Back to Journals » Clinical Ophthalmology » Volume 17
Impact of COVID-19 on Depressive Symptoms Among Patients with Low Vision and Blindness
Authors Tantirattanakulchai P , Hounnaklang N , Pongsachareonnont PF , Khambhiphant B, Hounnaklang S, Win N , Tepjan S
Received 16 December 2022
Accepted for publication 3 March 2023
Published 8 March 2023 Volume 2023:17 Pages 789—796
DOI https://doi.org/10.2147/OPTH.S401714
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Pankaew Tantirattanakulchai,1 Nuchanad Hounnaklang,1 Pear Ferreira Pongsachareonnont,2,3 Bharkbhum Khambhiphant,4 Suwanchai Hounnaklang,5 Nanda Win,1 Suchon Tepjan6
1College of Public Health Sciences, Chulalongkorn University, Bangkok, Thailand; 2Center of Excellence in Retina, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand; 3King Chulalongkorn Memorial Hospital, Bangkok, Thailand; 4Department of Ophthalmology, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand; 5Dhurakijpundit University, Bangkok, Thailand; 6VOICES-Thailand Foundation, Chiang Mai, Thailand
Correspondence: Nuchanad Hounnaklang, College of Public Health Sciences, Chulalongkorn University, Institute Building 2-3, Soi Chulalongkorn 62, Phyathai Road, Pathumwan, Bangkok, 10330, Thailand, Tel +66 2218 8205, Fax +66 2255 6046, Email [email protected]
Background: Disability is globally recognized as a key cause of depression. Likewise, the COVID-19 pandemic has significantly increased the vulnerability of patients with low vision to health and health-related issues, especially mental health. This study aimed to examine the association between the impact of COVID-19 and depressive symptoms in patients with low vision and blindness.
Methods: This cross-sectional study was conducted between February and July 2022 and involved face-to-face interviews. Patients with low vision and blindness diagnosed with depression were excluded. The following items were included in the questionnaire: sociodemographic information, Multi-Dimensional Scale of Perceived Social Support (MSPSS), the impact of COVID-19, and The Center for Epidemiological Studies-Depression (CES-D). Hierarchical linear regression analysis was used to examine the association between the impact of COVID-19 and depression.
Results: The prevalence of depression among patients with low vision and blindness was 43.0%. Three factors were associated with depressive symptoms: compliance with COVID-19 prevention strategies (β = 0.16, p< 0.01), anxiety during COVID-19 (β = 0.24, p< 0.001), and social support (β = − 0.16, p< 0.01).
Conclusion: The findings indicated that COVID-19 significantly increased depressive symptoms among patients with low vision and blindness. The psychological effects of the COVID-19 pandemic have been considered critical and emergent public health issues. Stakeholders, particularly public health organizations, need to urgently implement preventive and protective measures to help patients with physical and mental disabilities.
Keywords: COVID-19, depressive symptoms, low vision, blindness
Introduction
The COVID-19 pandemic was declared a global crisis by the World Health Organization (WHO) due to its widespread impact globally.1,2 The pandemic has had serious physical effects on people and has resulted in an increase in depression and anxiety globally.3 According to the WHO, there has been a 25% rise in the prevalence of mental health disorders. The impact of the pandemic has been felt across society, particularly in terms of health, economics, and social aspects. To control the pandemic, preventive and protective measures such as nationwide lockdowns, self-isolation, social distancing, and remote work were implemented.4 Although these measures were necessary to control the spread of the virus and maintain high standards of care for all, they have unavoidably affected the physical and mental health of individuals to an extent.5–7
In Thailand, the Ministry of Public Health requires that people susceptible to mental distress are provided counseling and mental health care access. Provincial program managers should be watchful and ready to take action on mental health disorders caused by COVID-19 such as anxiety, depression, and suicidal ideation.4
During the COVID-19 pandemic, Thai people experienced difficulties navigating their normal daily lives especially during the periods of mandatory quarantine and isolation.8 In addition, shortage of masks and medical protective equipment, limited access to information about COVID-19, limited access to hospital services and treatments, restriction of movement, and death of family members or relatives all affected their mental health.8,9 Previous studies indicated that approximately half of the participants had moderate to severe psychologically effects during the COVID-19 outbreak. The prevalence of moderate to severe depression, severe anxiety, and moderate to severe stress of the participants were found to be 16.5%, 28.8%, and 8.1%, respectively, and their poor self-rated health was significantly related to a greater psychological effect of COVID-19.10
A study reported that patients with disability or impairment had a higher risk of COVID-19 than those without.11 Approximately 253 million people a risk of COVID-19 globally.12 The measures against COVID-19, such as national lockdown, reduced social engagement, and social distancing, had effects on patients with visual impairment to the extent that their physical and mental health will be significantly affected in years to come.13
COVID-19 can increase the vulnerability of patients with low vision to general health and mental health issues. Older people with visual impairment are susceptible to a greater risk of depression than the general population.14,15 The WHO reported that people with depression can have low spirit, anhedonia, and tiredness and unnecessary worry, irritability, and exhaustion with anxiety.16 Moreover, people with low vision have a low level of social contact which can lead to more of depression and anxiety. A previous study reported that patients with blindness have a greater risk of contracting COVID-19 than those with mild or without visual impairment. Regarding healthcare accessibility, a study reported that participants who were blind had worries about the difficulty in receiving healthcare and support.17
There is currently limited evidence on the impact of COVID-19 on depression among Thai patients with low vision. Therefore, the present study aims to examine the association between the impact of COVID-19 on depressive symptoms in patients with low vision and blindness.
