Back to Journals » International Journal of Nanomedicine » Volume 17
Immune Repertoire and Advancements in Nanotherapeutics for the Impediment of Severe Steroid Resistant Asthma (SSR)
Authors Beeraka NM, Zhou R, Wang X, Vikram P R H, Kumar TP, Liu J , Greeshma MV, Mandal SP, Gurupadayya BM, Fan R
Received 2 March 2022
Accepted for publication 17 April 2022
Published 12 May 2022 Volume 2022:17 Pages 2121—2138
DOI https://doi.org/10.2147/IJN.S364693
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Yan Shen
Narasimha M Beeraka,1– 3 Runze Zhou,1 Xiaoyan Wang,4 Hemanth Vikram P R,5 Tegginamath Pramod Kumar,6 Junqi Liu,1 M V Greeshma,3 Subhankar P Mandal,5 B M Gurupadayya,1 Ruitai Fan1
1Department of Radiation Oncology, Cancer Center, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, 450052, People’s Republic of China; 2Department of Human Anatomy, Sechenov First Moscow State Medical University (Sechenov University), Moscow, 119991, Russia; 3Center of Excellence in Molecular Biology and Regenerative Medicine (CEMR), Department of Biochemistry, JSS Academy of Higher Education and Research (JSS AHER), JSS Medical college, Mysuru, Karnataka, India; 4Endocrinology Department, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, 450052, People’s Republic of China; 5Department of Pharmaceutical Chemistry, JSS College of Pharmacy, JSS Academy of Higher Education and Research (JSSAHER), Mysuru, 570015, Karnataka, India; 6Department of Pharmaceutics, JSS College of Pharmacy, JSS Academy of Higher Education and Research (JSSAHER), Mysore, Karnataka, 570015, India
Correspondence: Ruitai Fan, Department of Radiation Oncology, Cancer Center, The First Affiliated Hospital of Zhengzhou University, 1 Jianshedong Str., Zhengzhou, 450052, People’s Republic of China, Email [email protected]
Abstract: Severe steroid-resistant asthma (SSR) patients do not respond to the corticosteroid therapies due to the heterogeneity, and genome-wide variations. However, there are very limited reports pertinent to the molecular signaling underlying SSR and making pharmacologists, and formulation scientists to identify the effective therapeutic targets in order to produce novel therapies using novel drug delivery systems (NDDS). We have substantially searched literature for the peer-reviewed and published reports delineating the role of glucocorticoid-altered gene expression, and the mechanisms responsible for SSR asthma, and NDDS for treating SSR asthma using public databases PubMed, National Library of Medicine (NLM), google scholar, and medline. Subsequently, we described reports underlying the SSR pathophysiology through several immunological and inflammatory phenotypes. Furthermore, various therapeutic strategies and the role of signaling pathways such as mORC1-STAT3-FGFBP1, NLRP3 inflammasomes, miR-21/PI3K/HDAC2 axis, PI3K were delineated and these can be considered as the therapeutic targets for mitigating the pathophysiology of SSR asthma. Finally, the possibility of nanomedicine-based formulation and their applications in order to enhance the long term retention of several antioxidant and anti-asthmatic drug molecules as a significant therapeutic modality against SSR asthma was described vividly.
Keywords: natural products, steroid resistance, asthma, inflammation, molecular signaling, nanomedicine, novel drug delivery systems
Graphical Abstract:
Introduction
Asthma is a chronic, inherited, heterogenous respiratory tract disorder exemplified by the presence of symptoms like cough, wheezing, stiffness of chest, respiratory tract inflammation, and remodeling. Global prevalence of asthma is increasing rapidly more than 350 million people worldwide.1 A global action plan is taken for mitigating asthma and management of noncommunicable diseases and the UN-2030 agenda.2 Despite the rising morbidity of asthma globally among both children and adults, there is no complete remedy for preventing this disease occurrence. This can be attributed to the heterogeneity nature of the disease.3
Therapies against asthma can modulate heterogeneous clinical symptoms with variable responses. Corticosteroids are one of the significant therapies in treating mild to severe asthma; and they have been prescribed in early and long-term treatments as well.4 Global initiative for asthma (GINA)-2021 in their strategy reported the usage of inhaled corticosteroids to the patients more than 6 years of age and adult asthmatic patients rather than compromising the treatment only with short-acting beta2 agonists.5 Furthermore, corticosteroid therapeutics are steroidal drug molecules used for the treatment of allergic asthma.6
However, despite the admitting prominence and effectiveness of corticosteroids therapy for asthma treatment, their usage is constrained by the development of corticosteroid resistance in asthma patients. The degree of unresponsiveness towards corticosteroid therapy in asthmatic patients is up to 10%.7 Corticosteroids can modulate the gene expression of certain genes to minimize the pathophysiology underlying asthma; in certain cases, the prolonged usage of corticosteroids can induce variable gene expression as a result, the asthma patients attain steroid resistance. Side effects arising from corticosteroid therapy worsen the quality of life in asthma patients and cause a higher socio-economic burden for the patients receiving regular therapeutic interventions. Therefore, it is crucial to unravel the underlying mechanisms pertinent to the immune repertoire and pathophysiology of asthma during resistance acquisition to corticosteroid therapy.
