Back to Journals » OncoTargets and Therapy » Volume 10
Immunotargeting relapsed or refractory precursor B-cell acute lymphoblastic leukemia – role of blinatumomab
Authors Queudeville M , Handgretinger R, Ebinger M
Received 21 March 2017
Accepted for publication 9 June 2017
Published 19 July 2017 Volume 2017:10 Pages 3567—3578
DOI https://doi.org/10.2147/OTT.S103470
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Chiung-Kuei Huang
Manon Queudeville, Rupert Handgretinger, Martin Ebinger
Department of Pediatric Hematology and Oncology, University Children’s Hospital, University of Tübingen, Tübingen, Germany
Abstract: Patients with refractory or relapsed (R/R) acute lymphoblastic leukemia (ALL) have a dismal prognosis of around 5% long-term survival when treated with cytotoxic chemotherapy and allogenic stem cell transplantation. T-cell immunobased strategies open up new therapeutic perspectives. Blinatumomab is the first of a new class of antibody constructs that was labeled bispecific T-cell engager (BiTE): it consists of two single chain variable fragment connected with a flexible linker, one side binding CD3, the other CD19. The tight binding and the close proximity to the CD19-positive B-cells and leukemic cells leads to non-major histocompatibility complex-restricted T-cell activation, polyclonal T-cell expansion and direct target cell killing. Applied by continuous infusion, blinatumomab achieves morphological complete response rates ranging from 39% to 69% in R/R ALL patients (compared to 25% after second-line chemotherapy) with prolonged overall survival (blinatumomab median overall survival, 7.7 months vs chemotherapy, 4.0 months). In comparison to conventional cytotoxic second-line protocols blinatumomab has a favorable safety profile. The main adverse event is related to the mode of action of blinatumomab: the induction of a cytokine-release syndrome that can be managed by interruption and/or the application of steroids or tocilizumab. Another typical complication is the occurrence of neurological side effects, such as seizures and encephalopathy. This neurotoxicity is reversible after application of steroids and/or withdrawal of blinatumomab. Blinatumomab has proven to be a powerful therapeutic option in R/R ALL patients both adult and pediatric because of its efficacy and limited toxicity.
Keywords: R/R precursor B-cell ALL, blinatumomab, T-cell, immunotherapy
Introduction
Precursor B-cell acute lymphoblastic leukemia (ALL) is a disease with ambiguous prognosis. Although in specific subgroups such as pediatric ALL the outcome is favorable, patients with refractory or relapsed (R/R) ALL or patients with persisting or resurfacing minimal residual disease (MRD) have a high risk of relapse. Progress has been achieved in risk stratification strategies based on MRD response assessment and identification of risk-associated genetic alterations.1 Furthermore, new classes of agents such as proteasome inhibitors and new tyrosine kinase inhibitors have been introduced into the therapy of R/R ALL.2,3 Despite these improvements, the prognosis remains poor for patients in high risk groups.
T-cell based therapeutic strategies offer a new approach for the treatment of ALL overcoming several obstacles in the R/R ALL patient group such as chemoresistance or organ damage limiting further intensive cytotoxic therapy. Bispecific T-cell engager (BiTE) monoclonal antibody constructs activate T-memory effector cells and conduct them toward target cells with a certain surface antigen. The most prominent and advanced agent of this group is blinatumomab (Blincyto®), a CD3/CD19-bispecific construct that has proven its clinical value in the therapy of R/R CD19-positive ALL. This review is dedicated to the clinical usage of blinatumomab. Other bispecific constructs are currently evaluated on the basis of the BiTE blueprint (eg, CD3/EpCAM)4 or further bispecific antibody constructs directed mainly against CD19, CD20 and CD22.5 All of these antibody-based approaches engage the patient’s immune system. T-cell activation appears to be the most effective strategy but requires a significant number of activated T-cells. In patients with heavy pretreatment or recent allogenic hematopoietic stem cell transplantation (HSCT) blinatumomab may fail to induce sufficient T-cell activation to eliminate the CD19 positive cells.6,7
An alternative approach is the direct genetic manipulation of a patient’s T-cells: collected and purified CD3 positive T-cells are stably transfected by retro- or lentiviruses with a chimeric T-cell receptor (CAR). This CAR has an extracellular single chain variable fragment (scFv) head that binds with the specificity of a monoclonal antibody and can be chosen ad libitum. The intracellular part of the CAR mediates T-cell receptor (TCR) signaling when the scFv binds and leads to activation of the modified T-cell.8,9
Acute lymphoblastic leukemia
Epidemiology and biology
ALL is a hematological malignancy characterized by proliferation of immature lymphoid progenitor cells. The incidence of ALL is as low as 1.7/100,000 per year. It shows two peaks: the first one in preschool age with an incidence of 4.5/100,000 per year and the second one starting to increase at an age of around 50 years (incidence of 2/100,000 per year).10 Although ALL is the most common malignancy in childhood accounting for nearly 30% of all pediatric cancer cases and 80% of all leukemias, it is rare in higher age and constitutes <1% of all malignancies regarding all age groups.10
This review focuses on the role of blinatumomab in the therapy of ALL and, therefore, on CD19-positive precursor B-cell ALL. CD19 is expressed throughout long phases of B-cell development, expression starting at late pro-B-cell stage (CD34+CD10+CD19+), it persists during the complete B-cell development and does not disappear until maturation into plasma cell stage.11 Almost every precursor B-cell ALL expresses CD19 consistently on the malignant blasts.12 CD19 mediates costimulatory signals with CD21 and CD81 and seems to be essential for maintenance of the malignant proliferation by enhancing PI3K- and RAS-signaling.13
ALL risk stratification used to be based on clinical, biological and genetic parameters. Age, sex, white blood cell count and central nervous system (CNS) involvement at diagnosis have a prognostic value. Immunophenotype, cytogenetic and molecular features still play an important role, but detection of MRD has proven to be the single most significant prognostic parameter and has replaced most of the clinical prognostic factors.14 Besides the situation of frank relapse, the assessment of MRD is used to determine the indication for therapy with blinatumomab.
