Back to Journals » Open Access Emergency Medicine » Volume 15
Immunomodulation Effect of Convalescent Plasma Therapy in Severe – Critical COVID-19 Patients
Authors Fitriah M, Putri AE, Semedi BP, Atika A, Tambunan BA
Received 20 January 2023
Accepted for publication 18 April 2023
Published 24 April 2023 Volume 2023:15 Pages 109—118
DOI https://doi.org/10.2147/OAEM.S405555
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Hans-Christoph Pape
Munawaroh Fitriah,1 Aditea Etnawati Putri,1 Bambang Pujo Semedi,2 Atika Atika,3 Betty Agustina Tambunan1
1Department of Clinical Pathology, Faculty of Medicine, Universitas Airlangga – Dr. Soetomo General Academic Hospital, Surabaya, Indonesia; 2Department of Anesthesiology and Reanimation, Faculty of Medicine, Universitas Airlangga – Dr. Soetomo General Academic Hospital, Surabaya, Indonesia; 3Faculty of Medicine, Universitas Airlangga, Surabaya, Indonesia
Correspondence: Betty Agustina Tambunan, Department of Clinical Pathology, Faculty of Medicine, Universitas Airlangga – Dr. Soetomo General Academic Hospital, Jl. Mayjend Prof. Dr. Moestopo No. 6-8, Airlangga, Gubeng, Surabaya, East Java, 60286, Indonesia, Tel +6231-5023865, Email [email protected]
Introduction: Convalescent plasma therapy (CPT) is an alternative therapy for managing COVID-19, but its use is still controversial.
Objective: Analyzing the effectiveness of CPT in modulating immune responses based on SARS-COV-2 anti-spike protein receptor-binding domain (s-RBD) IgG, inflammatory cytokines (IL-6 and IL-4), and mortality in severe-critical COVID-19 patients.
Methods: This study was an observational analytical with a prospective cohort design. The number of participants was 39 patients from June to December 2020. The participants received CPT and was tested for blood analysis such as IL-4, IL-6 and s-RBD IgG. The data were taken a day before CPT, 1st day, 2nd day, and 7th day after CPT. The analysis included Friedman, Pearson correlation, and Mann–Whitney test which is significant if p < 0.05.
Results: The value of participant’s s-RBD IgG before CPT was 91.49 (0.43– 3074.73) AU/mL and the 7th day post-CPT, s-RBD IgG value of 1169.79 (6.48– 5577.91) AU/mL (p < 0.001). The IL-4 value before CPT was 1.78 (0.85– 5.21) ng/mL and the 7th day post-CPT, IL-4 value of 1.97 (0.87– 120.30) ng/mL (p = 0.401). The condition was also found in IL-6 value, in which the IL-4 value participant before CPT was 109.61 (0.73– 4701.63) ng/mL and the 7th day post-CPT, IL-6 value of 1.97 (0.87– 120.30) ng/mL (p = 0.401). No significant correlation found between increased s-RBD IgG level with increased IL-4 and decreased IL-6 before and after CPT in severe-critical COVID-19 patients (p > 0.05). No significant correlation was also found between increased s-RBD IgG levels, IL-4 too, and decreased IL-6 after CPT therapy between deceased and alive patients, both in 1st, 2nd, and 7th days (p > 0.05).
Conclusion: No correlation between the increase in s-RBD IgG levels and changes in IL-4 and IL-6 levels. Changes in s-RBD IgG, IL-4, and IL-6 levels are not associated with mortality in severe-critical COVID-19 degree post CPT recipients.
