Back to Journals » Integrated Pharmacy Research and Practice » Volume 11
Impact of Clinical Pharmacist-Led Interventions on Drug-Related Problems Among Pediatric Cardiology Patients: First Palestinian Experience
Authors Elhabil MK, Yousif MA, Ahmed KO , Abunada MI, Almghari KI, Eldalo AS
Received 11 May 2022
Accepted for publication 12 August 2022
Published 26 August 2022 Volume 2022:11 Pages 127—137
DOI https://doi.org/10.2147/IPRP.S374256
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Jonathan Ling
Mohammed Kamel Elhabil,1 Mirghani Abdelrahman Yousif,1 Kannan O Ahmed,1 Mohamed Ibrahim Abunada,2 Khaled Ismail Almghari,3 Ahmed Salah Eldalo3
1Department of Clinical Pharmacy and Pharmacy Practice, Faculty of Pharmacy, University of Gezira, Wad Medani, Sudan; 2Department of Pediatrics, Faculty of Medicine, Islamic University of Gaza, Gaza, Palestine; 3Department of Pharmacy, Faculty of Medicine and Health Sciences, University of Palestine, Gaza, Palestine
Correspondence: Mohammed Kamel Elhabil, Department of Clinical Pharmacy and Pharmacy Practice, Faculty of Pharmacy, University of Gezira, Hospital Street No. 1, Wad Medani, 21112, Sudan, Tel/Fax +249 0511842726, Email [email protected]
Background: Discovery and resolution of drug-related problems (DRPs) are taken as the cornerstone in the entire pharmaceutical care process to improve patient outcomes. Very limited reports on the analysis of DRPs in pediatric cardiology have been released worldwide.
Objective: The aim of this study was to disclose the impact of clinical pharmacist’s interventions on DRPs among pediatric cardiology patients in Palestine.
Methods: Between January and September 2021, a prospective interventional study involving clinical pharmacist’s care was implemented in the cardiology ward of Al-Rantisy Specialized Pediatric Hospital in Gaza, Palestine. Pharmaceutical Care Network Europe model 9.1 was used to identify DRPs, causes of the problem, clinical pharmacist’s interventions, cardiologist’s acceptance, and outcomes.
Results: A total of 309 DRPs were identified in 87 patients, representing a mean of 3.55 problems per patient. The most common DRPs were “Treatment effectiveness” (50.8%) and “Treatment safety” (30.4%), while the main causes of these DRPs were “Errors in dose timing instructions” (9.4%) and “Inappropriate combination of drugs” (13.7%), respectively. Analysis revealed that 96.7% of the interventions suggested by the clinical pharmacist were accepted by cardiologists and that 92.1% of problems were fully resolved with improved patient outcomes.
Conclusion: Interventions offered by the clinical pharmacist successfully addressed DRPs and positively impacted treatment outcomes in pediatric cardiology patients. With the high acceptance of pediatric cardiologists to the clinical pharmacist’s experience in Palestine, there is a growing need to integrate clinical pharmacists into cardiology teamwork care to optimize drug therapy and patient safety.
Keywords: clinical pharmacist, drug-related problems, pediatric cardiology, Palestine
Introduction
Cardiovascular disease (CVD) is more prevalent in pediatric patients and has a negative outcome.1 In the United State, nine out of every thousand newborns have a congenital heart condition, and 2.3 out of every thousand will undergo a surgical procedures or die during their first year of life.2 A variety of cardiovascular drugs are also among the most common causes of numerous types of drug-related problems (DRPs).3 The increased choice and complexity present in this medication group are likely to explain these problems.4
Based on the Pharmaceutical Care Network Europe (PCNE), DRPs are “events or circumstances involving pharmacotherapy that actually or potentially interfere with desired health outcomes”.5 In 2020, the updated model 9.1 of the PCNE allotted to every DRP 5 complementary areas: the problem, the cause of the problem, the intervention had to solve or prevent the problem, the acceptance of the intervention, and the outcome.5,6
Previous reports have indicated the undesirable effect of DRPs on pediatric patients.7 Increased admissions and readmissions to emergency wards, extended ward stays, more prescriptions, and extra healthcare charges had been effects of DRPs.8 The incidence of DRPs in pediatric patients ranges from 25.6 to 59.8%,9,10 with reported incidences of up to 76.4% in children with cardiovascular diseases.11 The cornerstone of a clinical pharmacist’s intervention is the discovery, identification, and resolution of DRPs.12 Even optimization of pharmacotherapy by a clinical pharmacist can be assessed by the overall number of DRPs treated or averted and by the assessment of patient outcomes.13
Very few publications were found in the literature that assessed the impact of a clinical pharmacist on DRPs among cardiology children worldwide. Most of these publications were retrospective, only assessed the characteristics of DRPs without clinical pharmacist involvement within healthcare teams in rounds, and the majority of studies were conducted in adult and outpatient settings.12,14,15,16 In Palestine, the implementation of clinical pharmacy service in the healthcare structure, especially in cardiology, is still in its preliminary stage until now.16,17 Thus, the aim of this prospective interventional study was to evaluate the impact of clinical pharmacist’s interventions on drug-related problems among pediatric cardiology patients admitted to Al-Rantisy Specialized Pediatric Hospital in Gaza, Palestine.