Furthermore, novel drug delivery systems through liposome-based drug delivery or nanoformulated drug delivery through different conventional routes into the asthma patients with steroid resistance is a significant innovative strategy in the coming years. In this review, we have substantially described the molecular immune repertoire and underlying signaling pathways pertinent to SSR asthma, and anticipated therapeutic strategies formulated using the applications of nanomedicine as an alternative approach to produce good clinical outcomes through the targeted specific delivery of therapeutic molecules across secondary bronchi in asthma patients. This kind of delivery systems can enhance drug delivery, solubility, bioavailability, and pharmacokinetic profiles in order to minimize the acquired steroid resistance or to enhance the overall clinical outcomes in the asthma patients.8–11
Literature Search
We have extensively performed literature search in PubMed, National Library of Medicine (NLM), google scholar for the several published reports pertinent to the asthma patients who are with corticosteroid resistance, and the mechanisms underlying this resistance acquisition; furthermore, the literature pertinent to the various novel drug delivery systems including application of nanomedicine to treat this corticosteroid therapy resistance was also collected and deciphered vividly in this review.
Multiple Signaling Involved in Severe Steroid Resistant (SSR) Asthma
Exposure to higher ozone levels could induce the development of glucocorticoid insensitivity during asthma treatment.12 In this study, the signaling mechanism pertinent to corticosteroid resistance in asthmatic patients with the increased exposure to ozone was delineated.12 For instance, T2-low asthmatic condition was induced by the infiltration of neutrophils in asthma models by exposure to ovalbumin and ozone. Inflammatory and pro-inflammatory cytokines expression, lung resistance, etiology of lungs, modulation in the levels of GR and p-GR receptors, Nr3c1 mRNA, (STAT3), (SOCS3), and CXCL1 were ascertained for inducing corticosteroid resistance. Results of this study depicted respiratory tract inflammation due to the infiltration of neutrophils mediated by Th17 cells. A hormonal corticosteroid dexamethasone was used in the treatment of asthma conditions. A positive correlation in IL-6 and STAT3 levels and negative correlation between SOCS3 and STAT3 thus concluding that STAT3/IL-6 signaling pathway could be a significant factor for corticosteroid resistance. STAT3/IL-6 signaling pathway can be considered as a crucial therapeutic target to mitigate corticosteroid resistance.12
Severe asthma is characterized by the induction of resistance to glucocorticoids. Severe asthma results from heterogenous immune phenotypes hindering the development of effective therapeutic targets. So, any novel therapeutic target for severe asthma requires in-depth understanding of cellular, molecular and signaling mechanisms underlying the phenomenon of corticosteroid resistance. Severe asthma is characterized by multiple factors like T-helper cell-2 low and T-helper cell-17 high, neutrophils infiltration causing inflammation of respiratory tract. Inflammatory cytokines like IL-17, IL-33 could be possible therapeutic targets. Sex and population type of asthmatic patient should also be taken into consideration during therapy. Most patients require combinatorial therapy due to the presence of different immune phenotypes.7 For instance, a study conducted on asthmatic patients by dividing them into different groups like mixed asthmatics (eosinophilic/neutrophilic), paucigranulocytic, neutrophilic with usual FEV1, and neutrophilic with less FEV1 (asthmatics with smoking are included). Macrophage counts were estimated and observed a specific incline in the interferon regulatory factor 5 (IRF5+) levels and decline in the interferon regulatory factor 10 (IRF 10) levels. In addition, another signaling through purinergic receptors also plays a key role in the physiological processes in lungs for which ATP acts as a signaling messenger. A review discussed about possibility of purinergic receptors (P2RX1, P2RX4, P2RX7, P2RY1, P2RY11, and P2RY14) as therapeutic targets in asthma conditions.13 Pentraxins receptors are soluble and exhibit a significant role in the pattern perception and modulation of innate immune responses. These receptors could be considered as biomarkers for various immune related diseases including respiratory disease like asthma, as they cause enhancement in the inflammatory response by interacting with various other proteins.14
Therapies Against Corticosteroid Resistant Asthma
Macrolides
Macrolides are well known for their antibacterial properties but recent investigations to repurpose them as anti-inflammatory molecules with steroid sparing effect are specifically reported. For example, clarithromycin, a macrolide antibiotic which can be administered in combination with dexamethasone showed repression of lymphocytes. In addition, clarithromycin decreased inflammation and airway hyper-responsiveness by decreasing Th2 responses and TNF-α thereby leading to decrease in interleukin 17 responses that cause steroid-resistant asthma in mouse models.15 Another study conducted on 232 asthmatic patients developed IgG antibodies to C. pneumonia when the patients were given treatment with roxithromycin for 6 weeks during the asthmatic condition. In a study on asthmatic children with confirmed chlamydia infection were treated with clathrithromycin showed typically decrease in the overall timespan of wheezing. Clarithromycin is also effective for the treatment of acute episodes of bronchospasm in children with a history of recurrent wheezing.16 A randomized, double blind placebo controlled trial on 55 asthmatic patients reported a significant reduction in TNF-alpha, IL-5, and IL-12 levels in the respiratory tract. A study conducted on mouse models with steroid resistant asthma and H. influenzae infection induced by Th2 responses were controlled by clarithromycin treatment and the study concluded that H. influenzae infection shows synergistic effect on asthmatic patients.15,17 In a study conducted on 45 steroid resistant asthmatic patients with clarithromycin treatment showed a significant mitigation in the IL-8, neutrophils, neutrophil elastase, matrix metalloproteinase-9 (MMP-9) concentrations and improved quality of life in asthmatic patients.18 A study conducted on 420 patients concluded that azithromycin can be used as an adjunct therapy to treat steroid resistant asthma and enhanced quality of life in asthmatic subjects by decreasing symptoms. Hence, the nanoformulation composed of clarithromycin or combinatorial regimen of clarithromycin with other FDA-approved anti-asthmatic drugs may enhance the overall therapeutic window and bioavailability of the therapeutic molecules across the airways.