Prognosis
Although >80% of pediatric patients survive the disease for 5 years and longer, the 5-year survival rate in adults is around 40% in developed countries.10 With intense, pediatric-derived and mainly BFM-based protocols, >95% of pediatric and up to 80% of adult patients may achieve a first complete remission (CR).15 Although this approach results in improved outcome for young adults,16 it is associated with a high morbidity and mortality in elderly patients resulting in over 20% therapy-related deaths during induction treatment. Therefore, most protocols for elderly patients involve multidrug combinations that are less toxic than the BFM-based protocols.15 The outcome drops significantly after first relapse: in adult ALL the 3-year survival rate after first relapse treatment is 11%, after second 6% and after third or higher salvage 4%.17 The same holds true for pediatric ALL on a higher level with a 10-year survival rate of around 40% after first relapse.18 These proportions demonstrate that for high risk groups new therapeutic strategies such as the T-cell-based immunotherapeutic approaches are urgently needed.
Blinatumomab
Mechanism of action
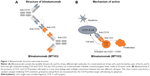
Blinatumomab is the first agent of a new class of antibody constructs that was labeled BiTE.19 It contains two scFv, each formed by a pair of variable domains from heavy and light immunoglobulin chains. The two scFv proteins are connected with a flexible linker made of 25 amino acids (Figure 1A). Compared to the 150 kDa of a complete IgG antibody, the 55 kDa BiTE molecule is a small protein.
BiTE is a construct out of the fast growing field of bispecific antibodies which exhibit several advantages over monoclonal antibodies: adding CD3 as one component redirects T-cells to the target cell to enhance killing. Bispecific antibodies are able to bind two antigens simultaneously for either blocking two pathways or enhancing the specificity of the interaction.20 The most frequent approach is the combination of a CD3 binding site with a tumor-specific target antigen, such as CD20, CD33, CEA, HER2 or CD2.21 Bispecific antibodies in an IgG-like format have the advantage of a relatively long serum half-life, yet smaller molecules such as the scFv-based BiTEs are less immunogenic and have a better tissue penetration.21 The first and till now only bispecific antibody that has advanced to Phase III trials is blinatumomab.22
For blinatumomab the BiTE construct was armed with a mouse antibody recognizing CD3ε on one end, the constant part of the TCR complex that mediates its signaling. Therefore, blinatumomab is able to activate different fractions of T-cells including both CD8+ and CD4+ T-cells and regulatory T-cells (Tregs).23 Binding itself does not activate the TCR unless a target cell is bound to the other scFv of the BiTE molecule. The second scFv construct of blinatumomab is the variable fragment of a murine anti-CD19 antibody. Application of blinatumomab in the presence of T-cells and CD19-positive cells (physiological B-cells as well as precursor B-cell leukemic blasts) leads to a very close linkage with multiple connections between the two different cell types which is caused by the small size and the flexibility of the internal linker of blinatumomab (Figure 1B). This close contact zone forms a cytolytic synapse and can be visualized by staining activated PKCθ which mediates the TCR activation: granzymes and perforin are exocytosed into the CD19 positive target cell and thereby induce its apoptosis.24 The multiple, bivalent binding leads to a strong stimulus of the engaged T-cell which is independent of the TCR specificity and of major histocompatibility complex (MHC) class I antigen presentation or other costimulatory factors.23 The strong activation of engaged T-cells leads to direct and serial lysis. Furthermore, blinatumomab induces polyclonal proliferation of activated T-cells which leads to an increased activity of blinatumomab 1–2 days after onset of application.7,25
Efficacy of blinatumomab in R/R ALL
In Phase II trials, blinatumomab was evaluated in patient cohorts with primary resistant or relapsed B-lineage ALL (R/R ALL), patient cohorts with poor outcome irrespective of the therapeutic regimen applied (Table 1). The first Phase II trial was conducted by the German Multicenter Study Group for Adult ALL (GMALL; NCT00198991 and NCT00198978): patients with persistent or relapsed MRD were included (15 patients with persistent, 5 patients with relapsed MRD). Within 4 cycles of blinatumomab with a dosage of 15 μg/m2/d, 80% of the patients became MRD negative. Five patients with BCR-ABL translocation resistant to tyrosine kinase inhibition (imatinib and/or dasatinib) were included, three of whom turned MRD negative. Nine of the responding patients received allogenic HSCT after blinatumomab treatment. After a median of 33 months, 61% of the patients persisted in CR.26 The same study group, GMALL, was able to demonstrate a remarkable efficacy in R/R ALL with an open relapse of the leukemia, albeit not as good as in MRD level patients (NCT01209286). Because this trial was a Phase I with dose finding and because of a grade 4 cytokine-release syndrome (CRS) in one of the first 5 patients, the dosage of blinatumomab was lowered to 5 μg/m2/d for the first 7 days. Twenty-five of 36 patients (69%) achieved a complete or partial hematologic recovery within the first two blinatumomab cycles, of which 22 patients (61%) turned MRD negative. Thirteen of the responders (36% of the included patients) were able to proceed to allogenic HSCT. Median overall survival was 13.0 months with a medium follow-up time of 28.9 months, and median relapse-free survival was 8.8 months. Ten of the 36 patients (28%) were long-term survivors. All long-term survivors were MRD responders and showed a stronger T-cell expansion compared to the other patients.27