Keywords: convalescent plasma, COVID-19, infectious disease, s-RBD
Introduction
SARS-CoV-2 infection causes damage to lung tissue, which triggers a local immune response thereby inducing monocytes and macrophages that release inflammatory cytokines and inducing an adaptive immune response.1 Inflammatory cytokines such as Interleukin-1 (IL-1), Interleukin-6 (IL-6) and tumor necrosis alpha (TNF-α) play a role in the initial response, while anti-inflammatory cytokines such as Interleukin-4 (IL-4) and Interleukin-10 (IL-10) produced during infection plays a role in immune system homeostasis.2 Severe inflammation characterized by an increase in cytokines, known as a cytokine storm, triggers critical conditions such as acute respiratory distress syndrome (ARDS) and death due to multiorgan failure.3
IL-6 plays a vital role in the pathogenesis of COVID-19. The SARS-CoV-2 virus stimulates inflammation and antiviral responses through the production and secretion of cytokines such as IL-6. Together with other inflammatory cytokines, IL-6 triggers a cytokine storm and tissue damage due to a dysregulated immune response. Increased IL-6 levels, a pro-inflammatory molecule, cause a decrease in NK cell activity and a decrease in granzyme and perforin levels.4 IL-6 can be used as a predictor of disease severity in COVID-19 patients.2 IL-4 is the main cytokine of the Th2 immune response. It has an anti-inflammatory role by causing up-regulation of several cytokine inhibitors and scavenging receptors.5,6 A significant increase in IL-4 serum levels was found in recovered COVID-19 patients, whereas in COVID-19 patients and severe cases, there were no significant changes compared to the control group, indicating the role of the Th2 response in recovery.7
Specific and most effective therapies and vaccines for managing COVID-19 infection have not been widely reported, as antivirals are still used as the primary therapies.8 Due to the dearth of therapeutic options during the pandemic, convalescent plasma therapy (CPT) played a significant part in the management of patients with severe COVID-19.9 CPT is a classic intervention that has become an option in treating COVID-19.10 Passive immunization strategies have been widely used in managing and preventing infectious diseases since the 20th century.11 The CPT has been used in various pandemics, such as the Spanish flu, SARS-CoV-19, West Nile virus and Ebola.10,12,13 Administering CPT during the pandemic influenza A showed reduced mortality in severe patients and decreased IL-6 levels compared to the control group.14 The CPT is obtained through an apheresis procedure in survivors with a history of infection with certain specific pathogens who have specific antibodies against disease agents. The main target is to neutralize the pathogen to achieve the eradication of pathogen. During apheresis, neutralizing antibodies and other essential components such as anti-inflammatory cytokines, blood clotting factors, natural antibodies, defensins, pentraxins and various other proteins can be harvested from the donor.10,15
CPT is expected to provide further benefits such as immunomodulation through amelioration of severe inflammatory responses, in which in cases of COVID-19 that experience over-activation of the immune system and are accompanied by systemic hyper-inflammation or cytokine storms due to IL-1β, IL-2, IL-6, IL-17, IL-8, TNF-α.10,16 Neutralizing antibodies in CPT neutralize the virus by binding to the SARS-CoV-2 spike receptor binding domains (RBDs), so that the virus cannot bind to the angiotensin-converting enzyme 2 (ACE 2) receptor and the virus cannot enter cells.11 The lack of data on the effectiveness of CPT in COVID-19 makes its use controversial. Several studies have shown that giving CPT can improve the clinical outcome of COVID-19 patients, but other studies have shown no benefit in giving CPT to patients with severe degrees of COVID-19,17 whereas previous studies have stated that CPT is not associated with reduced mortality or clinical outcomes.18 Increased IL-6 compared to before CPT and an increase in the ratio of pro-inflammatory and anti-inflammatory cytokines (IL-6/IL-10 and IL-6/IL-4).19 This study aimed was to analyze the effectiveness of CPT as a therapy option for the mortality of COVID-19 patients in the critical-severe group by evaluating changes in levels of neutralizing antibodies, IL-6 and IL-4 cytokines as an immunomodulating effect of CPT administration.
Methods
Study Design
This study was an observational analytical study with a prospective cohort design. The study population was COVID-19 patients admitted to the hospital’s isolation ward. The number of participants in this study were 39 severe to critical patients from June to December 2020 (Figure 1). Ethical approval was obtained from the health research ethics committee of the hospital. The participants received CPT and performed blood analysis such as IL-4, IL-6, and SARS-COV-2 Anti-Spike Protein Receptor-Binding Domain (s-RBD) IgG. Data collection was carried out a day before CPT, 1st day, 2nd day, and 7th day after CPT. The demographics and clinical characteristics of the participants were evaluated on day-1 before CPT and the patients’ outcomes were monitored for 28 days after CPT transfusion.