Marine-Derived Therapeutics vs SSR Asthma
Extract isolated from Korean marine algae, Ulva pertusa composed of hydroxy-2,3-dimethyl-2-nonen-4-olide can impair the generation of proinflammatory cytokines generated from BM-derived dendritic cells; these molecules could be implicated in targeting SSR asthma through the nanoformulation strategies by studying their efficacy in preclinical and clinical studies.19–21 Other fatty acids ‘(E)-9-Oxooctadec-10-enoic-acid and (E)-10-Oxooctadec-8-enoic-acid’ isolated from Gracilaria verrucosa, can impair the generation of inflammatory biomarker production such as NO, IL-6, and impair the NF-kB.22 Other marine molecules such as Ogipeptins A-D (Japanese marine bacterium, Pseudoalteromonas sp. can block the generation of TNF-α from human U937 monocytic cells.23 Chrysamides, A–Cisolated from Penicillium chrysogenum SCSIO41001 can impair the generation of proinflammatory IL-17 cytokine production.24 Two marine bacteria produce diketopiperazine products such as, “cyclo(L-Pro-D-Val), cyclo(L-Pro-L-Tyr), and cyclo(L-pro-D-Leu),” which can impair the generation of TNF-α, IL-6, NF-kB, and ERK1/2 by blocking MAPK activation.25 These molecules should be examined for the anti-SSR asthma efficacy either as monotherapy or combinatorial regimen with other anti-asthma therapeutics through several nanoformulations.
Targeting HDAC2 Axis and SSR Asthma
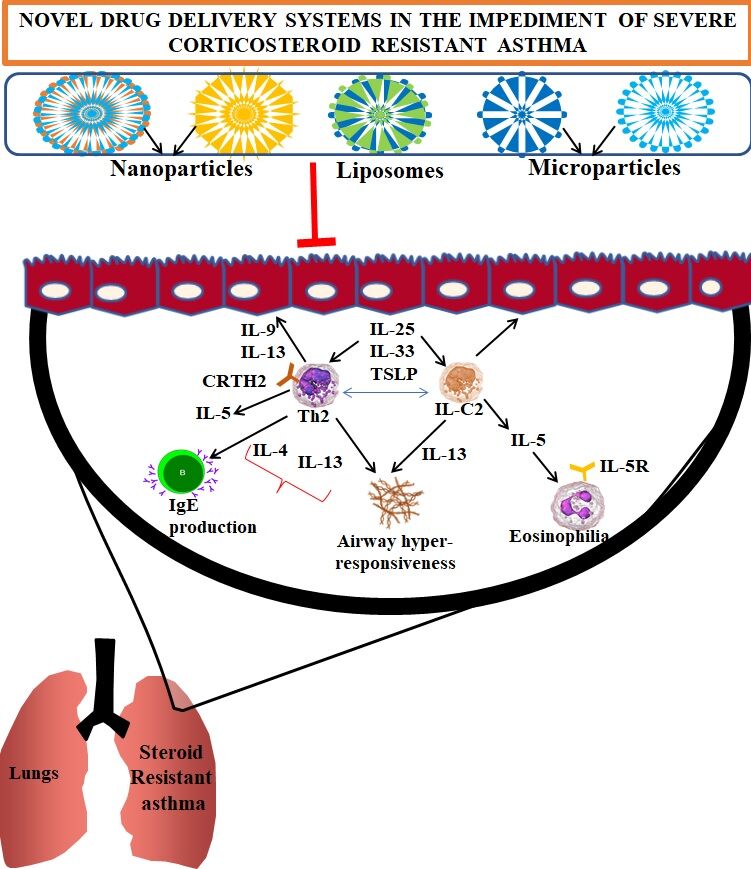
To reverse steroid resistance in SSR asthma after the glucocorticoids administration, it is crucial to develop therapies targeting the signaling molecules that stimulate or suppress the glucocorticoids activity; For instance, HDAC enzyme that deacetylates histone proteins and suppresses proinflammatory cytokines production. Glucocorticoid receptor mediated activity is enhanced by the steroid intake against asthma consequently suppress the proinflammatory gene expression by recruiting HDAC2 (Figure 1); therefore, any decline in the HDAC2 could induce a higher inflammatory cytokine production linked to a significant role of HDAC2 in the acquisition of steroid resistance in bronchial asthma patients,26–30 therefore, targeting this HDAC2 axis may be a crucial strategy to mitigate the pathophysiology of SSR.