In the development and assessment of new agents, a decrease of efficacy with increase of included patients and treatment centers is a frequent observation. The same holds true for blinatumomab. The international multicenter trial NCT01466179 included 189 patients suffering from R/R ALL. Inclusion criteria were more stringent than in the previous studies: first relapse within 12 months of first remission, relapse within 12 months after allogenic HSCT or no response to or relapse after salvage therapy.7,26,28,37 In this trial a fixed, not body surface area dependent dosage, was applied: a reduced dosage of 9 μg/day for the first 7 days, then the full dosage of 28 μg/day to reduce the risk of severe CRS or neurological toxicity. The following cycles started with 28 μg/day. A complete response was shown by 33% of the patients and an additional 10% of the patients showed a partial hematological response. For all included patients, median overall survival was 6.1 months after a median follow-up of 9.8 months. Thirty-two patients achieving complete or partial hematological recovery (40% of the responders) were able to proceed to allogenic HSCT.28
Out of this patient cohort, Barlev et al conducted an estimation study to compare the long-term survival with blinatumomab to survival without this option: at month 60, an estimated 12.6% of the blinatumomab-treated patients were alive compared to 5.4% without blinatumomab (historical data). The mean overall survival increased from 39.8 months before blinatumomab-treatment to 76.1 months afterwards.29
A phase III randomized controlled trial TOWER (NCT02013167) compared blinatumomab with conventional second-line standard chemotherapy (FLAG, fludarabine + high-dose cytarabine arabinoside ± anthracycline or HiDAC, high-dose cytarabine arabinoside-based regimen ± anthracycline or high-dose methotrexate-based regimen or clofarabine based regimens) in patients with R/R ALL. The inclusion criteria were similar to the above-mentioned NCT01466179 trial and blinatumomab dosing was the same: 9 μg/day ×7 days, followed by 28 μg/day ×21 days, subsequent cycles 28 μg/day ×28 days. Both groups received up to 5 cycles of blinatumomab or chemotherapy for induction and consolidation. Maintenance therapy, when indicated, consisted of 4-week-blinatumomab cycles every 3 months or low dose chemotherapy for 1 year.30
The data of the blinatumomab group (271 patients) correspond well with the NCT01466179 trial results: the rate of complete response ± hematological recovery was 44%, the median overall survival 7.7 months (median follow-up of 11.7 months). This was significantly superior to the chemotherapy group data (134 patients): complete response ± hematological recovery rate was 25%, median overall survival 4.0 months. In both groups, 24% of the patients proceeded to allogenic HSCT. The Kaplan–Meier curves for overall survival separated significantly within the first 3 months with longer survival for blinatumomab. Interestingly, the two curves converged again between 15 and 18 months.30
The first clinical trial (Phase I/II) on pediatric ALL was recently published by von Stackelberg et al (NCT01471782, Table 1). The same body surface area adjusted dosage as in adults was confirmed: 5 μg/m2/d for 7 days after the onset of blinatumomab, followed by 15 μg/m2/d for the remaining 3 weeks of the first and the subsequent cycles. Seventy patients received the complete dosage. After the first two cycles, 27 patients (39%) had achieved CR, 14 patients in this group (20%) became MRD negative. The numbers seem to be similar to the results in R/R adult ALL, yet it is too early to draw long-term conclusions.7
The restriction of the approval for blinatumomab on Philadelphia chromosome-negative (Ph−) ALL is not based on biological reasons but motivated by the existence of an alternative specific treatment, the tyrosine kinase inhibitor class of drugs. Blinatumomab shows the same efficacy in CD19 positive Ph+ ALL than in Ph− ALL. Eighty-nine percent of the Ph+ ALL patients in the ALCANTARA trial reached a MRD response in case of achieving CR, irrespective of resistance to chemo- and tyrosine kinase inhibitor-therapy.31
Pharmacokinetics and dosage
