Eligibility
Patients with DME, who were treated with one or more intravitreal dexamethasone implant and had a follow-up of at least 6 months.
Inclusion criteria included age ≥18 years; a diagnosis of type 1 or 2 diabetes; DME (naïve or previously treated) with central macular thickness (CMT) ≥300 µm, a BCVA of 20/200 (35 letters in the Early Treatment Diabetic Retinopathy Study (ETDRS) charts) and had medical chart data available for at least 6 months after receiving the first DEX. Patients were excluded if they had macular edema due to any other condition; history of major ocular surgery, except non-complicated cataract surgery, within the previous 6 months or an intraocular pressure (IOP) ≥ IOP >21 mm Hg with one medication or any use of 2 or more medications. DME patients who received treatment with intravitreal triamcinolone ≤6 months before baseline or intravitreal bevacizumab, ranibizumab, or aflibercept ≤3 months before baseline were also excluded.
Cataract was assessed according to the lens Opacities Classification System.21
Study Variables
For each patient, data collected at the baseline visit included demographics; mean BCVA [letters in the Early Treatment Diabetic Retinopathy Study (ETDRS) charts]; CMT; IOP; IOP lowering medications; duration of diabetes; duration of DME; HbA1c; previous treatment of DME; DME perfusion status and lens status.
Variables collected during the follow-up included proportion of implants with BCVA improvement ≥15 letters (ETDRS) from baseline; time to ≥15 letters (ETDRS) improvement from baseline; proportion of implants with BCVA improvement ≥10 letters (ETDRS) from baseline; time to ≥10 letters (ETDRS) improvement from baseline; mean change in BCVA from baseline; time to peak improvement in BCVA from baseline; mean change in CMT from baseline; time to peak improvement in CMT from baseline; proportion of implants with BCVA reduction ≥15 letters (ETDRS) from baseline; time to ≥15 letters (ETDRS) reduction from baseline; proportion of implants with BCVA reduction ≥10 letters (ETDRS) from baseline; time to ≥10 letters (ETDRS) reduction from baseline and number of implants.
DEX 0.7 mg was injected into the vitreous cavity using standard protocols.9
After the DEX implantation, retreatment was judged necessary at the scheduled follow-up visits if recurrence of DME and/or intraretinal (IRF)/subretinal (SRF) were present or if BCVA decreased due to recurrence of macular edema.
Retreatment criteria were based on a pro-re-nata treatment regime. There were two anatomic criteria and one functional criterion for retreatment. The anatomic criteria were a CMT increase of 50 µm in OCT measurements or the onset of new cysts as compared to the previous visit. The functional criterion was a BCVA loss ≥5 ETDRS letters due to DME and not secondary to other causes, such as cataract or optical changes.
Outcomes
The main outcome measure was the proportion of DEX achieving an improvement ≥15 letters in BCVA and risk factors significantly associated with BCVA improvement ≥15 letters.
Secondary outcome measures included the mean change in BCVA from baseline, mean change in CMT from baseline; risk factors significantly associated with BCVA improvement ≥10 letters; proportion of implants achieving a BCVA improvement ≥10 letters; time to ≥15 letters (ETDRS) improvement from baseline; time to ≥10 letters (ETDRS) improvement from baseline; time to peak improvement in BCVA from baseline; time to peak improvement in CMT from baseline; proportion of implants with BCVA reduction ≥15 letters (ETDRS) from baseline; time to ≥15 letters (ETDRS) reduction from baseline; proportion of implants with BCVA reduction ≥10 letters (ETDRS) from baseline; time to ≥10 letters (ETDRS) reduction from baseline; number of implants and adverse events.
Statistical Analysis
A standard statistical analysis was performed using SAS ® version 9.3 (SAS Institute, Cary North Carolina) and the statistical program MedCalc version 20.008 (MedCalc Software bvba, Ostend, Belgium; http://www.medcalc.org; 2021).
Descriptive statistics number (percentage), mean [standard deviation (SD)], mean [95% confidence interval (95% CI)], mean [standard error (SE)] or median (95% CI) were used, as appropriate.