Targeting phosphoinositide-3-Kinase (PI3K) Pathway and SSR Asthma
Increased PI3K-p110α levels can cause decline in the RA-inducible gene-I, interferons, and inflammatory cytokine responses subsequently invoke pathophysiological exacerbation in airway inflammation and enhance the susceptibility to viral infection. PI3K axis can be a therapeutic target in steroid resistant asthma due to its substantial role in modulating the levels of inflammatory cytokines in the asthma patients.31,32
Novel Treatment Strategies for Severe Steroid Resistant Asthma (SSR): miR-21/PI3K/HDAC2 Axis
Several miRNAs have been implicated in the pathogenesis of asthma.33,34 For instance, the miR-21 is known to be important in allergic airways disease (AAD) pathogenesis. The miR-21-deficient (miR-21−/−) mice with AAD are associated with decline in the eosinophilic inflammation and IL-4 levels, and elevated IFN-γ responses.33,35 Biological network-based transcriptome analysis of OVA (ovalbumin)-challenged miR-21−/− mice determined that dysregulation of IL-12/IFN-γ plays a significant role in the observed phenotype and miR-21 considered as a key regulator of IFN-γ signalling and T-cell polarization. Thus, the loss of miR-21 augments T-helper cell type 1 (Th1)-related delayed hypersensitivity.36 Interestingly, miR-21 can induce downregulation of PTEN expression, an endogenous suppressor of PI3K.37 A study by Kwak et al37 demonstrated that the mice with ovalbumin (OVA)-induced AAD mitigated PTEN levels across bronchiolar epithelial layer; the adenovirus-mediated overexpression of PTEN in AAD resulted in the increased IL-4 and IL-5 levels in the airways and reduced airway hyper-responsiveness (AHR). Furthermore, intratracheal administration of pharmacological pan-PI3K inhibitors could induce the impairment of bronchial inflammation and AHR in mice with AAD, which concluded the significant potential to implicate PI3K inhibitors (eg.wortmannin) to treat severe asthma.38
Novel Treatment Strategies for mORC1-STAT3-FGFBP1, and SSR Asthma
A recent study delineated the efficacy of poly-L-arginine in enhancing the asthma angiogenesis by modulating the mORC1-STAT3-FGFBP1 signaling in the airway epithelium but this pathway yet to be explored more during the SSR asthma in order to develop novel therapeutic targets to target this pathway.39
Furthermore, the role of miR-21 has been observed to delineate pathogenesis of SSR asthma and previously deciphered its potential role to target Chlamydia, H. influenzae, influenza and Respiratory SyncystialVirus (RSV) infection-induced SSR asthma.40 As per this study, miRNA-21 is an overexpressed gene in mouse models but the miR-21 expression in AAD is not reduced by steroid treatment. Additionally, the putative targets of miR-21 and PTEN expression are mitigated during upregulated expression of miR-21; these changes are associated with higher PI3K responses (Figure 2). A significant association was observed between HDAC2 nuclear levels and the NR3C1 lung expression implicated in the SSR. Thus, a novel miR-21/PTEN/PI3K/HDAC2 signaling was described in the steroid resistant asthma. This was concluded by the administration of antagomiR-21, a miR-21 inhibitor, which impaired the lung miR-21 expression, and enhanced PTEN expression consequently mitigated PI3K activity, and enhanced HDAC2 levels. However, the antagomiR-21 can impair disease pathophysiology associated with severe SSR asthma.
This antagomiR-21 has not exhibited any effect on airway inflammation but retrieved the sensitivity to steroid treatment during severe steroid resistant allergic airway disease (SSRAAD). The treatment of pan-PI3K inhibitor LY294002 also impaired the SSRAAD features. Targeting miR-21 or PI3K could be considered as an effective way which can be implicated in the SSR asthma, eosinophilic asthma, and neutrophilic asthma or several other endotypes of SSR asthma.40 Nanoformulations carrying these gene-based therapeutic miRNAs may effectively enhance the therapeutic window in the patients with SSR, which yet require substantial preclinical and clinical studies.
NLRP3 inflammasome formation significantly involved in the neutrophilic asthma; for instance, the higher NLRP3 and caspase-1 in airways enhanced IL-1β responses followed by the substantial rise in the Th17, and IL-17 generation implicated in AHR.41–46 In addition, the NLRP3, caspase-1 and IL-1β responses could be extensively higher during the experimental models of SSR asthma induced through Chlamydia infection;41 administration of NLRP3 inflammasome blocker, MCC950 could induce ameliorative effects in SSR asthma. So, the therapeutic molecules with nanoformulations which can target NLRP3 signaling might be a beneficial option to mitigate SSR asthma. Mepolizumab treatment can mitigate the requirement of oral steroids in severe eosinophilic asthma.47,48 A specific link between innate type 2 inducers and TSLP (thymic stromal lymphopoietin), T-helper cells, with steroid resistance has also been reported.49,50 In the following sections, we have vividly discussed the possible options of nanomedicine-based formulations, which could be implicated in the mitigation of pathophysiology of SSR.