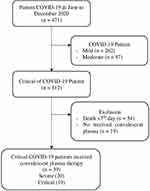 |
Figure 1 Collecting participant process. |
Data Collection and Participant
Participants in this study were critical or severe degree of COVID-19 patient received CPT. Inclusion criteria were confirmed positive for COVID-19 through polymerase chain reaction (PCR) examination of the nasopharyngeal swab,20,21 and experiencing severe or critical degrees and undergoing therapy at the Isolation Ward. Criteria for the severity of COVID-19 included: a) Shortness of breath with a respiration rate of 30 breaths per minute, b) Oxygen saturation (SpO2) 93% in room air, c) PaO2/FiO2 ratio 300 mmHg, and d) Worsening of chest X-ray lesions >50% in 24–48 hours. While, criteria for critically ill COVID-19 patients included: a) experiencing severe pneumonia with rapid progression which periodically experiences an increase in viral load despite receiving standard COVID-19 therapy, b) experiencing acute respiratory distress syndrome (ARDS) with a ratio of PaO2/FiO2 <300, c) Requires or is receiving mechanical ventilator therapy.21,22 Exclusion criteria participants included a) patient allergy to plasma and other bloods component, b) sequential organ failure assessment (SOFA) score of >11,23 and c) diagnosed with primary immunodeficiency, HIV/AIDS, and malignancy.
Convalescent Plasma Therapy (CPT)
The donated CPT which was transfused to patients in this study was obtained from apheresis donation using Haemonetics MCS®+ machine equipment according to National Standards for Blood Transfusion Service and blood bank guidelines. From each donor, 200–400 mL of CPT were collected and each recipient was transfused with 200 mL of CPT for two consecutive days.
CPT donor inclusion criteria were: a) Aged 17–60 years; b) recovered from COVID-19 after a period of hospitalization confirmed by two consecutive negative PCR results of naso-oropharyngeal swab; c) No symptoms of COVID-19 or experiencing a symptom-free period of at least 14 days prior to plasma donation; d) Outpatients and self-isolation patients who were negative on re-PCR results of naso-oropharyngeal swabs 24 hours prior to plasma donation; e) No comorbidities such as Diabetes Mellitus, Hypertension with target organ damage (stroke, coronary heart disease, kidney disease), CKD, and difficult vascular access; f) Free from Transmitted Infections Through Blood Transfusion (IMLTD) evaluation, namely HBV, HCV, HIV, Syphilis (Negative for hepatitis B, hepatitis C, human immunodeficiency virus, and syphilis during transfusion microbiology screening); g) SARS-CoV-2 specific serum antibody titer more than 1:320 based on rapid test assay using PANBIO™ COVID-19 IG G rapid test device. Donors with incomplete clinical information during COVID-19 treatment were excluded.
Blood Analysis
Participants were taken to collect 5 mL of venous blood using an SST tube (serum separator tube). Blood samples were sent to the laboratory, then centrifuged at 3000 rpm for 15 minutes. The serum obtained after centrifugation was stored in the freezer −80°C.
The SARS CoV-2 s-RBD IgG antibody was measured by the Chemiluminescence method using a Maglumi® analyzer (Snibe Diagnostic, China) according to the procedure. The 1AU/mL cut off was used to differentiate non-reactive (<1AU/mL) and reactive (>1AU/mL) results. IL-6 was measured by the Cytometric Beads Array (CBA) method using the BD CBA Human IL-6 Flex set reagent which was read on a BD FACS Calibur (BD Biosciences, USA). The measurement results were then analyzed with the FCAP Array™ software. IL-4 was measured by the Enzyme Linked Immunosorbent Assay (ELISA) method with the Sandwich ELISA principle using the Quantikine HS IL-4 ELISA kit (R&D system, USA). All tests were carried out according to the instructions for each inspection kit.