Phase I clinical studies of blinatumomab have revealed a short serum half-life of 2–3 hours.23 Therefore, application of blinatumomab was established as continuous infusion. This was facilitated by using a portable pump. Steady-state levels of blinatumomab were reached within 24 hours and persisted over the prolonged application period. Pharmacological studies revealed a mean volume of distribution of 4.52 L (±2.89) under steady-state conditions, mean half-life of 2.11 h (±1.42) and mean clearance of 2.92 L/h (±2.83).32 These parameters are not influenced by age, sex, weight or renal function (if creatinine clearance is ≥30 mL/min). Patients with severe renal impairment or receiving dialysis were not included in the Phase I/II studies, so no recommendations for this patient group can be made. Yet renal excretion was low and, therefore, usage in selected cases might be justified. Hepatic dysfunction had no influence on blinatumomab clearance.32,33 Blinatumomab exhibits such a high efficacy that a dosage of 5 μg/m2 body surface per day (μg/m2/d) leads to a prompt and prolonged eradication of CD19-positive B-cells.34 In patients with ALL, the increase of the dosage up to 30 μg/m2/d led to a significant rise of mainly neurotoxic but also inflammatory side effects.7,35 In patients with non-Hodgkin lymphoma (NHL) higher doses of blinatumomab were evaluated up to 90 μg/m2/d. Neurologic events led to the maximum-tolerated dose of 60 μg/m2/d in B-NHL, and stepwise dose escalation was tolerated better than a flat start with the target dose.34 This trial by Bargou et al demonstrated a dose dependency of blinatumomab in inducing response in CD19-positive NHL.34
Blinatumomab is applied as a 28-day cycle with a dosage of 28 μg/d respective (resp). 15 μg/m2/d in pediatric patients followed by a 14-day off-period before start of the following cycle. In order to prevent a CRS the first cycle is started with a dosage of 9 μg/d resp. 5 μg/m2/d in children for the first 7 days. Up to 5 cycles have been applied.7,28 Recently, the TOWER trial proposed a maintenance therapy of 4 additional cycles every 3 months.30 It was observed repeatedly that peripheral T-cell levels of both CD4 and CD8 positive T-cells declined within a few hours after onset of blinatumomab due to redistribution of T-cells into the tissue and consumption by strong cytotoxic commitment. T-cell levels returned to baseline by days 1–2 and surmounted the initial level in many patients during the next days. This was explained by a polyclonal T-cell increase and is most probably the result of a T-cell proliferation induced by the initial activation by blinatumomab.6,25,35 Treatment with blinatumomab led predominantly to an expansion of CD4+ T-cells with CD45RA/CCR7 phenotype and of effector memory CD8+ T-cells.34,36 In parallel, proinflammatory cytokines correlated with T-cell activation such as interferon gamma, tumor necrosis factor alpha, interleukin-2, interleukin-6, and interleukin-10 increased within day 1 and were detectable at elevated levels for 2 days. This transient elevation was often accompanied by fever which led to a CRS (see section below) in severe cases. This cytokine elevation was not observed in subsequent cycles.35
Safety and tolerability
Since the first publications of clinical application of blinatumomab two specific adverse reactions stand out from the multitude of side effects in this heavily pretreated patient cohort. The first adverse reaction is the induction of a CRS which is directly correlated to the mode of action. The second striking side effect is frequent triggering of neurotoxicity.19,34 Systematic evaluation in clinical trials confirmed these observations.
Cytokine-release syndrome
In the trial of Topp et al with 36 patients, blinatumomab had to be terminated or discontinued due to severe CRS in 2 patients (6%). In total, 29 of the patients (81%) experienced fever/pyrexia ≤ grade 3.37 In the large trial with 189 enrolled patients published in 2015 by Topp et al, only 3 patients (2%) developed grade 3 CRS.28 Similarly, the TOWER trial reported CRS ≥ grade 3 in 13/267 (5%) patients receiving blinatumomab.30
In a pediatric Phase I/II trial, grade 4 CRS was observed in 4 out of 49 patients (9%) of the dose escalation cohort. In Phase II extension cohort with the regular dosage of 5 μg/m2/d for the first 7 days and 15 μg/m2/d for the further treatment, only 4 of 70 patients (6%) developed ≥ grade 3 CRS.7
The development of a CRS has to be expected within the first week of the first treatment cycle or the first days of the second cycle. Therefore, inpatient treatment is strongly advised during the first week of the first cycle and the first 5 days of the second cycle. CRS can be treated efficiently by withdrawal of blinatumomab, application of high-dose steroids and/or application of tocilizumab, a monoclonal IL-6-receptor antibody, the latter most probably not interfering with the efficacy of blinatumomab.38
Neurotoxicity
Neurotoxic events such as seizures, irritability, disorientation, tremor and encephalopathy may be induced by activated T-cells binding CD19-positive cells in the CNS. The resulting cytokine release leads to local inflammation and disruption of the blood–brain barrier. The symptoms are, generally, reversible.28,38,39 Neurotoxicity of blinatumomab is increased by but not limited to the occurrence of a CRS and appears after the first week of blinatumomab application.28,38 Topp et al reported neurological events causing a halt of blinatumomab application in 6 of 36 patients (17%).37 The following larger study with 189 patients observed neurologic events grade 3 in 20 (11%) and grade 4 in 4 (2%) patients.28 A pediatric Phase I/II study described 2 of 70 patients (3%) with grade 2 seizures which led to interruption of blinatumomab treatment.7 The rate of neurotoxic events ≥ grade 3 in the randomized controlled TOWER trial was comparable between blinatumomab and chemotherapy with 25/267 (9%) vs 9/109 (8%), respectively.30
Since neurotoxic adverse events also seem to be inflammation mediated, the therapeutic options are similar to those for the treatment of CRS: interruption of blinatumomab, application of high-dose steroids and/or application of tocilizumab.