Application of NDDS in Corticosteroid-Resistant Asthma
Novel Drug Delivery Systems
NDDS are modern therapeutic options through different routes of administration for asthma treatment due to their salient features like target-specific drug action, prolonged release accompanied by specific targeted drug deposit, sustained release, biodegradability, decreased dosage frequency and lesser particle size; In addition, a higher surface area, enhanced solubility, stability, bioavailability making them a very good choice for the formulation scientists in formulating the drugs to treat heterogeneous diseases like asthma. NDDS includes liposomes, niosomes, nanoparticles, implants, solid lipid nanoparticles, polymeric micelles, dendrimers, and microparticles. Several nanomedicine based therapeutic formulations to mitigate the pathophysiology of SSR asthma or AAD were depicted in Figure 3. Application of NDDS to asthma is still in developmental stage, the operation of DDS in steroid resistant asthma is yet require substantial studies. The challenges in employing NDDS to asthma treatment and their implications in steroid resistance in asthma patients were described below.
Mucoadhesive Microparticles
The mucoadhesive polymer-based formulations are previously described for chronic obstructive pulmonary disease (COPD) but the efficacy of these formulations is yet to be explored against steroid resistant asthma as these mucoadhesive polymers possess a high affinity to mucosal surfaces. They possess amino groups in the chitosan, which can form electrostatic interactions with the amino groups of mucus’s anionic groups; subsequently enhance the adhesiveness in order to release the drug moieties across the alveolar region during asthma conditions, which yet to be examined for the corticosteroid asthma.51
Solid lipid microparticles (SLMs) mainly alginate and chitosan-based mucoadhesive SLMs can produce potential capability to form hydrogen bonding with mucin layers subsequently induce drug release. This strategy can be a remarkable strategy to treat asthma patients during corticosteroid therapy.52 For instance, previous studies depicted the application of SLMs made of 3.5–4.0 μm induced the effective delivery of fluticasone across secondary bronchi for treating COPD.52 Another study reported the delivery of budesonide mucoadhesive microparticles containing hyaluronic acid; these kinds of microparticles are in the range of 3.12–5.35 μm, and effectively inhalable range which can prolong Tmax and delay absorption, and prolong the budesonide retention. Furthermore, this phenomenon was also proven in inducing the higher bioavailability in an animal model because of hyaluronic acid’s mucoadhesive ability.53 However, these microparticles are very effective in delivering the antioxidant drug molecules through sustained release pattern, and the also enhancing the drug encapsulation efficiency, which could be an effective strategy to treat corticosteroid resistance in asthma patients.
Nanoparticles
In recent times, nanoparticles are employed to deliver drugs across the alveolar region of the lungs to mitigate the pathophysiology of several airway diseases.54 For instance, the nanoparticles are mainly preferred for modulating the activity of endothelium cell adhesion receptors on endothelial lining of respiratory tract during asthma. In addition, the NADPH oxidase inhibitors, antioxidants such as SOD and CAT were formulated in the nanoparticles formulation for effective drug delivery across pulmonary airway in COPD or asthma.55
Solid lipid nanoparticles (SLNs) are biocompatible and exhibit better stability when compared to liposomes. These are non-toxic when compared to polymeric nanoparticles, and production can be easily scaled up. By using melt-emulsion method, Castellani et al formulated SLNs by melt emulsion technique with proanthocyanidins as a drug that reduced oxidative stress via lowering ROS generation. SLNs were nontoxic towards epithelial cells of respiratory tract. During in vitro studies, the uptake of SLNs with proanthocyanidins was better than those of drugs administered by conventional route of administration.56 Carvalho et al formulated SLNs by fusion-emulsification technique with carvacrol as a drug that reduced the inhalational injury by decreasing malondialdehyde levels causing decline in the histopathological changes, which may be a potential implication in SSR asthma. SLNs typically exhibited polydispersed index of 0.126 ± 0.015 and average particle size of 78.72 ± 0.85 nm.57 The limitations of SLNs could be uncertain drug release pattern and possibility of gelation (in case of polymorphism exhibited by solid lipids).58,59 Hence, these patterns of SLN-based anti-asthmatic formulation can ameliorate the pathophysiology of SSR asthma when the therapeutic molecules combined even in multiple combinations.
Lipid-polymer hybrid nanoparticles (LPNs) possess similar features similar to that of polymeric nanoparticles and liposomes, in terms of stability aspects and biocompatibility. LPNs are composed of poly(lactic-co-glycolic acid) due to its substantial biodegradability; it can produce a suitable core to load several drugs including novel anti-asthma drugs. LPNs can effectively deliver drugs to cellular level . A study conducted by Thanki et al on H1299, lung carcinoma cell line with LPNs loaded with polylactic-co-glycolic acid, a biodegradable polymer and lipidoid showed gene silencing properties on the cell line.60 LPNs were employed to deliver siRNA into the cell effectively as a part of therapy for COPD.61 LPNs formulated with PLGA and an antioxidant Mn-porphyrin dimer (MnPD) were employed in treating COPD patients and showed higher HDAC2 levels inside the cell.62 Lipidoid possesses the alkylated tetraamine backbone, many secondary and tertiary amines that makes easier interaction with anionic siRNA molecules. This kind of LPN-based drug formulation could be other alternate approaches to target SSR asthma. Simplification of preparation methods and effort to scale up LPNs production could be possible in the future perspective.