Statistical Analysis
Statistical analysis was performed with the software of Microsoft Excel® and Statistical Package for the Social Sciences (SPSS) 25.0 version (IBM Corp., Armonk, NY, USA). Data normality test used the Shapiro–Wilk test. Comparison of neutralizing antibody levels, IL-4 and IL-6 between examination days was tested by the Friedman test, if significantly different it was continued with the Wilcoxon signed rank test. The association between s-RBD IgG antibodies, IL-4 and IL-6 was tested with the Pearson Correlation test if the data were normally distributed, or the Spearman correlation test if the data were not normally distributed. Analysis of the association between s-RBD IgG, IL-4, and IL-6 used the Mann Whitney test, with p <0.05 being statistically significant.
Results
Characteristic of Participant
The average age of the participants was 49.85 ± 9.27 years, with a median of 52.00 (28.00–65.00) years. The participant’s average BMI was 25.96 ± 4.00 kg/m2, with a median of 25.60 (19.31–40.00) kg/m2. The participant’s average length of stay (LOS) in the intensive care unit (ICU) was 11.26 ± 4.44 days, with a median of 10.00 (3.00–24.00) days. Most of the participants were male as much as 74.4% and as much as 51.3% in the critical category. Most participants had comorbid diseases (71.8%) such as diabetes mellitus (35.8%), hypertension (33.3%), and obesity (17.9%). Most participants also experienced complications (94.9%) including respiratory failure (92.3%), septic shock (25.6%), non-specific hepatitis (12.8%), acute kidney injury (15.3%), hypoalbuminemia (12.8%), and others (10.2%; Table 1).
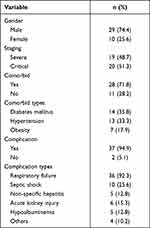 |
Table 1 Characteristic of Participant |
Anti-Spike Protein Receptor-Binding Domain (S-RBD) IgG Value
The participants’ s-RBD IgG value before CPT and 7th days post-CPT was 91.49 (0.43–3074.73) AU/mL and 1169.79 (6.48–5577.91) AU/mL (p <0.001), respectively. Post-CPT, delta s-RBD IgG value included 222.41 ± 765.46 AU/mL (1st day), 441.07 ± 1008.85 AU/mL (2nd day), and 1150.77 ± 1645.06 AU/mL (7th day). In addition, there were significant differences between each s-RBD IgG delta included z = 4.563 with p <0.001 (1st day vs 2nd day), z = 4340 with p <0.001 (1st day vs 7th day), and z = 3.349 with p = 0.001 (2nd day vs 7th day). There was a significant difference in the participant’s s-RBD IgG value on each measurement day (Table 2 and Table 3).
 |
Table 2 Value of s-RBD, IL-4 and IL-6 in COVID-19 Patient Severity |
 |
Table 3 Comparison Between s-RBD, IL-4 and IL-6 Values on 1st, 2nd, and 7th Day in COVID-19 Patient Severity |
IL-4 and IL-6 Value
The participants’ IL-4 value before CPT and 7th day post-CPT was 1.78 (0.85–5.21) ng/mL and 1.97 (0.87–120.30) ng/mL (p = 0.401), respectively. Post-CPT, delta IL-4 value included 0.07 ± 0.66 ng/mL (1st day), 0.20 ± 0.57 ng/mL (2nd day), and 3.20 ± 18.45 ng/mL (7th day; p = 0.380). In addition, there was no significant difference between each IL-4 delta included z = 0.377 with p = 0.706 (1st day vs 2nd day), z = 0.232 with p = 0.816 (1st day vs 7th day), and z = 0.464 with p = 0.642 (2nd day vs 7th day). There was no significant difference in IL-4 participant values on each measurement day (Table 2 and Table 3).
Similar conditions were also found for the IL-6 value, in which IL-6 value for participants before CPT and 7th day post-CPT was 109.61 (0.73–4701.63) ng/mL and 1.97 (0.87–120.30) ng/ mL (p = 0.401), respectively. Post-CPT, delta IL-6 value included 96.63 ± 1427.79 ng/mL (1st day), 107.19 ± 1116.48 ng/mL (2nd day), and 151.97 ± 1884.01 ng/mL (7th day; p = 0.819). In addition, there was no significant difference between each IL-6 delta included z = 0.377 with p = 0.706 (1st day vs 2nd day), z = 0.232 with p = 0.816 (1st day vs 7th day), and z = 0.464 with p = 0.642 (2nd day vs 7th day). There was no significant difference in participant IL-6 values on each measurement day (Table 2 and Table 3).