Materials and Methods
Participants and Setting
This was a cross-sectional study. The face-to-face interviews were conducted between February 2022 and July 2022 at the eye center, King Chulalongkorn Memorial Hospital, Bangkok, Thailand. Participants were selected using convenience sampling, and they consisted of 284 patients with low vision and blindness who were aged 18 years and above and were willing to participate. Participants with severe psychological illness, auditory deficits, and cognitive impairment were excluded. The WHO defines low vision as best-corrected visual acuity worse than 6/18 to 3/60 in the better eye and blindness as visual acuity worse than 3/60.18
Measurements
Sociodemographic Variables
The sociodemographic variables included age, gender, religion, level of education, monthly income, employment status, marital status, and eye diseases.
The Impact of COVID-19 Questionnaire
The questionnaire used in this study to explore the impact of COVID-19 on visual impairment was developed to suit the Thai culture and local context. It used the Thai language and was based on the patient questionnaire used in the study of the impact of COVID-19 on visual impairment.17 The 23 items for the questionnaire on the impact of COVID-19 on visual impairment belong to five domains including compliance with COVID-19 prevention strategies, accessibility to service or treatment, visibility with COVID-19, visual service and treatment, and anxiety during COVID-19. The responses were scored using a five-point Likert scale from “not at all” to “completely”. The total score ranged from 23 to 115. (Appendix 1). Higher scores indicated a greater impact of COVID-19. In our study, the Cronbach’s alpha internal consistency coefficient was 0.82.
Multi-Dimensional Scale of Perceived Social Support
We employed the Thai version of the Multi-Dimensional Scale of Perceived Social Support (MSPSS) to quantify the degree of social support from family, friends, and others.19 The questionnaire contains 12 items ranked on a seven-point Likert-type scale varying between 1 (very strongly disagree) to 7 (very strongly agree). (Appendix 2). In this research, the Cronbach’s alpha internal consistency coefficient was 0.88.
The Center for Epidemiological Studies-Depression (CES-D)
The Thai version of the Center for Epidemiological Studies-Depression (CES-D) questionnaire20 used in this study includes 20 items for assessing depressive symptoms. Depression refers to a common mood disorder, characterized by sadness, loss of interest or pleasure, feelings of guilt or low self-worth, disturbed sleep or appetite, tiredness, and poor concentration with everyday life.21–23 The study participants were invited to provide information about the frequency of depression symptoms during the previous week. The answers were reported using a five-point Likert scale, where 0 indicated rarely or never and 3 indicated almost all the time. Additionally, the CES-D scores were rated from 0 to 60 with a cut-off point at 16. (Appendix 3). In this research, the Cronbach’s alpha internal consistency coefficient was 0.89.
Data Analysis
SPSS version 28.0 was used to analyze the data. Descriptive analyses were used to establish the characteristics of patients with low vision and blindness and assess the prevalence of depression. Hierarchical linear regression analysis was performed with depressive symptoms as the dependent variable and two models of predictors. The first model included five domains for the impact of COVID-19 (anxiety during COVID-19, compliance with COVID-19 prevention, accessibility to service or treatment, visibility with COVID-19, visual service, and treatment). Social support was included in the second model. To illustrate the effect size changes when adjusting the model’s various variables, we have provided the regression coefficients (standardized β). Statistical significance was set at p-values of <0.05.