Other severe adverse events
In a trial including 189 patients with R/R ALL, three deaths caused by severe infections (Escherichia coli, candida and unknown) were attributed to the therapy with blinatumomab by the investigators.40 A heavily pretreated 5-year-old boy succumbed to cardiac failure under a grade 4 CRS and tumor lysis syndrome in Phase I part of the pediatric blinatumomab trial conducted by von Stackelberg et al.7,41 The same trial reports a fatal respiratory failure related to a grade 4 CRS.7 Common adverse events were related to the mode of action of blinatumomab, such as pyrexia, fatigue, headache, tremor, edema, nausea and diarrhea. The most frequent grade ≥3 adverse events were anemia (36%), thrombocytopenia (21%) and hypokalemia (17%).28,37,42
The TOWER trial compared blinatumomab therapy with intensive second-line chemotherapy in a randomized controlled fashion. In a cohort of 405 patients with R/R ALL it described adverse events ≥ grade 3 in 87% of patients in the blinatumomab group and in 92% in the chemotherapy group. Fatal events were reported in 19% vs 17% of the patients. After adjustment for treatment duration, the event rate for serious adverse events was 3.5 per patient-year in the blinatumomab cohort vs 6.4 per patient-year in the chemotherapy group.30
CD19-depletion
Therapy with blinatumomab leads to a profound depletion of CD19-positive cells. CD19 expression is restricted to B-cell lineage committed cells. CD19 deficiency seems to be a very rare condition in humans. Only very few cases are described: in four patients of two unrelated families, a frameshift mutation leads to loss of function of the cytoplasmatic domain of the CD19 receptor.43 Apart from hypogammaglobulinemia, no nonimmunological pathology was observed. The long-term off-target effects of blinatumomab, therefore, seem to be restricted to the impairment of the B-cell compartment. As we know from therapy with rituximab, a CD20 monoclonal antibody, recovery of the B-cells could take over a year with the need of long-term immunoglobulin substitution.44
Escape mechanisms
By binding to the constant CD3ε component of the TCR, blinatumomab is capable of engaging multiple T-cell subsets such as CD3 positive CD8 and CD4 positive T-cells as well as regulatory (Tregs) T-cells.23 The engaged T-cell may execute direct cytolysis or initiate its own proliferation; however, both effects are not restricted to specific T-cell subsets, specific TCRs, MHC class I molecules, neither are they dependent on a specific immune environment.23 Therefore, the broad mode of T-cell engagement of blinatumomab may overcome escape mechanisms of other immune therapies induced by immune editing, MHC downregulation or modulation of the environment by specific selection pressure.25,45
Activity of blinatumomab depends on the expression of CD19 on the target cells. Emergence of CD19 negative ALL blast populations has been observed under CD19 targeted approaches such as blinatumomab or CD19-directed chimeric antigen receptor (CAR) T-cell therapy. Clinical data from the first trials and clinical observations show a CD19 negative relapse in 10%–20% of the patients.26,37 An important mechanism of CD19 escape relapse seems rather to be due to disrupted CD19 membrane trafficking than to the outgrowth of a CD19-negative progenitor cell or myeloid lineage shift.46 MLL-rearranged ALL is a disease originating from very immature B-cell precursors. In a child with MLL-rearranged ALL, a CD19 negative relapse with myeloid phenotype was recently described under therapy with blinatumomab.47 It will be essential to further describe the underlying biological processes of CD19 loss to predict and prevent relapses.
Aldoss et al have observed a number of CD19-positive ALL relapses in an extramedullary, lymphoma-like pattern.48 Such manifestations might be overcome by higher dosages of blinatumomab, as recommended for NHL.34
Prediction factors
Therapy with blinatumomab is highly expensive. Furthermore, it prohibits any simultaneous immunosuppressive cytotoxic therapy for the period of application. Therefore, it would be of high interest to predict patient cohorts which will benefit from such a high-priced therapy and those in which it will only lead to a delay of further cytotoxic therapy. We learned from the exploration of MRD that the initial therapeutic response of ALL has a strong correlation with outcome.49 Zugmaier et al described a similar observation: of 10 long-surviving patients, all belonged to the group of 25 patients who had reached MRD negativity within the first two cycles in a cohort of 36 R/R ALL patients. In addition, long-term survivors had a stronger T-cell expansion compared to the patients with an overall survival of <30 months.27
In a recent study, Duell et al were able to predict the response to blinatumomab with the level of regulatory T-cells at start of blinatumomab therapy. Regulatory T-cells were T-cells defined by coexpression of CD4/CD25/FOXP3. Patients responding had a mean of 4.8% of regulatory T-cells (22 patients, 95% confidence interval: 1.8%–8.3%) compared to 10.3% in nonresponders (20 patients, 95% confidence interval: 3.4%–65.9%). The cutoff of 8.525% regulatory T-cells was able to identify all blinatumomab responders and excluded 70% of the nonresponders. The study described a 1.7-fold upregulation of PD-1 on the regulatory T-cells in vitro under exposure to blinatumomab. To overcome the regulatory T-cell-mediated resistance to blinatumomab the authors propose a prior in vivo depletion of regulatory T-cells and discuss the feasibility of a simultaneous therapy with a PD-1 inhibitor.50
An interesting in vitro study of ALL patient samples suggests a correlation between PD-L1 expression on ALL blasts and resistance to blinatumomab therapy: PD-L1 expression on leukemic cells was higher in relapsed or blinatumomab-refractory ALL patients compared to leukemic cells of primary, nonblinatumomab treated ALL patients. Correspondingly, the expression of the T-cell exhaustion marker PD-1 was higher in ALL patients compared to healthy controls. Kohnke et al described a patient resistant to blinatumomab, who displayed an increase of PD-L1 expressing B-precursor ALL cells.51 These observations support a model in which the PD-1/PD-L1-mediated T-cell suppression leads to resistance to blinatumomab therapy (Figure 2A). Treatment of patient-derived cells with a PD-1 inhibitor was able to re-sensitize those cells to the action of blinatumomab (Figure 2B). In fact, one ALL patient refractory to blinatumomab showed a response after the simultaneous application of pembrolizumab, a PD-1 inhibitor with blinatumomab.52 These data are promising starting points for further research in the prediction of individual blinatumomab efficacy.