Methods
Study Setting and Design
This was a prospective interventional study design. The study was conducted over nine months from January to September 2021 in the cardiology ward of Al-Rantisy Specialized Pediatric Hospital. This 90-bed governmental hospital is the only tertiary care center, fully equipped with advanced medical services for the pediatric population, located in Gaza city, Palestine.
Ethical Approval
Permission to use the PCNE V.9.1 was obtained from PCNE-DRP group by contacting them through their official website. Furthermore, ethical approval (No, PHRC/HC/787/20) was obtained from the Palestinian Health Research Council in accordance with the Declaration of Helsinki. Additionally, written informed consent was also obtained from a parent or legal guardian of the patient to participate in the study prior to data collection. Data confidentiality and security were ensured throughout the study period.
Data Collection Procedures
All medical files of patients admitted to the cardiology ward were routinely screened by a registered clinical pharmacy specialist with three years experience in pediatric cardiology. A specially designed form was used to collect data on demographic details, complaint presentation, previous medical and medication history, diagnosis, prescribed medications, length of stay, and laboratory findings for each patient. Data were collected prospectively by the clinical pharmacist from medical records, patient/parent interviews, cardiologists’ rounds, and the multidisciplinary discussion on each patient. Patients attended the emergency ward without admission, were hospitalized for diagnostic purposes only, and those who died during hospitalization were excluded.
To discover any potential drug-related problem, the clinical pharmacist assessed therapeutic effectiveness, dosage regimen, drug safety, unnecessary treatment, drug availability, incompatibilities, dose adjustments, and cost-appropriateness. In this regard, the latest therapeutic strategies recommended in the evidence-based guidelines and the latest online version of Medscape Drugs and Diseases (Copyright © 1994–2021 by WebMD LLC) and UpToDate® (by Walters Kluwer) have been used. Also, Lexicomp® software (by Wolters Kluwer) was used to identify drug interactions within each patient’s prescribed medications.
Discovered DRPs were peer-reviewed and validated by an expert focus group consisting of two professors of clinical pharmacy, a pediatric consultant, and the clinical pharmacist who reported the data. Consensus discussion and agreement within the group sought to reach a final decision on the identification and resolution of DRPs. During daily ward rounds and whenever needed, the clinical pharmacist led a discussion with the cardiology healthcare team to make appropriate recommendations for managing drug therapy problems. Cardiologists’ acceptance of intervention was documented, and the outcome was also evaluated by the focus group.
Identified DRPs were then categorized using the Pharmaceutical Care Network Europe (PCNE) model 9.1.5 The PCNE system allotted to every DRP five complementary areas: (1) Problems involved three domains including treatment effectiveness, treatment safety, and others; (2) The causes of the problem were divided into nine domains, and they were related to drug selection, drug form, dose selection, duration of treatment, dispensing, drug administration process, patients, patient transfer, and others; (3) Clinical pharmacist’s interventions needed to resolve the discovered problem were classified in five domains as interventions made at the level of the prescriber, at the level of the patient, and at the level of drug, in addition to those categorized as others and non-intervention; (4) Acceptance of the intervention falls into three domains as accepted, not accepted, and unknown; (5) Outcomes of clinical pharmacist’s interventions were ranked in four domains as problems were fully resolve, partially solved, and unresolved, or the outcome was unknown.
Data Analysis
Data were analyzed using the Statistical Package for the Social Sciences (SPSS), Version 21.0 (IBM Corporation, Armonk, New York, USA), and summarized in tables and pie chart. Descriptive analysis was performed to show the mean, standard deviation (SD), and the range of different characteristics. Frequency of categorical variables was reported as a percentage of the total [n (%)].