Correlation of Convalescent Plasma Therapy and s-RBD, IL-4, and IL-6
There was no significant correlation between increased s-RBD IgG antibody levels and increased IL-4 and decreased IL-6 before and after administration of CPT in the critical-severe group of COVID-19 patients (Table 4). There was no significant difference in the increase in s-RBD IgG antibody levels, IL-4 and decreased IL-6 after CPT between the deceased and alive patients, both on the 1st, 2nd, and 7th days. This also indicated no association between increased levels of s-RBD IgG antibodies and IL-4 and decreased IL-6 after CPT with mortality (Table 5).
 |
Table 4 Comparison of s-RBD on IL-4 and IL-6 in COVID-19 Patients’ Severity |
 |
Table 5 Comparison of s-RBD, IL-4, and IL-6 Levels on Mortality in COVID-19 Patient Severity |
Discussion
CPT is a form of passive immunization that contains neutralizing antibodies from recovered donors to reduce the risk of developing a worse disease through a virus neutralization mechanism24 and has immunomodulatory effects such as anti-inflammatory cytokines, clotting and/or anti-clotting factors, albumin protein C, protein S and complement.25 Previous studies have shown CPT from recovered individuals to have anti-RBD SARS-CoV-2 antibodies with potent antiviral activity. RBD-binding monoclonal antibodies (mAb) consisting of C121, C144 and C135 are potent neutralizing antibodies against SARS-CoV-2 with an inhibition concentration (IC50) <5 ng/mL. mAbs that target RBD will form a mAb-RBD complex that binds RBD to ACE2. In general, antibodies that target the RBD of the viral S protein as a binding site are more potent at neutralizing than antibodies that target other regions.26,27
The humoral immune response of SARS-Cov-2 is similar to that of SARS-CoV, mediated by antibodies against surface protein glycoproteins, especially Spike (S) glycoproteins and nucleocapsid (N) proteins. The first B-cell response appears to the Nucleocapsid protein, 4–8 weeks after the onset of symptoms, antibody responses to the S protein begin to occur. Antibodies against the s-RBD can neutralize so that they can eliminate the virus and play a role in preventing infection.28 Several studies have shown a strong positive correlation between RBD-specific IgG levels and viral neutralization. Patients receiving CPT experienced an increase in neutralizing antibodies up to a titer of 1:640 on the 7th day.29,30
Evaluation of IL-4 levels in CPT administration showed no significant change in IL-4 levels 5 days after CPT administration. The increase in IL-4 beyond the upper limit of normal occurs together with the improvement of lymphopenia, indicating its role in the healing process.19 IL-4 levels were higher on days 15–42 compared to days 0–14 indicating the anti-inflammatory role of IL-4 as a slow-phase cytokine that increases at the end of infection or the beginning of healing.31 IL-6 plays a vital role in inflammatory reactions. Observing the dynamics of IL-6 levels during the SARS-CoV-2 infection process can provide a comprehensive understanding of the dynamic changes in inflammation that are useful for monitoring and treating COVID-19 patients. Increased ratio of IL-6/IL-4 which may be caused by an increase in systemic inflammatory cytokines in ARDS and MODS which cannot overcome the damage caused by inflammation immediately after administration of CPT.19,32