Results
Descriptive Statistics
This study involved 284 patients with low vision and blindness, with ages ranging between 18 and 96 years with a mean age of 59.7 years (SD 17.67). Most participants were aged 60 years and above (57.0%), female (58.5%), Buddhist (89.0%), unemployed (63.7%), and married (47.5%). For most patients, the level of education was lower than the bachelor’s degree (79.6%), and they perceived their income to be insufficient (64.1%). For eye diseases, 20.1%, 35.9% and 47.9% of the patients had glaucoma, macular degeneration, and cataract, respectively (Table 1).
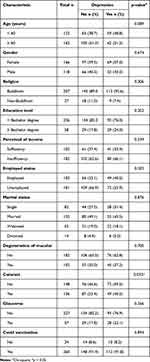 |
Table 1 Demographic Characteristics of the Participants (n = 284) |
Simple Linear Regression Analysis
Simple linear regression analysis showed that the impact of COVID-19 on anxiety during COVID-19 was the strongest predictor of depressive symptoms with standardized β = 0.246 and R2 = 0.060. A negative correlation was found between social support and depressive symptoms with standardized β = −0.141 and R2 = 0.020 (Table 2).
 |
Table 2 Hierarchical Linear Regression Analysis for Predicting Depressive Symptoms |
Hierarchical Linear Regression Analysis
Table 2 shows the strengths of the crude and adjusted associations from the simple and hierarchical linear regression analyses of the covariates and depressive symptoms. The hierarchical linear regression analysis incorporated two blocks of predictors for depressive symptoms as a dependent variable. The final Model II showed that the impact of COVID-19 on anxiety was significantly and positively associated with depressive symptoms (standardized β = 0.240, P <0.001). Compliance with COVID-19 prevention was also significantly and positively associated with depressive symptoms (standardized β = 0.164, P = 0.005). Perceived social support was significantly and negatively associated with depressive symptoms (standardized β = −0.160, P = 0.005).
Discussion
This study was designed to examine the association between COVID-19 and depressive symptoms in patients with low vision and blindness. Our findings indicated associations between perceived social support and depressive symptoms. Notably, we confirmed an association between COVID-19 and depressive symptoms.
In this study, 43.0% of patients with low vision and blindness had experienced depression; 57.0% had not. This was compared to the percentages reported by a study of disabled Ethiopians before COVID-19 (38.3%),24 visual impairment among patients with cataracts in Australia (31.2%),25 older adults with visual impairment in England (29.6%),26 older people with visual impairment in Spain (27.5%),27 older persons with vision loss in Netherlands (26.5%)28 and older adults with visual impairment living in residential care in India (20.9%).29 The prevalence of depression in our study was lower than those reported during the COVID-19 pandemic for older patients with visual impairment in Bangladesh (58.1%)30 and patients with visual impairment in Brazil (53.9%).31
It is well recognized that depression is a key cause of disability globally and contributes to the global burden of disease.32 The risk factors of depression are obvious; however, only a few people clearly understand what makes patients recover, let alone what gives them immunity against depression. A study concluded that social support is important in managing depression.33 Generally, social support includes the perceived and received forms, which refer to the support given to depressed individuals in specific situations and periods. Nonetheless, social support can also be perceived negatively.