Results
During the 9-month study, the clinical pharmacist closely followed a total of 110 cases. Among them, 87 experienced DRPs were included in this study. The mean age of the cases at first hospitalization was 2.32 years (range, 2 months −11 years), and 58.6% (n=51) were males. The total number of prescriptions was 548, which comprised a mean of 5.27 drugs per case (ranged, 3–14 different drugs). Five or more medications were prescribed daily in 57.5% (n=50) of cases and fewer than five medications were prescribed in 42.5% (n= 37) of cases (Table 1).
 |
Table 1 Demographic and Clinical Characteristics of the Study Cases |
Types of Discovered Drug-Related Problems
A total of 309 DRPs were identified in the 87 cases of the present study, representing a mean of 3.55 problems per case. Among these DRPs, the main problem was “P1 -Treatment effectiveness” (50.8%, n=157). The major subcategory of this problem was “P1.2 Effect of drug treatment not optimal” (36.2%, n=112). The next subcategory was “P1.3 Untreated symptoms or indication” (10.4%, n=32). “P1.1 No effect of drug treatment despite correct use” (4.2%, n=13) constituted the third subcategory. The different types of DRPs are given in Table 2. The second identified DRP was “P2 - Treatment safety” including “P2.1 possibly occurring adverse drug event” (30.4%, n=94). DRPs classified as “P3 – Others” represented 18.8% (n=58) of all detected DRPs and they related to those issues that were not included in any category of the PCNE classification system. “P3– Others” problems comprise two subcategories. First; “P3.1 Unnecessary drug-treatment” (8.7%, n=27). Second; “P3.2 Unclear problem/complaint” (10.1%, n=31) which can be expressed by: (1) Missing prescribing data; (2) Non-compliance with treatment; (3) Physical drug incompatibilities; and (4) Pharmaceutical logistic problems.
 |
Table 2 Types of Drug-Related Problems According to the PCNE V9.137 |
Major Drugs Involved in Drug-Related Problems
As shown in Table III, the ten major drugs were involved in 77.0% (n=238 out of 309) of the DRPs that occurred during this study. The drugs most commonly related to DRPs were furosemide (17.1%), captopril (13.6%), and spironolactone (10.0%). Other drugs are illustrated in Table 3.
 |
Table 3 Major Drugs Involved in Drug-Related Problems |
Relationship Between Drug-Related Problems and Causes
Analysis indicated that 329 causes were involved in the occurrence of the 309 DRPs detected in the cases as demonstrated in Table 4. The main causes of “P1 - Treatment effectiveness” were “C3.5 Dose timing instructions errors” (9.4%, n = 31), “C2.1 Inappropriate drug form” (8.5%, n = 28), and “C1.5 No or incomplete drug treatment” (8.2%, n = 27). Nine causes led to “P2 - Treatment safety” and the most common cause was “C1.3 Inappropriate combination of drugs, or drugs and food” (13.7%, n = 45). The DRP classified as “P3 – Others” was mainly caused by “C1.2 No indication for drug” (4.3%, n=14), “C2.1 Inappropriate drug form” (3.0%, n=10), and “C4.2 Duration of treatment too long” (2.7%, n=9).
 |
Table 4 Relationship Between Drug-Related Problems and Causes According to the PCNE V9.137 |
Level of Clinical Pharmacist’s Interventions in Drug-Related Problems
A total of 329 interventions were performed by the clinical pharmacist to manage the 309 DRPs found in the study cases. The highest proportion 47.7% (n=157) of the interventions were done at the “prescriber level”, where the clinical pharmacist discussed these interventions with the prescriber comprehensively as shown in Figure 1. The next common interventions were made at the “drug level” (39.2%, n = 129). This was followed by interventions provided at the patient/caregiver level (13.1%, n = 43), in which the clinical pharmacist’s recommendations were made by speaking directly to a family member/caregiver thoroughly.