Passive antibody transfer via CPT connotes potential therapeutic efficacy. S-specific IgG and RBD play an important role in neutralizing the virus. The rate of increase of S-specific IgG and RBD is a consideration of the efficacy of CPT. Antibody levels in CPT donors are positively correlated with changes in antibodies in recipients which IgG sRBD levels are able to neutralize antibodies.19,33 In this study, the increase in s-RBD IgG did not directly affect changes in IL-4 and IL-6 levels on the seventh day after administration of CPT. The anti-inflammatory effect of CPT was inadequate to compensate for the high increase in pro-inflammatory cytokines in the advanced phase or critical degree on the 7th day after administration of CPT. Neutralizing antibodies to CPT are very effective in the early phase, when viral replication predominates but are ineffective in the later phase, possibly s-RBD IgG is not the only factor influencing CPT efficacy.34 In line with various studies showing that the efficacy of CPT is affected by the time of administration and earlier administration is recommended within 72 hours after the onset of symptoms with high anti s-RBD IgG titers >1:1350.35 The success of CPT is also influenced by factors such as disease severity, comorbidities, complications and length of stay.29,35–37
There was no association between changes in s-RBD IgG, IL-4 and IL-6 levels after administration of CPT and mortality. IL-6 is an adequate predictor of disease severity but is not correlated with mortality.38 The causes of mortality in COVID-19 are very diverse, especially due to MODS caused by cytokine storms. In this study, the median age of the patients was >45 years and were in the late course of the disease, most of them had comorbidities and received mechanical ventilation. Administration of CPT may be effective in eliminating the virus but not sufficient to reduce lung damage due to inflammation in patients with advanced or critical stages of COVID-19.37
This study has several limitations, including the limited number of subjects conducted at the start of the COVID-19 pandemic, so the choice of therapy and examination was still limited. Monitoring of biomarkers was only carried out until the 7th day after CPT without following until the end of clinical outcome monitoring, the time from the onset of the first symptoms to hospital admission and obtaining CPT was different for each patient and was not a criterion for analysis so that the patient was not known in the immunopathological phase when CPT was administered. This study did not compare with the group that was not given CPT and did not perform paired analysis of s-RBD IgG on the CPT obtained and the recipient, so it was not known whether the recipient already had antibodies that were higher than the donor obtained.
Conclusion
The role of CPT immunomodulation is not seen on the 7th day after administration of CPT in patients with severe – critical COVID-19 degrees. No association exists between the increase in s-RBD IgG levels and changes in IL-4 and IL-6 levels. Changes in IgG levels of s-RBD IgG, IL-4 and IL-6 are not associated with mortality in severe-critical degrees of COVID-19 CPT recipients.
Ethical Approval
This study has been reviewed by the Health Research Ethics Committee of Dr Soetomo General Academic Hospital, Surabaya, Indonesia (0073/KEPK/IX/2020).
Consent
All participants received an explanation regarding the rights and obligations during study. Written informed consent was obtained from the participant or guardian for publication of the study based on Declaration of Helsinki.
Acknowledgment
We would like to thank our editor, “Fis Citra Ariyanto” and all the laboratory technician staff of the Clinical Pathology Laboratory in Dr. Soetomo General Academic Hospital, Surabaya, Indonesia.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Schultze JL, Aschenbrenner AC. COVID-19 and the human innate immune system. Cell. 2021;184(7):1671–1692. doi:10.1016/j.cell.2021.02.029
2. Dhar SK, Gujar S, Gujar S, Das M, Das M. IL-6 and IL-10 as predictors of disease severity in COVID-19 patients: results from meta-analysis and regression. Heliyon. 2021;7(2):e06155. doi:10.1016/j.heliyon.2021.e06155
3. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi:10.1016/s0140-6736(20)30183-5
4. Abbasifard M, Khorramdelazad H. The bio-mission of interleukin-6 in the pathogenesis of COVID-19: a brief look at potential therapeutic tactics. Life Sci. 2020;257:118097. doi:10.1016/j.lfs.2020.118097
5. Schuerwegh AJ, Dombrecht EJ, Stevens WJ, Van Offel JF, Bridts CH, De Clerck LS. Influence of pro-inflammatory (IL-1 alpha, IL-6, TNF-alpha, IFN-gamma) and anti-inflammatory (IL-4) cytokines on chondrocyte function. Osteoarthritis Cartilage. 2003;11(9):681–687. doi:10.1016/s1063-4584(03)00156-0
6. Vaz de Paula CB, de Azevedo MLV, Nagashima S, et al. IL-4/IL-13 remodeling pathway of COVID-19 lung injury. Sci Rep. 2020;10(1):18689. doi:10.1038/s41598-020-75659-5
7. Merza MY, Hwaiz RA, Hamad BK, Mohammad KA, Hama HA, Karim AY. Analysis of cytokines in SARS-CoV-2 or COVID-19 patients in Erbil city, Kurdistan Region of Iraq. PLoS One. 2021;16(4):e0250330. doi:10.1371/journal.pone.0250330
8. Rojas M, Monsalve DM, Pacheco Y, et al. Ebola virus disease: an emerging and re-emerging viral threat. J Autoimmun. 2020;106:102375. doi:10.1016/j.jaut.2019.102375
9. Dhawan M, Priyanka PM, Angural S, Choudhary OP, Choudhary OP. Convalescent plasma therapy against the emerging SARS-CoV-2 variants: delineation of the potentialities and risks. Int J Surg. 2022;97:106204. doi:10.1016/j.ijsu.2021.106204
10. Rojas M, Rodríguez Y, Monsalve DM, et al. Convalescent plasma in Covid-19: possible mechanisms of action. Autoimmun Rev. 2020;19(7):102554. doi:10.1016/j.autrev.2020.102554
11. Marano G, Vaglio S, Pupella S, et al. Convalescent plasma: new evidence for an old therapeutic tool? Blood Transfus. 2016;14(2):152–157. doi:10.2450/2015.0131-15
12. Mair-Jenkins J, Saavedra-Campos M, Baillie JK, et al. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis. J Infect Dis. 2015;211(1):80–90. doi:10.1093/infdis/jiu396
13. Planitzer CB, Modrof J, Kreil TR. West Nile virus neutralization by US plasma-derived immunoglobulin products. J Infect Dis. 2007;196(3):435–440. doi:10.1086/519392
14. Hung IF, To KK, Lee CK, et al. Convalescent plasma treatment reduced mortality in patients with severe pandemic influenza A (H1N1) 2009 virus infection. Clin Infect Dis. 2011;52(4):447–456. doi:10.1093/cid/ciq106
15. Garraud O, Heshmati F, Pozzetto B, et al. Plasma therapy against infectious pathogens, as of yesterday, today and tomorrow. Transfus Clin Biol. 2016;23(1):39–44. doi:10.1016/j.tracli.2015.12.003
16. Lünemann JD, Nimmerjahn F, Dalakas MC. Intravenous immunoglobulin in neurology--mode of action and clinical efficacy. Nat Rev Neurol. 2015;11(2):80–89. doi:10.1038/nrneurol.2014.253
17. Wenjing L, Yuanzheng F, Li JY, Tang LV, Yu H. Safety and efficacy of convalescent plasma therapy in severely and critically ill patients with COVID-19: a systematic review with meta-analysis. Aging. 2020;13(1):1498–1509. doi:10.18632/aging.202195
18. Janiaud P, Axfors C, Schmitt AM, et al. Association of convalescent plasma treatment with clinical outcomes in patients with COVID-19: a systematic review and meta-analysis. JAMA. 2021;325(12):1185–1195. doi:10.1001/jama.2021.2747
19. Zeng H, Wang D, Nie J, et al. The efficacy assessment of convalescent plasma therapy for COVID-19 patients: a multi-center case series. Signal Transduct Target Ther. 2020;5(1):219. doi:10.1038/s41392-020-00329-x
20. Suryananda TD, Yudhawati R. Association of serum KL-6 levels on COVID-19 severity: a cross-sectional study design with purposive sampling. Ann Med Surg (Lond). 2021;69:102673. doi:10.1016/j.amsu.2021.102673