Our findings suggested that perceived social support was significantly and negatively associated with depressive symptoms. The findings are supported by reports that greater negative social support and lower positive social support in adults with visual impairment are strong predictors of depression.34 Moreover, our study results are consistent with the reports of previous studies in that satisfaction with available perceived social support is negatively related to depression.35 An earlier study involving adults with visual impairment also indicated that negative social support can lead to higher levels of depression although the severity of vision loss and functional disability are regulated.36
It is well-known that low vision and blindness are key causes of disability. In addition, people with visual challenges are more likely to contract COVID-19 than those with normal vision.11 The current study showed a significantly positive association between COVID-19 and compliance with COVID-19 prevention strategies, anxiety during COVID-19, and depressive symptoms in patients with low vision and blindness. The findings also showed that patients with visual impairment have various shortcomings that potentially make them susceptible to poorer quality of health.37 Moreover, concurrent disabilities were reportedly found in approximately 40% of people with visual challenges; 60% of whom were contracted to COVID-19.38 However, the scope of challenges and effects that may be experienced by people with low vision and visual impairment, especially related to their physical and mental health consequences in the long term, have not been established.13
Among patients with visual impairment, the lack of adequate knowledge about COVID-19 related to its cause, transmission, and strategies for prevention resulting from limited accessibility and lack of suitable media for information access for patients with visual impairment can cause overwhelming stress leading to mental health problems such as depression.13,17 COVID-19-related announcements designed for the general public are not suitable for individuals with visual impairment. Moreover, these patients have challenges in obtaining COVID-19-related information on websites. A study found that visually impaired and blind people have to depend on touch screen technology and physical sensations in their daily life activities, which can increase their risks of COVID-19.13
This study showed that anxiety during COVID-19 was significantly and positively associated with depressive symptoms. The outbreak of COVID-19 has been a significant concern globally. Governments and organizations immediately developed preventive measures, policies, and strategies to address and tackle this new outbreak.39 However, these measures inevitably led to stress related to confusion, fear, anger, insomnia, and anxiety as psychological responses to the impact of COVID-19.40 Additionally, anxiety and depressive disorders, which are common psychiatric illnesses, have frequently been found to co-exist. A global survey reported that approximately 45.7% of patients with lifetime depression have a lifetime history of one or more anxiety disorders.41 Therefore, psychological counseling is recommended to provide affected patients mental care service and decrease the psychological effects of COVID-19.42,43
The two key limitations of our study are as follows. First, as a cross-sectional design was adopted, we inferred association between the predictor variables and depressive symptoms. It has to be acknowledged that the risk factors and outcomes are measured concurrently in a cross-sectional survey. Therefore, the order of exposure and disease is not easy to determine. Nonetheless, under certain circumstances a cross-sectional design can be applicable when studying potential associations. For example, if the exposure is assumed to be stable over time, a cross-sectional design may be valid. Second, the convenience sampling technique was used to enlist participants; consequently, our samples may not be representative of the population. Convenience sampling involves stopping people at random, which means that not everyone has an equal chance of being selected depending on the place, time, or day the data are collected. We acknowledge that using convenience sampling approach does not allow us to generalize the result to a larger population. However, in some cases, it is the only feasible option that can be employed. Moreover, it may sometimes be the only viable method when a researcher cannot obtain contact details for individuals in a large population. In this case, convenience sampling was the viable option.
Conclusion
Our study showed that COVID-19 significantly increased depressive symptoms among people with low vision and blindness. Because the mental health effects of COVID-19 are recognized as evolving public health issues, preventive measures should be developed and mental health care provision should be improved. A key strategy for tackling this problem is the efficient collaboration between public health service providers and organizations to provide care for individuals mentally and physically affected by the pandemic.
Data Sharing Statement
Data is available upon request to the corresponding author.
Ethics Approval and Informed Consent
This study was approved by the Institutional Review Board (IRB) Human Research, Faculty of Medicine, Chulalongkorn University (COA No. 1638/2021) on November 8, 2021. To protect the identities of the participants and maintain confidentiality, written consent was obtained before the interview. For those unable to read owing to their vision, the interviewer read the research objectives and details of the consent form. Those who agreed to participate provided the verbal consent bearing a witness signature. This study has been performed in accordance with the principles stated in the Declaration of Helsinki.
Acknowledgments
The authors would like to acknowledge the 100th Anniversary Chulalongkorn University Fund for Doctoral Scholarship and the 90th Anniversary Chulalongkorn University Fund (Ratchadapiseksomphot Endowment Fund) for supporting this research project. We also thank Ms. Reberta Adele Steer Huthart for her kind support during this research.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Cucinotta D, Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomed. 2020;91(1):157–160. doi:10.23750/abm.v91i1.9397
2. Choi E, Lee J, Lee SA. Validation of the Korean version of the obsession with COVID-19 scale and the coronavirus anxiety scale. Death Stud. 2022;46(3):608–614. doi:10.1080/07481187.2020.1833383
3. World Health Organization. COVID-19 pandemic triggers 25% increase in prevalence of anxiety and depression worldwide; 2022. Available from: https://www.who.int/news/item/02-03-2022-covid-19-pandemic-triggers-25-increase-in-prevalence-of-anxiety-and-depression-worldwide.