Comparisons between pre-intervention and post-intervention values were performed for BCVA and central macular thickness (CMT).
Normal distribution of continuous variables was evaluated by means D’Agostino-Pearson test.
As data were normally distributed, the two-way paired samples Student’s t-test was used to compare means between pre-intervention and post-intervention values.
A logistic regression model was used to assess and test factors for their association with achieving an improvement in BCVA ≥ 15 letters and ≥10 letters.
For the logistic regression analysis, the study subjects were divided according to their lens status (phakic vs pseudophakic); DME perfusion status (non-ischemia vs ischemia); DME status (naïve vs previously treated); duration of DME (<6 months vs ≥6 months); HbA1c (≤8% vs >8%) and DEX implanted (first DEX vs subsequent ones).
A p value <0.05 was considered as statistically significant.
Results
The study analyzed 192 DEX implants administered to 97 eyes (65 patients). Their main demographic and clinical characteristics are shown in Table 1.
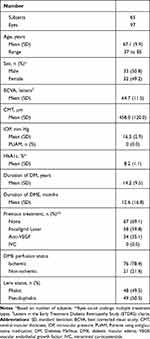 |
Table 1 Baseline Demographic and Clinical Characteristics of the Study Sample |
Mean age was 67.1±9.9 years and 32 (49.8%) patients were women. Mean HbA1c was 8.2%±1.1%. Sixty-seven (69.1%) eyes were naïve and 30 (30.9%) ones had undergone previous DME treatment.
Among the 192 DEX analyzed, 57 (29.7%) implants achieved a BCVA improvement ≥15 letters (ETDRS) from baseline, with a mean time for achieving such improvement of 89.2±39.7 days. Eighty-four (43.8%) implants achieved an improvement in BCVA ≥ 10 letters (ETDRS) from baseline and the mean time for achieving that improvement was 93.6±36.8 days.
On the negative side, as compared to baseline, 24 (12.5%) implants presented a reduction in BCVA ≥ 15 letters (ETDRS) and 31 (16.1%) ones a reduction in BCVA ≥ 10 letters.
The mean (SD) change in BCVA from baseline was 7.5±14.5 letters, p < 0.0001 (Figure 1).
The mean change in CMT from baseline was −128.0±151.0 µm, p < 0.0001 (Figure 2). The mean number of DEX implanted was 1.9±0.8.
 |
Figure 2 Mean central macular thickness (CMT) at baseline and at the end of the study. *Statistical significance was determined using the two-way paired Student’s t-test. |
Main clinical outcomes are presented in Table 2.
 |
Table 2 Main Clinical Outcomes |
Baseline BCVA did not show no significant differences among the different comparisons, depending on lens status (Phakic vs Pseudophakic); perfusion status (without ischemia vs with ischemia); DME status (Naïve vs Previously treated); duration of DME (<6 months vs ≥6 months); HbA1c levels (≤8% vs >8%); or DEX (First vs Subsequent) (Figure 3).
As compared to baseline, mean change in BCVA was +5.8±16.7 and 9.1±11.9 letters in phakic and pseudophakic eyes, respectively (mean difference −3.3 letters; 95% CI: −8.5 to 2.0 letters, p = 0.2232); +6.8±14.1 and 10.2±15.9 letters in eyes without and with macular ischemia, respectively (mean difference −3.4 letters; 95% CI: −9.8 to 2.9 letters, p = 0.2860); +8.5±15.2 and +5.3±12.5 letters in naïve and previously treated eyes, respectively (mean difference 3.2 letters; 95% CI: −2.5 to 8.9 letters, p = 0.2729); +8.9±13.7 and +5.7±15.4 in eyes with a DME duration <6 months and ≥6 months, respectively (mean difference 3.2 letters; 95% CI: −2.1 to 8.5 letters, p = 0.2283); +8.8±15.4 and 6.7±14.0 letters in patients with HbA1c ≤8% and >8%, respectively (mean difference 2.1 letters; 95% CI: −3.3 to 7.5 letters, p = 0.4519); and +5.4±13.0 and 6.1±15.8 letters with the First and subsequent DEX implants, respectively (mean difference −0.7 letters; 95% CI: −5.1 to 3.7 letters, p = 0.7618).
