Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Impact of COVID-19 Vaccine and COVID-19 Infection on Vitiligo Activity and Progression
Received 8 September 2023
Accepted for publication 9 December 2023
Published 15 December 2023 Volume 2023:16 Pages 3581—3587
DOI https://doi.org/10.2147/CCID.S439045
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Rungsima Wanitphakdeedecha
Xiaoyi Shi, Yifang Sun, Xiaolan Ding
Department of Dermatology, Peking University People’s Hospital, Beijing, People’s Republic of China
Correspondence: Xiaolan Ding, Department of Dermatology, Peking University People’s Hospital, No. 11 Xizhimen South Street, Xicheng District, Beijing, 100044, People’s Republic of China, Tel +8613522658992, Fax +861088325474, Email [email protected]
Background: Coronavirus disease 2019 (COVID-19) has given rise to several new onset or exacerbated dermatologic disorders including vitiligo. However, the relationship between COVID-19 infection or its associated vaccines and vitiligo progression is unclear.
Aim: We investigate the impact of COVID-19 infection and its associated vaccines on vitiligo progression.
Methods: A cross-sectional study was performed among patients who visited Department of Dermatology, Peking University People’s Hospital between 2022.1 and 2023.6. Detailed information including demographic characteristics, vitiligo clinical features, information on COVID-19 infection and vaccination and disease progression was collected by an electronic questionnaire.
Results: Overall, 314 patients with vitiligo completed the questionnaire. 47.5% were males, with an average age of 25.5± 15.9 years. 266 (84.7%) patients had received COVID-19 vaccination, and 70.3% of the patients reported progression of vitiligo after vaccination, mostly within 3 months. 55.6% of the patients had disease progression after the second dose of vaccine. 270 patients experienced COVID-19 infection, and 30.7% of these patients had progression of vitiligo after infection, most of the progression occurred within 1– 2 months. 184 patients (68.2%) interrupted treatment. Analysis results indicated patients in active stage had a higher risk for vitiligo progression after COVID-19 infection and vaccination.
Keywords: COVID-19, infection, vaccination, vitiligo, disease progression
Introduction
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has been causing a worldwide pandemic since 2019.1 The clinical manifestations of COVID-19 infection are complex and diverse, and the whole systems may be affected, including the skin. Vitiligo is an acquired chronic depigmenting disorder of the skin resulting from selective destruction of melanocytes, and autoimmune abnormality is one of the main causes of vitiligo. Until now, there have been some case reports of new vitiligo or deterioration of original vitiligo after COVID-19 vaccination and infection, but large scale epidemiological data are lacking. In a recent study, the author reported active vitiligo vulgaris following the administration of the Oxford–AstraZeneca (AZD1222) vaccine against SARS-CoV-2. They believe that immune activation during SARS-CoV-2 infection may increase vitiligo disease activity.2 In another study, 15 cases (88.2%) had new-onset or worsening vitiligo after COVID-19 vaccination and two cases (11.8%) developed vitiligo following primary COVID-19 infection. Patients who have comorbid autoimmune diseases were more likely to develop vitiligo.3
COVID-19 vaccination rate and COVID-19 infection rate are both more than 80% in China. The impact of COVID-19 vaccine and COVID-19 infection on vitiligo progression in Chinese patients is still unknown. Therefore, we conducted an online questionnaire survey among vitiligo patients, in order to investigate the impact of COVID-19 vaccine and COVID-19 infection on vitiligo progression, providing guidance for the treatment of vitiligo under the epidemic.
Methods
Study Sample
This cross-sectional study was conducted in the Department of Dermatology of Peking University People’s Hospital. The study protocol conformed to the Declaration of Helsinki and was approved by the ethical committee of Peking University People’s Hospital and confirmed in the revised manuscript that all participants provided informed consent. Inclusion criteria: (1) patients of both gender, diagnosed as vitiligo, according to the “Vitiligo Diagnosis and Treatment Consensus (2021 edition)”; (2) Able to read and understand the text of the questionnaire. For patients aged <18 years, detailed information was taken from the patient or their parents; exclusion criteria: (1) Filling in the logical errors; (2) Short response time (less than 50s).