Theranostics, or the use of multifunctional nanomaterials that combine both therapeutic and imaging modalities, has brought a new age in current treatment techniques.63 Silk fibroin nanoparticles can be prepared using a one-step desolvation process. To generate self-assembled CeNP-CD@SFSNPs nanocomposites, these anionic SFSNPs were combined with CeNPs and PEI-passivated carbon dots (CDs). The synthesized self-assembled CeNP-CD@SNPs-based nanocomposite could effectively minimize the oxidative stress in allergic asthma conditions when formulated with sulfarophane, which can lower ROS levels.64 Such multifunctional nanocomposites could be great choices for delivering medications to the patients to prevent oxidative stress while also beneficial to mitigate disease pathophysiology including SSR asthma.
Nanotechnology adds a new dimension to customized medication delivery, with several advantages in COPD. However, nanomaterials for therapeutic delivery must be investigated for possible health risks, particularly as distinct NPs are reported to induce toxicity in proportion to their nanometer size, spawning a new area called nanotoxicology.65 Through direct and indirect biological interactions, NPs also contribute to oxidative stress. The interaction of nanoparticles with interior cellular components can cause oxidation, which worsens the severity of oxidative stress. NPs may also interact with cells indirectly, regulating ROS generation and modulate cellular phagocytic activity and oxidative burst. The oxidative stress generated by silica (SiO2) nanoparticles was studied by Liu et al. The fluorescent probe DCFH-DA was utilized to measure the fluorescence intensity and detect ROS. Remarkably, the fluorescence in cells exposed to silica NPs was 1.7 times greater than in cells exposed to micro-sized SiO2 particles. The study found that cells exposed to silica NPs suffered significantly higher oxidative damage than A549 cells exposed to micronized silica.66 Because silica NPs are smaller and have a greater surface area, they can interact with cellular or subcellular structures more efficiently. However, there is a high amount of evidence accumulated pertinent to the toxicity, which suggests that NPs can cause oxidative stress. When creating nanoparticles for the treatment of oxidative stress-related asthma, or SSR asthma, it is crucial to ascertain NPs-induced toxicity and oxidative stress.
Formulation innovations pertinent to NPs offer a powerful methodology for drug conveyance in asthma conditions. Nonetheless, a few issues still need to be settled before its utilization in a clinical setting. Until this point in time, no clinical investigations utilizing NPs in the treatment of oxidative stress have been enrolled in the ‘clinical trials.gov’ information database. NPs cannot be straightforwardly utilized for the inhalation since they are not in the ideal size range for inward breath (optimal middle mass streamlined measurement (MMAD) for the statement in the little aviation routes and alveoli of the lungs ought to be 1 to 5 µm). Moreover, NPs exhibit predominantly bigger surfaces, which may bring about an increment in their free energy and expand the association between particles. The substantial levels of association between nanoparticles can induce agglomeration of the therapeutic molecules, which is considered as one of the significant limitations in the implications of NPs in developing the anti-SSR formulations.
To resolve these issues, NPs could be formulated along with microparticles. The strong grid can forestall the connection between NPs, limit their versatility, and increment their drawn-out security. More significantly, it can further develop aerosolization properties for effective delivery of novel anti-SSR formulations.
Liposomes
Liposome-based formulations could also be effective therapeutic option in order to enhance the combinatorial drug release, bioavailability, biodegradability during COPD treatment. Hence, these formulations composed of lipid moieties which can have a very good compatibility across lung tissue and confer the effective intracellular delivery of drug molecules through fusion with plasma membrane lipids, receptor mediated endocytosis, and phagocytosis.67–69 In addition, liposomes (Table 1) are also utilised to confer effective release of hydrophilic, hydrophobic, and amphiphilic antioxidants, and antioxidant enzymes to mitigate the oxidative stress-induced damage including asthma.78 Yet, the efficacy of these kinds of formulations must be examined against SSR asthma.
For instance, the liposome-based formulations pertinent to ‘antioxidant-containing liposomes’ made of NAC, glutathione, tocopherol, SOD, CAT can be used as a therapeutic option for acute oxidant-related COPD. However, due to their unfavourable physicochemical properties, the efficacy of SOD and CAT is limited. Antioxidant efficiency of these enzymes is significantly higher by the liposome entrapment.81 For example, the NAC loaded liposomes could induce a higher efficacy by mitigating the lung permeability index during acute (4-h) CEES induced injury; in addition, there was a significant reduction in the levels of pro-inflammatory mediators in bronchoalveolar lavage fluids in the lungs exposed to substantial levels of oxidants.82 Curcumin-loaded liposomes combined with chitosan also synergistically exerted their efficacy against oxidant-induced lung damage.83 Translating the liposome treatment into the clinical aspects has been facing a lot of challenges due to the limited delivery, relative fragility in disease treatment like COPD. Therefore, the synthesis of liposome formulations of the specific anti-asthmatics requires special attention to avoid the difficulties in drug delivery in SSR asthma.84–86
Nanocomposite microparticles: SSR Asthma
Nanocomposite microparticles (NCMPs) have been used in treating respiratory diseases including COPD in order to mitigate oxidative stress effectively. The NCMPs can be prepared by combining both nanoparticles and microparticles; they can undergo dissociation at physiological conditions into original nanoparticles and also maintain nanocarrier properties in order to deliver the drug moieties.87,88 NCMPs combined with miR-146 formulated with PGA-co-PGL along with mannitol and leucine can enhance the therapeutic efficacy and bioactivity of miR-146a against COPD.89 It is easier to maintain the structure of nanoparticles as NCMPs atomized suspension made using biodegradable polymers and could be an effective treatment for COPD.89 Therefore, the extensive research studies requiring the formulation of the antioxidant molecules with a higher antioxidant capacity in the encapsuled NCMPs form can be considered as a very good therapeutic agent for SSR asthma to mitigate pathophysiology.