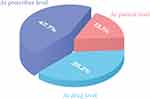 |
Figure 1 Level of interventions in drug-related problems according to the PCNE V9.1. |
Acceptance of the Clinical Pharmacist’s Interventions
As indicated in Table 5, 329 interventions were made by the clinical pharmacist and appropriate recommendations on drug therapy changes were suggested to the prescribers. The overall acceptance rate was 96.7% (n=318). Of these interventions, 86.9% (n=286) were accepted and fully implemented, 5.8% (n=19) were accepted and partially implemented, and 4.0% (n=13) were accepted but not implemented by the prescribers. Only 3.3% (n=11) of all interventions were not accepted by the prescribers. These were either due to disagreement with the clinical pharmacist’s suggestion (1.5%, n=5) or to other reasons (1.8%, n=6); such as unavailability of the prescribed drug, the comparative cost of the drug, and inappropriate drug form for the patient.
 |
Table 5 Acceptance and Outcome of Clinical Pharmacist’s Intervention According to the PCNE V9.137 |
Outcomes of Clinical Pharmacist’s Interventions
Analysis revealed that 92.1% (n=303) of the problems were totally resolved and positively impacted the patients’ outcomes (Table 5). Interestingly, in the majority (n = 17 out of 19) of accepted and partially implemented interventions, problems were totally resolved resulting in an increase in the total percentage of problems solved. In 3.4% (n=11) of cases, the problems were partially solved. However, in 0.9% (n=3) of cases, the problem status was unknown due to the patient’s discharge and loss of follow-up by the clinical pharmacist. Only 3.6% (n=12) of the problems were not resolved. Out of them, 0.9% (n=3) did not resolve problems due to caregivers/patients’ noncompliance. In 1.5% (n=5) of the interventions, problems were not resolved due to the non-consent of the prescriber. Also, in 1.2% (n=4) of the problems, there were no problem-solving possibilities; such as prescribing bosentan 62.5mg film-coated tablets to a patient with pulmonary arterial hypertension, which was not available at the time of prescription.
Discussion
Pediatric patients receiving cardiovascular medications are at risk of different types of drug-related problems (DRPs).3,11 Few studies have been published on the analysis of DRPs among hospitalized pediatric cardiology patients. Most publications, however, were conducted in the adult population.3,8 In Palestine, the integration of clinical pharmacists in the healthcare field especially in cardiology is limited till now.16 Therefore, the aim of this prospective interventional study was to explore the role of the clinical pharmacist in detecting and identifying DRPs and to assess his capacity to resolve these DRPs by making appropriate interventions at each stage in the pediatric cardiology ward.
Discovery and resolution of DRPs by the clinical pharmacist are taken as a cornerstone in the entire pharmaceutical care process to improve patient outcomes.18 A Brazilian study was performed in an educational hospital to evaluate the characteristics of DRPs in newborns with various heart diseases.11 The study reflected that the main identified problem was “P1: Treatment effectiveness” (49.0%), followed by “P2: Treatment safety” (46.7%), and “P3: Others” (3.3%). Our results came in the same order as their DRPs, with a relatively similar percentage of the “P1 -Treatment effectiveness” (50.8%), but we also observed a lower percentage of the “P2 - Treatment safety” (30.4%) than the one obtained by the Brazilian study. This low incidence of adverse events may be explained by the direct participation of a well-experienced clinical pharmacist in the cardiology team who made appropriate recommendations on the safe use of medications by the updated guidelines. In our study, also, a higher proportion (18.8%) of the problem classified as “P3 others” which included “P3.1 Unnecessary drug-treatment” (8.7%), and “Unclear problem/complaint” (10.1%) compared to those obtained by the Brazilian study.11 The following explanations can be taken into account to discuss this high rate of the “P3 others” problem. The first refers to the methodology currently in use that highlighted the identification of this “P3 other” problem that paid less attention before and was not defined entirely by the “PCNE classification system”. This contributed to the identification of the unclear problem known as “P3.2: Unclear problem/complaint”. The second explanation can be attributed to the identification of “P3.1: Unnecessary drug-treatment”, through a real role of the clinical pharmacist within the cardiology team in discontinuing the drugs without indication, inappropriately duplicated, and those with too long duration. The current data offers a significant need for the clinical pharmacist to integrate his knowledge within the medical team to stop unnecessary drug orders and enhance medication safety as indicated in previous publications.19–21
Furosemide (17.1%), captopril (13.6%), and spironolactone (10.0%) were the most frequent drugs combined with DRPs. Compared with the present study, a study by Nascimento et al in cardiology neonates showed that the most common drugs in DRPs were vancomycin (10.2%), meropenem (8.0%), and furosemide (7.1%).11 The difference in the diagnosis, the treatment guidelines used, and the level of the clinical pharmacist’s intervention could explain the difference between the two studies.