4. Ministry of Public Health. Strategic plan: Covid-19. Ministry of Public Health; 2021. Available from: en_ThailandCovid-19plan_MOPH_2021.pdf.
5. Javed B, Sarwer A, Soto EB, et al. The coronavirus (COVID-19) pandemic’s impact on mental health. Int J Health Plann Manage. 2020;35(5):993–996. doi:10.1002/hpm.3008
6. Singh S, Roy D, Sinha K, et al. Impact of COVID-19 and lockdown on mental health of children and adolescents: a narrative review with recommendations. Psychiatry Res. 2020;293:113429. doi:10.1016/j.psychres.2020.113429
7. Javed B, Sarwer A, Soto EB, Mashwani ZU. Is Pakistan’s response to coronavirus (SARS-CoV-2) adequate to prevent an outbreak? Front Med. 2020;21(7):158. doi:10.3389/fmed.2020.00158
8. Oxford Policy Management. Social impact assessment of COVID-19 in Thailand. Oxford Policy Management Limited; 2022. Available from: https://www.unicef.org/thailand/media/5071/file/SocialImpactAssessmentofCOVID-19inThailand.pd.
9. Srichannil C. The COVID-19 pandemic and Thailand: a psychologist’s viewpoint. Psychol Trauma. 2020;12(5):485–487. doi:10.1037/tra0000808
10. Wang C, Pan R, Wan X, et al. Immediate psychological responses and associated factors during the initial stage of the 2019 Coronavirus Disease (COVID-19) epidemic among the general population in China. Int J Environ Res Public Health. 2020;17(5):1729. doi:10.3390/ijerph17051729
11. Armitage R, Nellums LB. The COVID-19 response must be disability inclusive. Lancet Public Health. 2020;5(5):e257. doi:10.1016/S2468-2667(20)30076-1
12. Bourne RRA, Flaxman SR, Braithwaite T, et al. Magnitude, temporal trends, and projections of the global prevalence of blindness and distance and near vision impairment: a systematic review and meta-analysis. Lancet Glob Health. 2017;5(9):e888–e897. doi:10.1016/S2214-109X(17)30293-0
13. Senjam SS. Impact of COVID-19 pandemic on people living with visual disability. Indian J Ophthalmol. 2020;68(7):1367–1370. doi:10.4103/ijo.IJO_1513_20
14. Evans JR, Fletcher AE, Wormald RP. Depression and anxiety in visually impaired older people. Ophthalmology. 2007;114(2):283–288. doi:10.1016/j.ophtha.2006.10.006
15. Demmin DL, Silverstein SM. Visual impairment and mental health: unmet needs and treatment options. Clin Ophthalmol. 2020;14:4229–4251. doi:10.2147/OPTH.S258783
16. World Health Organization. International classification of diseases. World Health Organization; 2019. Available from: https://icd.who.int/browse10/2019/en.
17. Shalaby WS, Odayappan A, Venkatesh R, et al. The impact of COVID-19 on individuals across the spectrum of visual impairment. Am J Ophthalmol. 2021;227:53–65. doi:10.1016/j.ajo.2021.03.016
18. Dandona L, Dandona R. Revision of visual impairment definitions in the international statistical classification of diseases. BMC Med. 2006;4:7. doi:10.1186/1741-7015-4-7
19. Wongpakaran T, Ruktrakul R, Ruktrakul R. Reliability and validity of the Multidimensional Scale of Perceived Social Support (MSPSS): Thai version. Clin Pract Epidemiol Ment Health. 2011;7:161–166. doi:10.2174/1745017901107010161
20. Trangkasombat U, Larpboonsarp V, Havanond P. CES-D as a screen for depression in adolescents. J Psychiatr Assoc Thailand. 1997;42(1):2–13.
21. Salik I, Marwaha R. Electroconvulsive therapy. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022.
22. Singh R, Volner K, Marlowe D. Provider burnout. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022.
23. The National Institute of Mental Health. Depression; 2018. Available from: https://www.nimh.nih.gov/health/topics/depression/index.shtml.