Significantly higher FPF (51.33%) and MMAD of less than 5 µm of microparticles reported with the ability of NCMPs deposition across the lungs; but this kind of efficacy in drug delivery can be achieved by selecting suitable excipients, adjuvants, and drying parameters, and drug concentration. These strategies can enhance the longer tissue retention times, and sustained drug release, and substantial cellular update, which could be very effective NDDS to treat SSR asthma.
On the other hand, the drug-loaded nanoparticles embedded in the swellable microparticles can be another effective NDDS approach to enhance drug delivery across lung regions in COPD or SSR asthma conditions. For instance, the curcumin-loaded PLGA nanoparticles with pegylated chitosan can form hydrogel microspheres in the range of 3.1–3.9 nm, and 221–243 nm delivered the drug in a sustained manner with good biodegradation rate even upon substantial drug loading. These are easily respirable and exhibit low tendency to generate TNF-α and mitigated macrophage update.90 Yet, the swellable microparticle delivery for lung diseases requires substantial research, mainly the formulated swellable microparticles with antioxidant therapeutic molecules.
Formulation of lipid-polymer hybrid nanoparticles (LPNs) can exhibit typically both nanoparticles and liposomes due to their physical stability, subsequently they can produce a good efficacy to foster in vivo cellular delivery. For instance, the lipidoid-modified LPNs (Table 2) can effectively induce gene knocking effects in NSCLC cancer cells, H1229 and induce intracellular siRNA delivery in order to target gene expression related to COPD. 60 These lipidoid formulations contain secondary and tertiary amines for61 the effective interaction with siRNA without changing their net charge of LPNs. Formulation of LPNs with PLGA core with antioxidant Mn-porphyrin dimers combined with cationic lipid (DOTAP) shell effectively binds with pHDAC2. This kind of formulation can easily mitigate oxidative stress due to the multi-antioxidative capacity of MnPD.62 Therefore, the LPNs-based drug molecule formulation can mitigate the pathophysiology of SSR asthma comparatively with a higher rate of drug delivery than the formulations administered in the conventional dosage forms.
A tailored drug delivery can be attained by the formulations made of noble metals such as silver, gold, and inorganic elements such as carbon, silicon dioxide, and iron oxide through the formulation of nanoparticles (Table 2). These kinds of inorganic NPs can induce significantly higher biocompatibility through drug delivery across pulmonary regions for treating COPD. Cationic metallic NPs can be used for the delivery of genes as they can effectively bind to DNA/RNA as inorganic NPs are considered as the potential nanocarriers for treating COPD.91 Gold NPs can deliver the drug formulations92 across the endothelium as they can be easily formulated with antioxidant CAT, SOD; these inorganic NPs can protect drug formulations from proteolysis suggesting a significant therapeutic approach to treat SSR asthma due to their efficacy in inducing anti-oxidant and anti-inflammatory effects in animal models of acute inflammation or oxidative stress.86 The findings revealed that SOD/CAT and the nanocarriers work together to give a synergetic antioxidant effect. Nonetheless, in vivo research is needed to further investigate this strategy.93 Activity of endothelial cells can be modulated using antioxidant enzyme nanocarrier formulation to induce anti-inflammatory effects.
Inorganic nanoparticles exhibit inherent oxidant-producing capabilities. However, these limitations can constrain their usage but the formulation of antioxidant inorganic NPs could prevent NPs induced oxidative damage. Another significant strategy is the dendrimers-type of NPs which can easily release drug moieties across the lungs and the dendrimers can be made of the polymers such as “poly(amidoamine) (PAMAM), ploy(L -lysine) (PLL), polyamides, polyesters (PGLSA-OH), polypropylenimine (PPI), ploy (2,2-bis(hydroxyl methyl) propionic acid), and polyethers”.106 These formulations can mitigate toxicity, and increase pharmacokinetic profiles as well as the aqueous solubility when combined with PEGylation. For instance, the PEGylatedpoly(lysine) dendrimers could be referred to as the effective pulmonary delivery agents due to their ability to induce longer drug retention across the lungs; futher, they can enable effective biodistribution for regulated drug delivery in passive circulation.91,107 PAMAM dendrimers which have been shown to deliver biomacromolecules resembling siRNA.108,109 This kind of formulation strategies also can enhance the longer drug retention during the treatment of COPD or SSR asthma.