Regarding the logistic regression analysis, as compared with the first DEX, received an additional DEX was significantly associated with a greater probability of achieving a BCVA improvement ≥15 letters from baseline (Odds ratio: 2.55; 95% CI: 1.38 to 4.72; p = 0.0028). Additionally, eyes with a DME duration <6 months had a significant greater probability of achieving a BCVA improvement ≥15 letters from baseline (Odds ratio: 1.93; 95% CI: 1.02 to 3.67; p = 0.0434) (Table 3). However, lens status (phakic vs pseudophakic); DME perfusion status (non-ischemia vs ischemia); DME status (naïve vs previously treated); and HbA1c (≤8% vs >8%) were no significant predictors of achieving a BCVA improvement ≥15 letters from baseline (Table 3).
 |
Table 3 Impact of Different Study Variables on Achieving an Improvement in BCVA ≥ 15 Letters |
DME status (p = 0.0295); duration of DME (p = 0.0040); and received an additional DEX (p = 0.0002) were significantly associated with the probability of achieving a BCVA improvement ≥10 letters. Test factors and their association with achieving an improvement in BCVA ≥ 10 letters are shown in Table 4.
 |
Table 4 Impact of Different Study Variables on Achieving an Improvement in BCVA ≥ 10 Letters |
The proportion of DEX with a BCVA reduction ≥10 letters (22.3% vs 10.2%) and ≥15 letters (19.1% vs 6.1%) were significantly greater in the phakic than in the pseudophakic eyes, p = 0.0188 and p = 0.0054, respectively. Additionally, the DEX implanted in eyes with a duration of DME ≥ 6 months had a greater proportion of implants with a BCVA reduction ≥10 letters and ≥15 letters (25.9% and 20.0%, respectively) than those with a duration of DME < 6 months (8.7% and 6.8%, respectively), p = 0.0007 and 0.0037, respectively.
The time for achieving an improvement ≥15 letters in BCVA was significantly shorter in the naïve eyes than in the previously treated ones 76.9±32.0 vs 127±28.8 days, respectively, p < 0.0001. There was a 11% difference in the proportion of DEX achieving an improvement ≥15 letters in BCVA between naïve and previously treated eyes (33.3% vs 22.2%, respectively), although such a difference was not statistically significant (p = 0.1136).
In 3 (1.6%) DEX an IOP ≥ 25 mm Hg was observed and, as compared to baseline, 4 (2.1%) DEX experienced an IOP increased ≥10 mm Hg; all the cases were successfully managed with topical antiglaucoma medication (Table 5). Seven eyes had new onset lens opacity or progression of an existing opacity during the study follow-up (Table 5).
 |
Table 5 Overview of the Incidence of Adverse Events Over the Course of the Study Follow-Up |
Discussion
According to the results of the current study, receiving an additional DEX and a DME duration <6 months was significantly and positively associated with the probability of achieving a BCVA improvement ≥15 letters from baseline. Additionally, treatment naïve eyes; eyes with a DME duration <6 months, and those who received an additional DEX showed a significantly greater probability of achieving a BCVA improvement ≥10 letters from baseline.
Due to its good efficacy and safety profile,8–16 DEX has become a first-line therapy for treating patients with DME.
About such a point, the results of our study, did not significantly differ from those previously published.8–16 DEX Ozurdex® was able to significantly improve both functional (BCVA) and anatomical (CMT) outcomes.
Nevertheless, as far as we know, this is the first study evaluating DEX results according baseline characteristics, which might help to predict its efficacy in DME patients.
Comparing our results to that published by Castro-Navarro et al15 (a retrospective study conducted on Spanish population), we could observe that the mean change in BCVA and CMT found in our study did not significantly differ from that reported by Castro-Navarro et al (5.6 letters ETDRS and 103.2 µm, respectively).