The analysis identified the main causes involved in the occurrence of DRPs. Overall, the major DRP being “Treatment effectiveness” was mainly caused by “Errors in dose timing instructions (wrong, unclear or missing)”, “Inappropriate drug form”, and “No or incomplete drug treatment”. The most likely explanation for this relationship may be attributed to insufficient up-to-date knowledge of the physician in the pharmacotherapy of pediatrics. To fill this gap, the clinical pharmacist should be incorporated as an integral member of the cardiology teamwork care to make appropriate recommendations on drug therapy during ward rounds and wherever needed.22,23 Between May 2016 and April 2018, a prospective study was conducted in a Brazilian teaching hospital among adult patients admitted to medical wards including cardiology, by reviewing medication orders possibly containing DRPs by clinical pharmacists.8 The most important difference observed between our study and the Brazilian study is the level of the DRP cause known as “Inappropriate drug form”, which was higher in this study compared to the Brazilian one (8.5%, n=28 versus 0.21%, n=7). The main justification for this difference is that the current study included children and that most oral medications available in hospital pharmacies and/or community pharmacies as solid dosage forms were difficult for children to take. Drug use in children may be accompanied by problems not seen in adults,24 due to differences in pharmacodynamic and pharmacokinetic properties.25
It is important to note that drug and/or drug and food interactions (13.7%) were the main cause of adverse drug events (ADEs) in patients of this study. Similarly, the findings of an Egyptian study involving pediatric heart patients indicated that drug-drug interaction was the primary reason responsible for the occurrence of ADEs.15 Also, an analysis conducted among Indian pediatric patients reflected that the majority of prescription-identified DRPs were associated with drug-drug interactions.26 These findings raise an urgent call for paying attention to this problem to improve drug safety in children with cardiovascular diseases. Among the recent approaches for detecting drug-drug/food interactions, the application of a computerized prescribing system that has shown a beneficial role in reducing the error rate during drug prescribing.27,28
Additionally, the current study identified the main causes of the two DRP subcategories classified as “P3 – Others” (“unnecessary drug treatment” and “unclear problem/complaint”). “Unnecessary drug treatment” was mainly caused by no indication for the drug and the prolonged duration of treatment. This was supported by the findings of Xiao-Xiao et al.19 Previous research has shown that physicians always exaggerate the healthcare they want properly for their patients. The doctor usually wants the treatment to cover all aspects of the existing disease and the patient wants to care, even if it is not necessary.29,30 The main consequence of unnecessary treatment is not only more patient exposure to ADEs but also an increase in the overall cost of pharmacotherapy.31 The most likely solutions suggested by Lyu et al to this unnecessary therapy were training physicians in good prescribing, access to medical records, and more availability of new treatment guidelines.30
Regarding the causes behind the unclear problem/complaint, the majority were related to an inappropriate drug form, unavailable medication, and missing dose prescribing. This is similar to the results reported by Saldanha et al, (2020).8 In Palestine as a developing country, there is a persistent problem of unavailability of essential medicines in hospitals and medical healthcare centers affiliated with the Ministry of Health, which causes a real problem in the optimal treatment of patients, especially those hospitalized in pediatric cardiology wards.32 At the end of 2020, 47% of essential medicine stock was zero, resulting in inadequate medical care for the most vulnerable patients. Add to that, during our study, 50% of the medical staff were allocated for emergency treatment of COVID-19, adding to the burden on the already overwhelmed Palestinian MOH.32 To bypass the problem of drug unavailability, the doctor chose an alternative drug that might be less effective or compelled the patient’s family to purchase the medication.
The present data indicated that 329 interventions were provided by the clinical pharmacist to manage the 309 DRPs identified in the study cases. The majority of these interventions were thoroughly discussed with the physicians, followed by interventions performed at the “drug level” and then at the “patient level”. Parallel to our results, a new Turkish study was conducted in 2021 to evaluate the impact of clinical pharmacists’ interventions and found that most interventions were performed at the prescriber level followed by the drug level and the patient level.33 Two possible factors can be proposed to explain such a higher intervention on the prescriber. First, the clinical pharmacist was aware of the importance of direct intervention with the prescriber as the decision-maker throughout the patient therapeutic process. Second, the physician recognition of the complementary role of the clinical pharmacist in bridging the gap between himself and the patient to address DRPs and improve patient outcomes. These interpretations were supported by the study of Francis and Abraham (2014).22
Contrastingly, a study by Khan and Ahmad (2014) to identify DRPs in a Pakistani teaching hospital found that most recommendations were implemented at the drug level (50.93%), followed by those at the prescriber (40.37%). However, no recommendations were given to patients.34 Moreover, another study from India conducted in a tertiary care hospital showed that the recommendations of the clinical pharmacist were not implemented at the patient level. This indicates the possible lack of communication between the clinical pharmacist and the patient.35 Differently, the present study showed the direct relationship between the clinical pharmacist and the caregiver of the patient who took special care during his daily duties. This was to achieve patient involvement in the entire treatment process to improve medication adherence, otherwise, the treatment cycle could fail.