The electronic questionnaire was created using Wen Juan Xing (an online survey tool by Changsha Ranxing Information Technology Co., Ltd., Hunan, China). Each patient was only allowed to submit the questionnaire once, in order to avoid duplicate submissions, depending on the IP address. The main contents of the questionnaire were summarized into four parts as follows: (1) demographic characteristics: age, gender, height, weight; (2) vitiligo clinical features: duration of disease, lesion distribution, status of lesions, comorbidities, family history and current treatments; (3) information of COVID-19 vaccination: vaccine type, inoculation time for each dose, activity of vitiligo after COVID-19 vaccination; (4) information of COVID-19 infection: infection time, symptoms, activity of vitiligo after infection, treatment after infection. The assessment of vitiligo subtypes and disease activity was done by dermatologists.
Statistical Analysis
The data exported from the background of Wen Juan Xing were manually checked and screened to establish a database. Analyses were performed in SPSS version 27.0 (IBM, Armonk, NY, USA). Data were summarized as means ± standard deviation (SD) for quantitative variables with normal distribution and were compared with a t-test. Quantitative variables with skewed distributions were expressed as median and interquartile range (IQR), and a non-parametric rank-sum test was done to test the statistical difference between groups. Qualitative variables were presented for frequencies with proportions (%) and were compared using the chi-squared test. Logistic regression was done to calculate the odds ratio (OR) and 95% confidence interval (95% CI) to detect the independent factors for disease progression among patients with vitiligo. Values of p < 0.05 were considered statistically significant.
Results
Demographic and Vitiligo Clinical Features
Totally, 314 patients included in our study, 149 (47.5%) were males and 165 (52.5%) were females, with an average age of 25.5±15.9 years. Patients’ demographic and clinical features are in Table 1.
 |
Table 1 Demographic and Clinical Features of 314 Patients with Vitiligo |
Impact of COVID-19 Vaccine on Vitiligo Progression
Overall, 266 patients received COVID-19 vaccination and 97.3% of them got inactivated vaccine. 187 (70.3%) patients reported disease progression after vaccination, while 79 did not. Logistic regression analyses indicated that patients in active stage (OR=0.182, 95% CI: 0.099–0.336) had higher risk for vitiligo progression. The proportion of disease progression after the first, second, third and fourth doses. were 22.5%, 55.6%, 20.9% and 1.1% (Figure 1), and the proportion of those with disease progression after the second dose was higher than the other doses of vaccination, but no significant difference was found. Most of the disease progression occurred within 3 months after vaccination. Most of the patients (184, 98.4%) had new lesions located in the original site, and only 3 patients (3, 1.6%) had new lesions in other sites.
 |
Figure 1 The relationship between vitiligo progression and vaccine dose and vaccination time. |
Impact of COVID-19 Infection on Patients with Vitiligo
Overall, 270 patients were infected with COVID-19, 86.0% of them (232) were confirmed by the detection of COVID-19 antigen reagents. After COVID-19 infection, most of the patients (238, 88.2%) had fever and other clinical manifestations including upper respiratory tract infection, fatigue, joint muscle pain, headache, dizziness, changes in taste and/or smell, gastrointestinal manifestations, chest pain, palpitation, rash, conjunctivitis and lung imaging abnormalities. Symptoms disappeared within 1–2 weeks in 92.6% of patients, 7.4% of patients had symptoms that lasted longer than 2 weeks. The majority of patients resolved with symptomatic supportive treatment, with only one case being hospitalized due to the severity of the condition. To further identify the independent factors associated with vitiligo disease progression, logistic regression analyses were performed (Table 2). Patients in active stage (OR = 1.707, 95% CI: 0.360–8.100) had higher risk for vitiligo progression after COVID-19 infection.
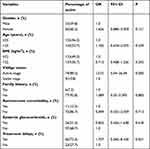 |
Table 2 Factors Associated with the Progression of Vitiligo After COVID-19 Infection |
During the epidemic, 184 patients (68.2%) interrupted treatment. More than half of patients (62.5%) interrupted phototherapy. 136 patients (73.9%) interrupted treatment for at least a week. Analysis results indicated that patients who interrupted treatment (OR = 1.707, 95% CI: 0.360–8.100) had higher risk for vitiligo progression but with no statistical significance. Thirty-five patients (42.2%) had disease progression within 1–2 months after COVID-19 infection, as shown in Figure 2. Most patients (82, 98.8%) had new lesions located in the original site. Sixty-two patients received treatment for the new lesions, and 64.5% of them got better.
 |
Figure 2 The relation between treatment interruption and vitiligo progression after COVID-19 infection. Abbreviations: w, week; m, month. |
Discussion
The COVID-19 pandemic has swept the world, becoming the largest and most widespread public health event of this century. COVID-19 infection can cause damage to multiple organs throughout the body with complex and varied clinical presentations. Vaccination against COVID-19 is an important way to prevent COVID-19 infection and reduce the percentage of severe cases in infected patients.