Moreover, our results in BCVA improvement and CMT reduction are in line with a systematic review search evaluating the pharmacological management of DME in real-life observational studies.22
Regarding the main outcome (the proportion of DEX achieving an improvement ≥15 letters in BCVA), it is extremely difficult to compare our results with the currently available scientific literature.8–16 All the studies evaluated the proportion of eyes achieving a BCVA improvement, while our study evaluated the proportion of DEX achieving such improvement.
The scientific evidence has suggested a better BCVA improvement in naïve eyes than in previously treated ones,8–16 in agreement with this finding our study found difference in the mean change in BCVA of 3 letters between naïve and previously treated eyes [8.5±15.2 vs 5.3±12.5 letters ETDRS, respectively], although such a difference was not statistically significant (p = 0.2729). Nevertheless, the time for achieving a BCVA improvement ≥15 letters, ≥10 letters or the peak BCVA improvement was significantly shorter in the naïve eyes than in the previously treated ones (p < 0.0001, p = 0.0006 and p = 0.0258, respectively).
This study did not find any relationship between the lens status, DME perfusion status, DME status, or HbA1c levels and the probability of DEX for achieving a BCVA improvement ≥15 letters.
Interestingly, as compared with the first DEX, received an additional DEX was significantly associated with achieving a BCVA improvement ≥15 letters from baseline. From our point of view, this finding is extremely important because it suggested that implanting a second DEX, even in eyes that did not exhibit a good initial respond, might suppose a high probability for obtaining a good functional outcome.
It has been reported that the subsequent DEXs did not have a lower effect, in terms of BCVA improvement, than the first one.8–16,23 Nevertheless, the results of our study suggested that subsequent DEXs might have even better results than the first DEX.
Additionally, a duration of DME < 6 months has been positively associated with the probability of achieving a BCVA ≥ 15 and ≥10 letters. Our results agreed with those reported by Rosenblatt et al,16 who found worse functional outcomes in eyes with longstanding DME, which indicated the role of DME duration as a predictor for visual outcomes.
In view of the results, it can be hypothesized that early DME may benefit from early treatment with DEX, although a chronic prolonged DME also respond to DEX.
Our study found that naïve eyes had a better probability for achieving a BCVA improvement ≥10 letters from baseline, this finding did not significantly differ from the currently available evidence.8–16
The finding that the proportion of DEX with BCVA reduction ≥10 letters and ≥15 letters were significantly greater in the phakic than in the pseudophakic eyes, from our point of view, would suggest that the reduction of BCVA observed in the phakic eyes might be related with either a new onset lens opacity or progression of an existing opacity during the study follow-up rather than with the efficacy of the DEX itself.
Regarding the adverse events, an IOP ≥ 25 mm Hg was observed in 3 (1.6%) DEX during the course of follow-up. In all cases, increased IOP was managed and controlled with topical medication or observation; none required surgery. These results are similar to those reported in other studies.9–13,24,25
There were no new or unexpected adverse events throughout the study.
This study has some limitations that should be taking into account when evaluating its results. The first one is its retrospective design. Although retrospective studies have inherent potential bias, the strict inclusion/exclusion criteria applied in our study might be able to minimize its impact. As a second limitation, it should be mentioned that this is a single center study with the possibility of including a limited number of patients. Nevertheless, the study sample (192 DEX implants administered to 97 eyes of 65 patients) was enough. Another limitation is the lack of information about the role of OCT data as predictors of DEX outcomes. As aforementioned in the introduction section, different OCT biomarkers have been identified as predictive factors of DEX outcomes.17–20 Additionally, integrity of external limiting membrane and ellipsoid zone was associated with the final BCVA improvement in patients with DME.26 Nevertheless, while recognizing the importance of OCT for the clinical management of patients with DME, our study focus on identifying predictive factors beyond OCT.
Conclusions
The results of the current study suggested that eyes who received an additional DEX and those who have a DME duration <6 months have a greater probability of achieving a BCVA improvement ≥15 letters from baseline. Additionally, the probability of achieving a BCVA improvement ≥10 letters from baseline was significantly greater in treatment naïve eyes, eyes with a DME duration <6 months, and those who received an additional DEX. The fact that a DME duration <6 months has been associated with a BCVA improvement ≥15 and ≥10 letters speaks in favor of early treatment of these patients.