One of the best findings observed in this study was the high cardiologist’s acceptance of the interventions suggested by the clinical pharmacist throughout the therapeutic process. The overall acceptance rate was 96.7%. Similar data with this high acceptance rate has been very limited across the pediatric cardiology literature. Yet, a lower acceptance rate was demonstrated in previous studies published in different countries. It was 92.1% in a Brazilian study of cardiac newborns in a maternity education hospital,11 and only 65% in an Egyptian study of pediatric cardiology patients.15 The current data strongly reflect that the clinical pharmacist has been recognized by cardiologists as an integral part of the multidisciplinary team especially during the ward round, as he can identify most DRPs while prescribing and providing real-time consultations and recommendations to cardiologists. More importantly, the clinical pharmacist has become the cornerstone of providing reliable information on drug therapy at the pediatric cardiology ward in Palestine. This points to the clinical pharmacist’s ability to integrate his knowledge into the cardiology ward previously held the ultimate authority of cardiologists.
Additional important results recorded in the study were the outcomes of the clinical pharmacist’s interventions. The analysis revealed that 92.1% (n=303) of the problems were completely resolved and positively impacted the patients’ condition. In 3.4% (n=11) of cases, the problems were partially resolved and only 3.6% (n=12) of the problems were not resolved. Resolution of DRPs identified in this study is attractively higher than that previously shown in different studies.26,34,36 Law et al conducted a study to assess the effect of clinical pharmacists’ recommendations among pediatric patients. The authors concluded that 89% of the problems were fully resolved while the remnants were partially resolved or the outcome was unknown.36 Another study was conducted by Daniel et al to identify DRPs among Indian pediatric patients at a tertiary care hospital. It recorded a relatively low resolution of the problems as follows; only 34.82% of the DRPs were fully resolved, 21.65% were partially resolved, and 43.53% of the outcome were considered unknown.26 The optimal outcomes observed in this study can be attributed to the fact that the clinical pharmacist always seeks up-to-date treatment references and guidelines when providing information about pharmacotherapy, as he investigates the problem as a whole to arrive at effective solving measures.
It is worth saying that the reasons behind the strengths of the present study are; First, this is the first project that prospectively investigated the impact of including a clinical pharmacist in pediatric cardiology care in the Palestinian health system. Second, the intervention process was comprehensive, as the clinical pharmacist’s attention was focused on the prescriber, the patient, and the drug, and none of the three levels was neglected, while most previous studies did not evaluate these three levels at the same time. Third, the excellent acceptance of the clinical pharmacist’s interventions by cardiologists and the successful implementation of his recommendations. Moreover, the hospital support and collaboration between the hospital director, the hospital pharmacy director, the head of the pediatric cardiology ward, and the nursing staff, all shared the success.
Limitation of the Study
Data were collected by one clinical pharmacist due to the limited number of clinical pharmacists employed in hospitals by the Palestinian Ministry of Health. However, two professors of clinical pharmacy oversaw the process of data collection, interpretation of results, and reporting of outcomes throughout the course of the study.
Also, a limitation in the distribution of the findings is that the current study was implemented in a single pediatric hospital, although it intersects a number of characteristics common in children’s hospitals worldwide. These include being a tertiary pediatric care center, having advanced medical services, and providing specialized cardiology services in Palestine. More multicenter studies in a similar discipline are warranted to extend the results to the entire population.