24. Necho M, Birkie M, Gelaye H, et al. Depression, anxiety symptoms, insomnia, and coping during the COVID-19 pandemic period among individuals living with disabilities in Ethiopia, 2020. PLoS One. 2020;15(12):e0244530. doi:10.1371/journal.pone.0244530
25. Palagyi A, Rogers K, Meuleners L, et al. Depressive symptoms in older adults awaiting cataract surgery. Clin Exp Ophthalmol. 2016;44(9):789–796. doi:10.1111/ceo.12800
26. Jackson SE, Hackett RA, Pardhan S, et al. Association of perceived discrimination with emotional well-being in older adults with visual impairment. JAMA Ophthalmol. 2019;137(7):825–832. doi:10.1001/jamaophthalmol.2019.1230
27. Gonzales-Turín JM, Rodríguez-Laso Á, Carnicero JA, et al. Relationship between self-reported visual impairment and worsening frailty transition states in older people: a longitudinal study. Aging Clin Exp Res. 2021;33(9):2491–2498. doi:10.1007/s40520-020-01768-w
28. van Nispen RM, Vreeken HL, Comijs HC, et al. Role of vision loss, functional limitations and the supporting network in depression in a general population. Acta Ophthalmol. 2016;94(1):76–82. doi:10.1111/aos.12896
29. Marmamula S, Kumbham TR, Modepalli SB, et al. Depression, combined visual and hearing impairment (dual sensory impairment): a hidden multi-morbidity among the elderly in residential care in India. Sci Rep. 2021;11(1):16189. doi:10.1038/s41598-021-95576-5
30. Rahman MS, Rahman MA, Ali M, et al. Determinants of depressive symptoms among older people in Bangladesh. J Affect Disord. 2020;264:157–162. doi:10.1016/j.jad.2019.12.025
31. Lopes N, Dias L, Ávila M, et al. Humanistic and economic burden of blindness associated with retinal disorders in a Brazilian sample: a cross-sectional study. Adv Ther. 2021;38(8):4215–4230. doi:10.1007/s12325-021-01672-3
32. World Health Organization. Depression; 2021. Available from: https://www.who.int/news-room/fact-sheets/detail/depression.
33. Gariépy G, Honkaniemi H, Quesnel-Vallée A. Social support and protection from depression: systematic review of current findings in Western countries. Br J Psychiatry. 2016;209(4):284–293. doi:10.1192/bjp.bp.115.169094
34. Papadopoulos K, Papakonstantinou D, Montgomery A, et al. Social support and depression of adults with visual impairments. Res Dev Disabil. 2014;35(7):1734–1741. doi:10.1016/j.ridd.2014.02.019
35. Guerette AR, Smedema SM. The relationship of perceived social support with well-being in adults with visual impairments. J Vis Impair Blind. 2011;105(7):425–439. doi:10.1177/0145482X1110500705
36. Cimarolli VR. Perceived overprotection and distress in adults with visual impairment. Rehabil Psychol. 2006;51(4):338–345. doi:10.1037/0090-5550.51.4.338
37. Swenor BK, Lee MJ, Tian J, et al. Visual impairment and frailty: examining an understudied relationship. J Gerontol a Biol Sci Med Sci. 2020;75(3):596–602. doi:10.1093/gerona/glz182
38. Rosenblum LP. Unprecedented times call for unprecedented collaboration: how two COVID-19 surveys were created with input from across the field of visual impairment to analyze the needs of adults, students, teachers, and orientation and mobility practitioners. J Vis Impair Blind. 2020;114(3):237–239. doi:10.1177/0145482X20927129
39. Batista P, Duque V, Luzio-Vaz A, et al. Anxiety impact during COVID-19: a systematic review. J Infect Dev Ctries. 2021;15(3):320–325. doi:10.3855/jidc.12730
40. Brooks SK, Webster RK, Smith LE, et al. The psychological impact of quarantine and how to reduce it: rapid review of the evidence. Lancet. 2020;395(10227):912–920. doi:10.1016/S0140-6736(20)30460-8
41. Kessler RC, Sampson NA, Berglund P, et al. Anxious and non-anxious major depressive disorder in the World Health Organization world mental health surveys. Epidemiol Psychiatr Sci. 2015;24(3):210–226. doi:10.1017/S2045796015000189
42. Yang Y, Peng F, Wang R, et al. The deadly coronaviruses: the 2003 SARS pandemic and the 2020 novel coronavirus epidemic in China. J Autoimmun. 2020;109:102434. doi:10.1016/j.jaut.2020.102434
43. Liu K, Chen Y, Wu D, et al. Effects of progressive muscle relaxation on anxiety and sleep quality in patients with COVID-19. Complement Ther Clin Pract. 2020;39:101132. doi:10.1016/j.ctcp.2020.101132
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