This study did not find any relationship between the lens status, DME perfusion status, DME status, or HbA1c levels and the probability of DEX for achieving a BCVA improvement ≥15 letters.
It should be noteworthy that the time for achieving an improvement ≥15 letters, ≥10 letters or the peak improvement was significantly shorter in the naïve eyes than in the previously treated ones.
Finally, the results of this study confirmed previous evidence suggesting that DEX is effective for improving BCVA and CMT in patients with DME.
Further studies, especially prospective, randomized and multicenter studies, are needed to elucidate potential factors associated with success of intravitreal dexamethasone implants in patients with diabetic macular edema.
Acknowledgments
Medical writing and Editorial assistant services have been provided by Antonio Martínez (MD) of Ciencia y Deporte S.L. and covered by a Grant from Allergan. Support for this assistance was funded by Allergan, an AbbVie company.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, agreed to the submitted journal, and agree to be accountable for all aspects of the work.
Funding
Logistic for writing services has been provided by Allergan, an AbbVie company. Allergan did not participate in either data analysis or redaction of the manuscript.
Disclosure
Dr. Patricia Udaondo reports grants from Allergan, an AbbVie company, during the conduct of the study. Dr. Amir Rosenblatt: Financial support and Lecturer – Allergan. The authors report no other conflicts of interest in this work.
References
1. Schmidt-Erfurth U, Garcia-Arumi J, Bandello F, et al. Guidelines for the management of diabetic macular edema by the European Society of Retina Specialists (EURETINA). Ophthalmologica. 2017;237(4):185–222. doi:10.1159/000458539
2. Bhagat N, Grigorian RA, Tutela A, Zarbin MA. Diabetic macular edema: pathogenesis and treatment. Surv Ophthalmol. 2009;54(1):1–32.
3. Al Dhibi HA, Arevalo JF. Clinical trials on corticosteroids for diabetic macular edema. World J Diabetes. 2013;4(6):295–302. doi:10.4239/wjd.v4.i6.295
4. Sohn HJ, Han DH, Kim IT, et al. Changes in aqueous concentrations of various cytokines after intravitreal triamcinolone versus bevacizumab for diabetic macular edema. Am J Ophthalmol. 2011;152(4):686–694. doi:10.1016/j.ajo.2011.03.033
5. Brooks HL
6. Ozurdex®. Summary of product characteristics. Available from: https://www.ema.europa.eu/medicines/human/EPAR/ozurdex.
7. Iluvien®. Summary of product characteristics; 2019. Available from: https://www.medicines.org.uk/emc/product/3061/smpc.
8. Escobar-Barranco JJ, Pina-Marin B, Fernandez-Bonet M. Dexamethasone implants in patients with naive or refractory diffuse diabetic macular edema. Ophthalmologica. 2015;233(3–4):176–185. doi:10.1159/000371770
9. Guigou S, Pommier S, Meyer F, et al. Efficacy and safety of intravitreal dexamethasone implant in patients with diabetic macular edema. Ophthalmologica. 2015;233(3–4):169–175. doi:10.1159/000381356
10. Chhablani J, Bansal P, Veritti D, et al. Dexamethasone implant in diabetic macular edema in real-life situations. Eye. 2016;30(3):426–430. doi:10.1038/eye.2015.246
11. Malclès A, Dot C, Voirin N, et al. Real-life study in diabetic macular edema treated with Dexamethasone implant: the Reldex study. Retina. 2017;37(4):753–760. doi:10.1097/IAE.0000000000001234
12. Pareja-Ríos A, Ruiz-de la Fuente-Rodríguez P, Bonaque-González S, López-Gálvez M, Lozano-López V, Romero-Aroca P. Intravitreal dexamethasone implants for diabetic macular edema. Int J Ophthalmol. 2018;11(1):77–82.