Conclusion and outlook
Blinatumomab is a BiTE construct that exhibits activity in adult and pediatric patients with R/R ALL in an MRD or open leukemic setting. Its efficacy is significantly higher in respect of induction of complete response and survival duration compared to cytotoxic second-line treatment protocols and displays a favorable safety profile.30 The main risk is the induction of a CRS that has to be expected within the first week of the first cycle or the first days of the second cycles. Neurotoxicity seems to be time related to CRS and also occurs during the first days of blinatumomab application. Therefore, inpatient treatment is strongly advised during the first days of blinatumomab application. The mode of application is comparably straightforward: Blinatumomab is applied continuously over 4 weeks by a small portable pump. As soon as the risk period of the first week is over, it can be administered in an out-patient setting. Blinatumomab shows efficacy in any malignoma expressing superficial CD19, irrespective of genetic alterations such as the Philadelphia translocation.31
A direct comparison to other T-cell therapies such as CAR-T-cell application is pending. Although first clinical CAR-T-cell trials report higher response rates, the severity of side effects (both CRS and neurotoxicity) seems to be a challenging obstacle of this approach. Furthermore, a CAR-T-cell graft has to be produced individually for each patient, a complex and technically demanding procedure compared to the standardized application of blinatumomab.
Different study groups aim to incorporate blinatumomab into frontline therapy of ALL (Table 2). Three trials (Phases II and III by the NCI and M.D. Anderson Cancer Center) are evaluating first-line induction therapy with and without blinatumomab in adult ALL (NCT02003222, NCT02143414, NCT02877303). As an immunotherapy inducing T-cell response blinatumomab is thought to be more active upfront than in the immunocompromised setting of a heavily pretreated patient with R/R ALL. The combination of blinatumomab with chemotherapy might reduce the risk of CD19 negative outgrowth of leukemic blasts, but clinical data supporting this assumption are pending. The mode of combination, simultaneous application or sequential application still has to be established. Any immunosuppressive chemotherapy counteracts an immunotherapy, yet the combination with targeted therapy and nonmyelosuppressive components such as tyrosine kinase inhibitors or L-asparaginase should be feasible.
Blinatumomab has proven to be a useful tool for bridging ALL high risk patients to HSCT because of its efficacy in R/R ALL and its limited toxicity. The same reasons turn blinatumomab into an attractive option for elderly patients and patients with multiple morbidities.
The application of blinatumomab is actually entering common clinical practice in R/R ALL patients. Even in such a heavily pretreated patient cohort this immunobased approach has proven strong efficacy. It still has to demonstrate its equivalence to other efficient T-cell-based approaches, such as CAR-T-cell therapy. But blinatumomab is paving its way down to the treatment of patient groups with lower risk profiles and into first-line therapy where the competitive pressure of complex CAR-T-cell therapies might be lower.
Disclosure
The authors report no conflicts of interest in this work.
References
Bassan R, Hoelzer D. Modern therapy of acute lymphoblastic leukemia. J Clin Oncol. 2011;29(5):532–543. | ||
Du XL, Chen Q. Recent advancements of bortezomib in acute lymphocytic leukemia treatment. Acta Haematol. 2013;129(4):207–214. | ||
Roberts KG, Li Y, Payne-Turner D, et al. Targetable kinase-activating lesions in Ph-like acute lymphoblastic leukemia. N Engl J Med. 2014;371(11):1005–1015. | ||
Deisting W, Raum T, Kufer P, Baeuerle PA, Munz M. Impact of diverse immune evasion mechanisms of cancer cells on T cells engaged by EpCAM/CD3-bispecific antibody construct AMG 110. PLoS One. 2015;10(10):e0141669. | ||
Klinger M, Benjamin J, Kischel R, Stienen S, Zugmaier G. Harnessing T cells to fight cancer with BiTE(R) antibody constructs – past developments and future directions. Immunol Rev. 2016;270(1):193–208. | ||
Schlegel P, Lang P, Zugmaier G, et al. Pediatric posttransplant relapsed/refractory B-precursor acute lymphoblastic leukemia shows durable remission by therapy with the T-cell engaging bispecific antibody blinatumomab. Haematologica. 2014;99(7):1212–1219. | ||
von Stackelberg A, Locatelli F, Zugmaier G, et al. Phase I/Phase II study of blinatumomab in pediatric patients with relapsed/refractory acute lymphoblastic leukemia. J Clin Oncol. 2016;34(36):4381–4389. | ||