21. Saputra GNR, Yudhawati R, Fitriah M. Association of soluble receptor for advanced glycation end-products (sRAGE) serum on COVID-19 severity: a cross-sectional study. Ann Med Surg (Lond). 2022;74:103303. doi:10.1016/j.amsu.2022.103303
22. Yudhawati R, Sakina S, Fitriah M. Interleukin-1β and interleukin-10 profiles and ratio in serum of COVID-19 patients and correlation with COVID-19 severity: a time series study. Int J Gen Med. 2022;15:8043–8054. doi:10.2147/ijgm.S381404
23. Asmarawati TP, Suryantoro SD, Rosyid AN, et al. Predictive value of sequential organ failure assessment, quick sequential organ failure assessment, acute physiology and chronic health evaluation II, and new early warning signs scores estimate mortality of COVID-19 patients requiring intensive care unit. Indian J Crit Care Med. 2022;26(4):464–471. doi:10.5005/jp-journals-10071-24170
24. Acosta-Ampudia Y, Monsalve DM, Rojas M, et al. COVID-19 convalescent plasma composition and immunological effects in severe patients. J Autoimmun. 2021;118:102598. doi:10.1016/j.jaut.2021.102598
25. Harvala H, Robb ML, Watkins N, et al. Convalescent plasma therapy for the treatment of patients with COVID-19: assessment of methods available for antibody detection and their correlation with neutralising antibody levels. Transfus Med. 2021;31(3):167–175. doi:10.1111/tme.12746
26. Pang NY, Pang AS, Chow VT, Wang DY. Understanding neutralising antibodies against SARS-CoV-2 and their implications in clinical practice. Mil Med Res. 2021;8(1):47. doi:10.1186/s40779-021-00342-3
27. Yang Y, Du L. SARS-CoV-2 spike protein: a key target for eliciting persistent neutralizing antibodies. Signal Transduct Target Ther. 2021;6(1):95. doi:10.1038/s41392-021-00523-5
28. Mortaz E, Tabarsi P, Varahram M, Folkerts G, Adcock IM. The immune response and immunopathology of COVID-19. Front Immunol. 2020;11:2037. doi:10.3389/fimmu.2020.02037
29. Duan K, Liu B, Li C, et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci U S A. 2020;117(17):9490–9496. doi:10.1073/pnas.2004168117
30. Shen C, Wang Z, Zhao F, et al. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020;323(16):1582–1589. doi:10.1001/jama.2020.4783
31. Lu Q, Zhu Z, Tan C, et al. Changes of serum IL-10, IL-1β, IL-6, MCP-1, TNF-α, IP-10 and IL-4 in COVID-19 patients. Int J Clin Pract. 2021;75(9):e14462. doi:10.1111/ijcp.14462
32. Wang Y, Huo P, Dai R, et al. Convalescent plasma may be a possible treatment for COVID-19: a systematic review. Int Immunopharmacol. 2021;91:107262. doi:10.1016/j.intimp.2020.107262
33. Xia X, Li K, Wu L, et al. Improved clinical symptoms and mortality among patients with severe or critical COVID-19 after convalescent plasma transfusion. Blood. 2020;136(6):755–759. doi:10.1182/blood.2020007079
34. RECOVERY Collaborative Group. Convalescent plasma in patients admitted to hospital with COVID-19 (RECOVERY): a randomised controlled, open-label, platform trial. Lancet. 2021;397(10289):2049–2059. doi:10.1016/s0140-6736(21)00897-7
35. Salazar E, Christensen PA, Graviss EA, et al. Treatment of coronavirus disease 2019 patients with convalescent plasma reveals a signal of significantly decreased mortality. Am J Pathol. 2020;190(11):2290–2303. doi:10.1016/j.ajpath.2020.08.001
36. Sewell HF, Glenn G, Bram K, Klein C. Vaccines, convalescent plasma, and monoclonal antibodies for covid-19. BMJ. 2020;370:m2722. doi:10.1136/bmj.m2722
37. Ostrovskaya E, Kostin A, Bulanov AY. Efficiency and safety of convalescent plasma therapy in patients with COVID-19: a systematic review. Ann Crit Care. 2022;2022(2):108–120. doi:10.21320/1818-474X-2022-2-108-120
38. Liu Y, Tan W, Chen H, et al. Dynamic changes in lymphocyte subsets and parallel cytokine levels in patients with severe and critical COVID-19. BMC Infect Dis. 2021;21(1):79. doi:10.1186/s12879-021-05792-7
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