13. Iglicki M, Busch C, Zur D, et al. Dexamethasone implant for diabetic macular edema in naive compared with refractory eyes: the International Retina Group Real-Life 24-month multicenter study. The IRGREL-DEX study. Retina. 2018;39(1):44–51. doi:10.1097/IAE.0000000000002196
14. Nagpal M, Mehrotra N, Juneja R, Jain H. Dexamethasone implant (0.7 mg) in Indian patients with macular edema: real-life scenario. Taiwan J Ophthalmol. 2018;8(3):141–148. doi:10.4103/tjo.tjo_62_17
15. Castro-Navarro V, Cervera-Taulet E, Navarro-Palop C, Monferrer-Adsuara C, Hernández-Bel L, Montero-Hernández J. Intravitreal dexamethasone implant Ozurdex® in naïve and refractory patients with different subtypes of diabetic macular edema. BMC Ophthalmol. 2019;19(1):15. doi:10.1186/s12886-018-1022-9
16. Rosenblatt A, Udaondo P, Cunha-Vaz J, et al.; ARTES Study Group. A collaborative retrospective study on the efficacy and safety of intravitreal Dexamethasone implant (Ozurdex) in patients with diabetic macular edema: the European DME registry study. Ophthalmology. 2020;127(3):377–393. doi:10.1016/j.ophtha.2019.10.005.
17. Zur D, Iglicki M, Busch C, Invernizzi A, Mariussi M, Loewenstein A; International Retina Group. OCT biomarkers as functional outcome predictors in diabetic macular edema treated with Dexamethasone implant. Ophthalmology. 2018;125(2):267–275. doi:10.1016/j.ophtha.2017.08.031
18. Fonollosa A, Zarranz-Ventura J, Valverde A, et al. Predictive capacity of baseline hyperreflective dots on the intravitreal dexamethasone implant (Ozurdex®) outcomes in diabetic macular edema: a multicenter study. Graefes Arch Clin Exp Ophthalmol. 2019;257(11):2381–2390. doi:10.1007/s00417-019-04446-4
19. Eandi CM, De Geronimo D, Giannini D, et al. Baseline SD-OCT characteristics of diabetic macular oedema patterns can predict morphological features and timing of recurrence in patients treated with dexamethasone intravitreal implants. Acta Diabetol. 2020;57(7):867–874. doi:10.1007/s00592-020-01504-w
20. Udaondo P, Adan A, Arias-Barquet L, et al. Challenges in diabetic macular edema management: an expert consensus report. Clin Ophthalmol. 2021;15:3183–3195. doi:10.2147/OPTH.S320948
21. Bullimore MA, Bailey IL, Friend J, McCarthy D, Wu SY. The lens opacities classification system III. The longitudinal study of Cataract Study Group. Arch Ophthalmol. 1993;111(6):831–836. doi:10.1001/archopht.1993.01090060119035
22. Kodjikian L, Bellocq D, Mathis T. Pharmacological management of diabetic macular edema in real-life observational studies. Biomed Res Int. 2018;2018:8289253. doi:10.1155/2018/8289253
23. Bucolo C, Gozzo L, Longo L, Mansueto S, Vitale DC, Drago F. Long-term efficacy and safety profile of multiple injections of intravitreal dexamethasone implant to manage diabetic macular edema: a systematic review of real-world studies. J Pharmacol Sci. 2018;138(4):219–232. doi:10.1016/j.jphs.2018.11.001
24. Bahadorani S, Krambeer C, Wannamaker K, et al. The effects of repeated Ozurdex injections on ocular hypertension. Clin Ophthalmol. 2018;12:639–642. doi:10.2147/OPTH.S148990
25. Hemarat K, Kemmer JD, Porco TC, Eaton AM, Khurana RN, Stewart JM. Secondary ocular hypertension and the risk of glaucoma surgery after Dexamethasone intravitreal implant in routine clinical practice. Ophthalmic Surg Lasers Imaging Retina. 2018;49(9):680–685. doi:10.3928/23258160-20180831-05
26. Castro-Navarro V, Monferrer-Adsuara C, Navarro-Palop C, Montero-Hernández J, Cervera-Taulet E. Effect of Dexamethasone intravitreal implant on visual acuity and foveal photoreceptor integrity in macular edema secondary to retinal vascular disease. Ophthalmologica. 2021;244(1):83–92.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