Kowolik CM, Topp MS, Gonzalez S, et al. CD28 costimulation provided through a CD19-specific chimeric antigen receptor enhances in vivo persistence and antitumor efficacy of adoptively transferred T cells. Cancer Res. 2006;66(22):10995–11004. | ||
Jensen MC, Popplewell L, Cooper LJ, et al. Antitransgene rejection responses contribute to attenuated persistence of adoptively transferred CD20/CD19-specific chimeric antigen receptor redirected T cells in humans. Biol Blood Marrow Transplant. 2010;16(9):1245–1256. | ||
Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2013, National Cancer Institute. Bethesda, MD. Available from: http://seer.cancer.gov/csr/1975_2013/. Accessed July 9, 2017. | ||
Sanz E, Munoz A, Monserrat J, et al. Ordering human CD34+CD10-CD19+ pre/pro-B-cell and. Proc Natl Acad Sci U S A. 2010;107(13):5925–5930. | ||
van Dongen JJ, Orfao A. EuroFlow Consortium: resetting leukemia and lymphoma immunophenotyping. Basis for companion diagnostics and personalized medicine. Leukemia. 2012;26(9):1899–1907. | ||
Rickert RC. New insights into pre-BCR and BCR signalling with relevance to B cell malignancies. Nat Rev Immunol. 2013;13(8):578–591. | ||
Mullighan CG, Jeha S, Pei D, et al. Outcome of children with hypodiploid ALL treated with risk-directed therapy based on MRD levels. Blood. 2015;126(26):2896–2899. | ||
Marks DI. The challenges of managing older patients with acute lymphoblastic leukemia. Am Soc Clin Oncol Educ Book. 2015;e343–e351. | ||
Lukenbill J, Advani AS. The treatment of adolescents and young adults with acute lymphoblastic leukemia. Curr Hematol Malig Rep. 2013;8(2):91–97. | ||
Gokbuget N, Dombret H, Ribera JM, et al. International reference analysis of outcomes in adults with B-precursor Ph-negative relapsed/refractory acute lymphoblastic leukemia. Haematologica. 2016;101(12):1524–1533. | ||
Tallen G, Ratei R, Mann G, et al. Long-term outcome in children with relapsed acute lymphoblastic leukemia after time-point and site-of-relapse stratification and intensified short-course multidrug chemotherapy: results of trial ALL-REZ BFM 90. J Clin Oncol. 2010;28(14):2339–2347. | ||
Nagorsen D, Bargou R, Ruttinger D, Kufer P, Baeuerle PA, Zugmaier G. Immunotherapy of lymphoma and leukemia with T-cell engaging BiTE antibody blinatumomab. Leuk Lymphoma. 2009;50(6):886–891. | ||
Wu J, Fu J, Zhang M, Liu D. AFM13: a first-in-class tetravalent bispecific anti-CD30/CD16A antibody for NK cell-mediated immunotherapy. J Hematol Oncol. 2015;8:96. | ||
Fan G, Wang Z, Hao M, Li J. Bispecific antibodies and their applications. J Hematol Oncol. 2015;8:130. | ||
Brinkmann U, Kontermann RE. The making of bispecific antibodies. MAbs. 2017;9(2):182–212. | ||
Offner S, Hofmeister R, Romaniuk A, Kufer P, Baeuerle PA. Induction of regular cytolytic T cell synapses by bispecific single-chain antibody constructs on MHC class I-negative tumor cells. Mol Immunol. 2006;43(6):763–771. | ||
Brischwein K, Schlereth B, Guller B, et al. MT110: a novel bispecific single-chain antibody construct with high efficacy in eradicating established tumors. Mol Immunol. 2006;43(8):1129–1143. | ||
Hoffmann P, Hofmeister R, Brischwein K, et al. Serial killing of tumor cells by cytotoxic T cells redirected with a CD19-/CD3-bispecific single-chain antibody construct. Int J Cancer. 2005;115(1):98–104. | ||
Topp MS, Gokbuget N, Zugmaier G, et al. Long-term follow-up of hematologic relapse-free survival in a phase 2 study of blinatumomab in patients with MRD in B-lineage ALL. Blood. 2012;120(26):5185–5187. | ||
Zugmaier G, Gokbuget N, Klinger M, et al. Long-term survival and T-cell kinetics in relapsed/refractory ALL patients who achieved MRD response after blinatumomab treatment. Blood. 2015;126(24):2578–2584. | ||
Topp MS, Gokbuget N, Stein AS, et al. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single-arm, phase 2 study. Lancet Oncol. 2015;16(1):57–66. | ||
Barlev A, Lin VW, Katz A, Hu K, Cong Z, Barber B. Estimating long-term survival of adults with Philadelphia chromosome-negative relapsed/refractory B-precursor acute lymphoblastic leukemia treated with blinatumomab using historical data. Adv Ther. 2017;34(1):148–155. | ||
Kantarjian H, Stein A, Gokbuget N, et al. Blinatumomab versus. chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med. 2017;376(9):836–847. | ||
Martinelli G, Dombret H, Chevallier P, et al. Complete molecular and hematologic response in adult patients with relapsed/refractory (R/R) Philadelphia chromosome-positive B-precursor acute lymphoblastic leukemia (ALL) following treatment with blinatumomab: results from a phase 2 single-arm multicenter study (ALCANTARA). Abstract #679. Presented at: The 2015 ASH Annual Meeting; December 7; 2015; Orlando, FL. | ||