Vitiligo is an acquired depigmentation skin disease. Autoimmune abnormalities play an important role in the pathogenesis of vitiligo. It is well-recognized that the skin-infiltration of melanocyte-specific CD8 + T cells mediates the progressive destruction of melanocytes and plays a key role in the pathogenesis of vitiligo.4 There have been reports of vitiligo or vitiligo progression after COVID-19 vaccination or COVID-19 infection in some cases, but epidemiological investigations in large samples are lacking.
In our study, we first investigated the correlation between COVID-19 vaccine and progression of vitiligo. Up to 70.3% of patients (187, 187/266) had disease progression after vaccination. Ahmed et al reported that a male had new-onset vitiligo after the administration of the first dose of the Oxford–AstraZeneca (AZD1222) vaccine.2 Tsai et al conducted a systematic review and reported that 12 patients had new-onset vitiligo, while the other two had preexisting vitiligo that was exacerbated by COVID-19 vaccination.5 In our study, we found that most patients (104, 55.6%) had disease progression within 3 months after the second dose. Patients who were in active stage (OR = 0.182, 95% CI: 0.099–0.336) had higher risk for further progression. Kaiqiao He et al described 35 patients had vitiligo progression within 1 month of vaccination, and 51.4% of these patients developed after the second dose of vaccination, which was consistent with our results.6 COVID-19 vaccines require two partial vaccinations to achieve the highest possible immunity.7 This may explain why most patients experience progression after the second dose of the vaccine. For those patients who were in active stage, delayed vaccination may prevent progression.
In addition, up to 97% (182/187) of patients with vitiligo experienced disease progression after receiving inactivated vaccines, which may be related to the predominance of inactivated vaccines approved in China or similar to the survivor bias, in which patients with disease progression are more likely to go to the hospital. There is no clear explanation for the relationship between COVID-19 vaccination and disease progression, and we hypothesized an autoimmune link. The following are several hypotheses about the possible pathogenesis. First, it could be explained as molecular mimicry, which is a similarity between specific pathogenic elements contained in the vaccine or vaccine adjuvants and specific human proteins.8 Moreover, vaccine components and adjuvants can induce potent innate immune responses that nonspecifically activate self-reactive T and B cells. Through these mechanisms, vaccines stimulate the immune system to produce antibodies against viral proteins as well as unintended antibodies against melanocytes.9
COVID-19 infection has been documented to cause a variety of skin problems such as urticaria, bullous pemphigoid-like disease and psoriasis. Vitiligo or progression of vitiligo after COVID-19 infection have also been reported. Pastukhova et al described a new onset of vitiligo post-COVID-19 infection.7 Schmidt et al found a new onset of vitiligo post-COVID-19 infection and present a patient who developed both eruptive halo nevi and vitiligo after a confirmed COVID-19 infection.10 In our study, 270 patients infected COVID-19, and 30.7% of them had progression of vitiligo. We found that patients in active stage (OR = 12.01, 95% CI: 5.54–26.04) had higher risk for vitiligo progression. And patients who interrupted treatment (OR = 1.707, 95% CI: 0.360–8.100) had higher risk for vitiligo progression, but no statistical significance. Xu et al conducted a retrospective study and determined treatment delays as the most important independent risk factor for disease progression and recurrence, and maintenance therapy (>2 years) as a protective factor against recurrence, during COVID-19 epidemic.10 The following were possible causes of vitiligo progression due to COVID-19 infection. Firstly, COVID-19 disrupts immune tolerance, resulting in increased inflammation potentiated by increased interferon gamma (IFN-γ) signaling and consequent cytotoxic destruction of melanocytes.11 Furthermore, Schmidt et al postulated a hyperstimulated CD8+ response and increased oxidative stress as catalysts.12 Finally, based on the shift of the immune system in non-segmental vitiligo toward adaptive type 1 (IFN-γ and CD8+T cells) and innate immune responses, immune activation during SARS-CoV-2 infection or COVID-19 disease may increase vitiligo disease activity.2
In summary, our study indicated that vitiligo progression was related to COVID-19 vaccination and COVID-19 infection, especially for those in active stage. Physicians should be aware of the possibility of aggravation of autoimmune skin diseases such as vitiligo following COVID-19 vaccination and infection. For patients with active vitiligo, a delay in vaccination may decrease the risk of disease progression.