Conclusion
The current Palestinian study revealed that DRPs were common across treatment effectiveness and treatment safety, and were mainly caused by inappropriate dose timing instructions and drug combination. The drugs mostly involved were furosemide, captopril, and spironolactone. DRPs were discovered early and effectively managed by the clinical pharmacist among pediatric cardiology patients. The acceptance rate of clinical pharmacist’s interventions was relatively high with a beneficial resolution of DRPs and improvement of patient outcomes. The observed positive impact as well as the good practitioner’s acceptance of this first Palestinian experience of the clinical pharmacist’s contribution to pediatric cardiology gives health policymakers support to incorporate clinical pharmacists into team-based cardiology care and expand their role in other specialties.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Ahmad FB, Anderson RN. The leading causes of death in the US for 2020. JAMA. 2021;325(18):1829–1830. doi:10.1001/jama.2021.5469
2. Watt K, Li J, Benjamin DK, et al. Pediatric cardiovascular drug dosing in critically ill children and extracorporeal membrane oxygenation. J Cardiovasc Pharmacol. 2011;58(2):126–132. doi:10.1097/FJC.0b013e318213aac2
3. Gelchu T, Abdela J. Drug therapy problems among patients with cardiovascular disease admitted to the medical ward and had a follow-up at the ambulatory clinic of Hiwot Fana Specialized University Hospital: the case of a tertiary hospital in eastern Ethiopia. SAGE Open Med. 2019;7:2050312119860401. doi:10.1177/2050312119860401
4. Tigabu BM, Daba D, Habte B. Drug-related problems among medical ward patients in Jimma university specialized hospital, Southwest Ethiopia. J Res Pharm Pract. 2014;3(1):1. doi:10.4103/2279-042X.132702
5. PCNE. Classification for drug-related problems V9.1; 2020. Available from: https://www.pcne.org/upload/files/417_PCNE_classification_V9-1_final.pdf.
6. Ahmed KO, Muddather HF, Yousef BA. Pharmaceutical Care Network Europe (PCNE) drug-related problems classification version 9.1: first implementation in Sudan. JPRI 2021;33(59A):699–706. doi:10.9734/jpri/2021/v33i59A34321
7. Rashed AN, Wilton L, Lo CCH, et al. Epidemiology and potential risk factors of drug‐related problems in H ong Kong paediatric wards. Br J Clin Pharmacol. 2014;77(5):873–879. doi:10.1111/bcp.12270
8. Saldanha V, Martins RR, Lima SIVC, et al. Incidence, types and acceptability of pharmaceutical interventions about drug related problems in a general hospital: an open prospective cohort. BMJ open. 2020;10(4):e035848. doi:10.1136/bmjopen-2019-035848
9. Leopoldino RD, Santos MT, Costa TX, et al. Drug related problems in the neonatal intensive care unit: incidence, characterization and clinical relevance. BMC Pediatr. 2019;19(1):1–7. doi:10.1186/s12887-019-1499-2
10. AlAzmi A, Ahmed O, Alhamdan H, et al. Epidemiology of Preventable Drug-Related Problems (DRPs) among hospitalized children at KAMC-Jeddah: a single-institution observation study. Drug Healthc Patient Saf. 2019;11:95–103. doi:10.2147/DHPS.S220081
11. Nascimento AR, Leopoldinoa RW, Santosa ME, et al. Drug-related problems in cardiac neonates under intensive care. Rev Paul Pediatr. 2020;38:e2018134. doi:10.1590/1984-0462/2020/38/2018134
12. Garin N, Sole N, Lucas B, et al. Drug related problems in clinical practice: a cross-sectional study on their prevalence, risk factors and associated pharmaceutical interventions. Sci Rep. 2021;11(1):883. doi:10.1038/s41598-020-80560-2
13. Viktil KK, Blix HS. The impact of clinical pharmacists on drug‐related problems and clinical outcomes. Basic Clin Pharmacol Toxicol. 2008;102(3):275–280. doi:10.1111/j.1742-7843.2007.00206.x
14. Eichenberger PM, Lampert ML, Kahmann IV, et al. Classification of drug-related problems with new prescriptions using a modified PCNE classification system. Pharm World Sci. 2010;32(3):362–372. doi:10.1007/s11096-010-9377-x
15. Sabry N, Farid S, Asrawi A, et al. Drug-related problems in cardiac children. Minerva Pediatr. 2016;68(2):89–95.
16. Nguyen TH, Le VTT, Quach DN, et al. Drug-related problems in prescribing for pediatric outpatients in Vietnam. Healthcare. 2021;9(3):227. doi:10.3390/healthcare9020227
17. Naseef H, Amria A, Asrawi A, et al. The acceptance and awareness of healthcare providers towards doctor of pharmacy (Phram D) in the Palestinian health care system. Saudi Pharmaceut J. 2020;28(9):1068–1074. doi:10.1016/j.jsps.2020.07.007
18. Westerlund T. Pharmaceutical care and the role of drug-related problems. In: The Pharmacist Guide to Implementing Pharmaceutical Care. Springer; 2019:11–22.