Zhu M, Wu B, Brandl C, et al. Blinatumomab, a bispecific T-cell engager (BiTE((R))) for CD-19 targeted cancer immunotherapy: clinical pharmacology and its implications. Clin Pharmacokinet. 2016;55(10):1271–1288. | ||
Kaplan JB, Grischenko M, Giles FJ. Blinatumomab for the treatment of acute lymphoblastic leukemia. Invest New Drugs. 2015;33(6):1271–1279. | ||
Bargou R, Leo E, Zugmaier G, et al. Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science. 2008;321(5891):974–977. | ||
Klinger M, Brandl C, Zugmaier G, et al. Immunopharmacologic response of patients with B-lineage acute lymphoblastic leukemia to continuous infusion of T cell-engaging CD19/CD3-bispecific BiTE antibody blinatumomab. Blood. 2012;119(26):6226–6233. | ||
Wu J, Fu J, Zhang M, Liu D. Blinatumomab: a bispecific T cell engager (BiTE) antibody against CD19/CD3 for refractory acute lymphoid leukemia. J Hematol Oncol. 2015;8:104. | ||
Topp MS, Gokbuget N, Zugmaier G, et al. Phase II trial of the anti-CD19 bispecific T cell-engager blinatumomab shows hematologic and molecular remissions in patients with relapsed or refractory B-precursor acute lymphoblastic leukemia. J Clin Oncol. 2014;32(36):4134–4140. | ||
Teachey DT, Rheingold SR, Maude SL, et al. Cytokine release syndrome after blinatumomab treatment related to abnormal macrophage activation and ameliorated with cytokine-directed therapy. Blood. 2013;121(26):5154–5157. | ||
Nagorsen D, Kufer P, Baeuerle PA, Bargou R. Blinatumomab: a historical perspective. Pharmacol Ther. 2012;136(3):334–342. | ||
Topp MS, Gockbuget N, Stein AS. Correction to lancet oncol 2015; 16: 60, 61. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multi-centre, single-arm, phase 2 study. Lancet Oncol. 2015;16(4):e158. | ||
Darvishi B, Farahmand L, Jalili N, Majidzadeh AK. Blinatumomab provoked fatal heart failure. Int Immunopharmacol. 2016;41:42–46. | ||
Topp MS, Kufer P, Gokbuget N, et al. Targeted therapy with the T-cell-engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival. J Clin Oncol. 2011;29(18):2493–2498. | ||
van Zelm MC, Reisli I, van der BM, et al. An antibody-deficiency syndrome due to mutations in the CD19 gene. N Engl J Med. 2006;354(18):1901–1912. | ||
Roberts DM, Jones RB, Smith RM, et al. Immunoglobulin G replacement for the treatment of infective complications of rituximab-associated hypogammaglobulinemia in autoimmune disease: a case series. J Autoimmun. 2015;57:24–29. | ||
Holzel M, Bovier A, Tuting T. Plasticity of tumour and immune cells: a source of heterogeneity and a cause for therapy resistance? Nat Rev Cancer. 2013;13(5):365–376. | ||
Braig F, Brandt A, Goebeler M, et al. Resistance to anti-CD19/CD3 BiTE in acute lymphoblastic leukemia may be mediated by disrupted CD19 membrane trafficking. Blood. 2017;129(1):100–104. | ||
Rayes A, McMasters RL, O’Brien MM. Lineage switch in MLL-rearranged infant leukemia following CD19-directed therapy. Pediatr Blood Cancer. 2016;63(6):1113–1115. | ||
Aldoss I, Song J, Stiller T, et al. Correlates of resistance and relapse during blinatumomab therapy for relapsed/refractory acute lymphoblastic leukemia. Am J Hematol. Epub 2017 May 11. | ||
Pongers-Willemse MJ, Verhagen OJ, Tibbe GJ, et al. Real-time quantitative PCR for the detection of minimal residual disease in acute lymphoblastic leukemia using junctional region specific TaqMan probes. Leukemia. 1998;12(12):2006–2014. | ||
Duell J, Dittrich M, Bedke T, et al. Frequency of regulatory T cells determines the outcome of the T-cell-engaging antibody blinatumomab in patients with B-precursor ALL. Leukemia. Epub 2017 February 24. | ||
Kohnke T, Krupka C, Tischer J, Knosel T, Subklewe M. Increase of PD-L1 expressing B-precursor ALL cells in a patient resistant to the CD19/CD3-bispecific T cell engager antibody blinatumomab. J Hematol Oncol. 2015;8:111. | ||
Feucht J, Kayser S, Gorodezki D, et al. T-cell responses against CD19+ pediatric acute lymphoblastic leukemia mediated by bispecific T-cell engager (BiTE) are regulated contrarily by PD-L1 and CD80/CD86 on leukemic blasts. Oncotarget. 2016;7(47):76902–76919. | ||
Ribera JM, Ferrer A, Ribera J, Genesca E. Profile of blinatumomab and its potential in the treatment of relapsed/refractory acute lymphoblastic leukemia. Onco Targets Ther. 2015;8:1567–1574. | ||
Gokbuget N, Zugmaier G, Klinger M, et al. Long-term relapse-free survival in a phase 2 study of blinatumomab for the treatment of patients with minimal residual disease in B-lineage acute lymphoblastic leukemia. Haematologica. 2017:102(4):e132–e135. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.