This study has some limitations. Firstly, the convenience sampling method used in this study has a degree of selection bias and may not be a good representation of the true picture of the overall vitiligo population. Secondly, an in-depth analysis of the exact relationship between COVID-19 vaccine or COVID-19 infection and vitiligo progression needs to be conducted in controlled clinical studies with large samples.
Conclusion
Up to 70.3% of the vitiligo patients had disease progression after COVID-19 vaccination and 30.7% of them had disease progression after COVID-19 infection. Active stage of vitiligo is a risk factor of vitiligo progression after COVID-19 vaccination and infection. Further investigations are required to clarify the relationship between COVID-19 infection or its associated vaccines and vitiligo progression.
Acknowledgments
Project (RDX2020-09) supported by Peking University People’s Hospital Scientific Research Development Funds.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Wu T, Li J, Hu Y, Tang LV. Hematological questions in personalized management of COVID-19 vaccination. J Pers Med. 2023;13(2):259. doi:10.3390/jpm13020259
2. Abdul-Aziz Ahmed A, Jaber GN. Active vitiligo vulgaris following the administration of the Oxford–AstraZeneca (AZD1222) vaccine against SARS-CoV-2. Our Dermatol Online. 2022;13(2):219–220. doi:10.7241/ourd.20222.27
3. Kasmikha LC, Mansour M, Goodenow S, Kessler S, Appel J. Vitiligo following COVID-19 vaccination and primary infection: a case report and systematic review. Cureus. 2023;15(9):e45546. doi:10.7759/cureus.45546
4. Boniface K, Seneschal J, Picardo M, Taïeb A. Vitiligo: focus on clinical aspects, immunopathogenesis, and therapy. Clin Rev Allergy Immunol. 2018;54(1):52–67. doi:10.1007/s12016-017-8622-7
5. Tsai TF, Ng CY. COVID-19 vaccine-associated vitiligo: a cross-sectional study in a tertiary referral center and systematic review. J Dermatol. 2023;50(8):982–989. doi:10.1111/1346-8138.16799
6. Kaiqiao H, Bozhang L, Shuli L. Impact of a novel coronavirus infection epidemic on disease management in patients with vitiligo: a cross-sectional study. China J Lepr Skin Dis. 2023;39(04):231–235.
7. Okan G, Vural P. Worsening of the vitiligo following the second dose of the BNT162B2 mRNA COVID-19 vaccine. Dermatol Ther. 2022;35(3):e15280. doi:10.1111/dth.15280
8. Hou X, Wu N, Xu M, et al. Demographic and clinical feature disparity between progress and non-progress patients with vitiligo after COVID-19 vaccination: a cross-sectional study. Exp Dermatol. 2023:
9. Segal Y, Shoenfeld Y. Vaccine-induced autoimmunity: the role of molecular mimicry and immune crossreaction. Cell Mol Immunol. 2018;15(6):586–594. doi:10.1038/cmi.2017.151
10. Xu X, Zhang C, Jiang M, Xiang LF. Impact of treatment delays on vitiligo during the COVID-19 pandemic: a retrospective study. Dermatol Ther. 2021;34(4):e15014. doi:10.1111/dth.15014
11. Pastukhova E, Ghazawi FM. Eruptive halo nevi and new-onset vitiligo post-COVID-19 infection. JAAD Case Rep. 2023;34:43–44. doi:10.1016/j.jdcr.2023.01.030
12. Schmidt AF, Rubin A, Milgraum D, Wassef C. Vitiligo following COVID-19: a case report and review of pathophysiology. JAAD Case Rep. 2022;22:47–49. doi:10.1016/j.jdcr.2022.01.030
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