19. Xiao-Xiao L, Zheng S-Q, Gu J-H, et al. Drug-related problems identified during pharmacy intervention and consultation: implementation of an intensive care unit pharmaceutical care model. Front Pharmacol. 2020;11:571906.
20. Penm J, Li Y, Zhai S, et al. The impact of clinical pharmacy services in China on the quality use of medicines: a systematic review in context of China’s current healthcare reform. Health Policy Plan. 2014;29(7):849–872. doi:10.1093/heapol/czt067
21. Tasaka Y, Tanaka A, Yasunaga D, et al. Potential drug-related problems detected by routine pharmaceutical interventions: safety and economic contributions made by hospital pharmacists in Japan. J Pharamaceut Health Care Sci. 2018;4(1):1–11. doi:10.1186/s40780-018-0125-z
22. Francis J, Abraham S. Clinical pharmacists: bridging the gap between patients and physicians. Saudi Pharmaceut J. 2014;22(6):600–602. doi:10.1016/j.jsps.2014.02.011
23. Ramos H, Pardo J, Sánchez R, et al. Pharmacist-physician interprofessional collaboration to promote early detection of cognitive impairment: increasing diagnosis rate. Front Pharmacol. 2021;12:579489. doi:10.3389/fphar.2021.579489
24. Stephenson T. How children’s responses to drugs differ from adults. Br J Clin Pharmacol. 2005;59(6):670–673. doi:10.1111/j.1365-2125.2005.02445.x
25. Kim C, Park K, McMahon AW, et al. Drug safety in labeling for pediatric drug development and dose selection in submissions to the US food and drug administration. J Clin Pharmacol. 2021;61(S1):S133–S140. doi:10.1002/jcph.1864
26. Daniel L, Rarichan L, Jose M, et al. An investigation on drug related problems in pediatrics of a Tertiary Care, Private, Teaching Hospital at Coimbatore. J Clin Case Rep Trials. 2018;1:01–07.
27. Jani YH, Ghaleb MA, Marks SD, et al. Electronic prescribing reduced prescribing errors in a pediatric renal outpatient clinic. J Pediatr. 2008;152(2):214–218. doi:10.1016/j.jpeds.2007.09.046
28. Pontefract SK, Hodson J, Slee A, et al. Impact of a commercial order entry system on prescribing errors amenable to computerised decision support in the hospital setting: a prospective pre-post study. BMJ Qual Saf. 2018;27(9):725–736. doi:10.1136/bmjqs-2017-007135
29. Carroll AE. The high costs of unnecessary care. JAMA. 2017;318(18):1748–1749. doi:10.1001/jama.2017.16193
30. Lyu H, Xu T, Brotman D, et al. Overtreatment in the United States. PLoS One. 2017;12(9):e0181970. doi:10.1371/journal.pone.0181970
31. Dalton K, Byrne S. Role of the pharmacist in reducing healthcare costs: current insights. Integr Pharm Res Pract. 2017;6:37. doi:10.2147/IPRP.S108047
32. Bizri NA, Alam W, Mobayed T, et al. COVID-19 in conflict region: the arab levant response. BMC Public Health. 2021;21(1):1–13. doi:10.1186/s12889-021-11580-4
33. Emre K, Burcu KC, Mesut S, et al. Impact of clinical pharmacist-led interventions in Turkey. Turk J Pharmaceut Sci. 2021;18(4):517. doi:10.4274/tjps.galenos.2020.66735
34. Khan MU, Ahmad A. The impact of clinical pharmacists’ interventions on drug related problems in a teaching based hospital. Int J Pharm Clin Res. 2014;63(63):276–280.
35. Alagiriswami B, Ramesh M, Basavanagowdappa H. A study of clinical pharmacist initiated changes in drug therapy in a teaching hospital. Indian J Pharm Pract. 2009;2:1.
36. Law A, Lo A, Stephenson E. Pharmaceutical interventions from paediatric pharmacists. Arch Dis Child. 2010;95(6):e1–e1. doi:10.1136/adc.2010.190322.10
37. PCNE-Classification v9.1. Published Online 2020. Available from: https://www.pcne.org/upload/files/417_PCNE_classification_V9-1_final.pdf.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