Another formulation strategy such as microparticulate powders designed in dimethyl fumarate, an Nrf-2 activator was formulated to treat pneumonitis. These are spray dried particles and utilize the D-mannitol as a spray execution enhancer due to MMAD and a thin molecular portion of 49%. This was proven effective in inducing ameliorative effects by treating COPD, and these forumation strategies yet to be examined against asthma.110 A comparative efficacy of co-spray dried powder was described by Trotta et al. In their review, inhalable microparticles containing budesonide, and resveratrol were described to create a multi drug inhalable plan for the treatment of COPD.111 This kind of formulation strategy against SSR asthma also can mitigate the underlying pathophysiology. The planning of microscale powder is basic and simple to increase in this formulation but drug stacking and brief term of activity are normally experienced difficulties.112
Translation of the NDDS from Bench Side to Bedside in SSR Asthma Patients
The lungs can act as a filtration unit removing all the pollutants and also harmful microbes thus protecting the respiratory system and also have a very composite structure. The lungs play a crucial role in the normal functioning of the respiratory system thus sometimes deposition of NPs is a problem for several functions like air humidification, temperature control across the thoracic and tracheobronchial regions, and gas exchange in the alveolar-interstitial region. Thus, the deposition of the NPs must cross the several barriers across lungs and also the physiology of lungs with high humidity (around 90%) during the phase of respiration may have interference with deposition and particle size. Initially, NCMPs need to attain the bloodstreams by moving across the lung obstruction; NCMPs tend to get retained by the lungs for longer duration which are associated with lining fluid or cells; and they are engulfed by macrophages in the phagocytosis process. Thus, the distribution of NPs must overcome several obstructions like epithelial tight junctions, immunological cells, and lung lining fluids must reach in order to induce sustained drug release.
Oxidative stress in COPD or SSR asthma can be treated with the help of inhalable microparticles. Several clinical studies have been conducted recently to prove the effectiveness of the inhalable microparticles. Particle replication process has been used in non-wetting Templates (PRINT) by Dumont et al to create uniform size and shaped dry powder microparticles which are undergoing Phase I clinical trial study. Ribavirin can be delivered effectively to the lung by two new inhalation formulations (Ribavirin-PRIN-CFI and Ribavirin-PRINT-IP) which can also minimize the bystander exposure. These can be examined against SSR asthma in order to mitigate the pathophysiology.
The main treatments of SSR asthma are the therapies to reduce the oxidative stress and drugs against inflammation. But frequent usage of the steroidal anti-asthmatic drugs could result in harmful side effects due to the development of steroid resistance. To minimize the associated side effects, safe and effective sustained release drugs should be used so that the drug administration could be minimized. In order to achieve the sustained release of drugs, the preparation of these drug molecules should be made in the form of biodegradable microparticles or NPs or other liposome-based nanoformulations which are required for the delivery to the pulmonary region.
Limitations
Normal surfactant-based drug delivery systems exhibit significant disadvantages of attaining premature drug releases due to the formation of micelle disruption if the micelle concentration declined below the critical micellar concentration.113 In case of spongosomes, and cubosomes, there are significant challenges observed in order to improve the loading capacities for enhancing the therapeutic window.113 Defects in the overall drug penetration are one of the prominent limitations when using nanoparticles in treating several diseases including cancers; this is due the perivascular accumulation and slow release, which consequently confer to the hindrance for effective drug delivery.
Conclusion
SSR asthma is a significant clinical problem and it should be addressed with the aid of more effective and competent therapies in those patients who are not responsive to the mainstay corticosteroid therapies. SSR asthma is also linked to the bacterial, viral respiratory infection, and the high-fat diet/obesity. Therefore, the strategies to target the signaling pathways such as mORC1-STAT3-FGFBP1, NLRP3 inflammasomes, miR-21/PI3K/HDAC2 axis, PI3K pathways with the aid of novel nanomedicine-based formulations may mitigate pathophysiology of SSR asthma and enhance the clinical outcomes.Several nanomedicine based formulations yet to be examined in the future studies against SSR asthma in order to identify and characterize their roles through therapeutic targeting.
Summary
- Immune signaling heterogeneity pertinent to the pathophysiology of SSR asthma is a predominant obstacle to choose efficient therapeutic modalities to overcome steroid resistance.
- The mORC1-STAT3-FGFBP1, NLRP3 inflammasomes, miR-21/PI3K/HDAC2 axis, PI3K pathways could contribute to the alterations in the immune heterogeneity during SSR asthma.
- Novel nanomedicine-based formulations may mitigate pathophysiology of SSR asthma by modulating immune heterogeneity and enhance the clinical outcomes.
Future Directions
As we discussed the immune repertoire of SSR, the exacerbation of SSR is exemplified by the presence of several specific cytokines such as IL-17, and IL-33 or immune effector elements, which could be used as specific molecular markers or therapeutic targets to treat SSR. A detailed investigation yet considered as unmet need in the clinical sector to prefer a personalized therapy for treating SSR. Albeit the respiratory system is highly accessible to several therapies to treat respiratory diseases, the gene therapy can satisfy the colloidal stability across the physiological fluids but may not overcome nucleases-mediated degradation. Therefore, nanomedicine-based delivery vectors may confer protection against degradation of these therapeutics when treating chronic respiratory diseases. In this scenario, several nanomedicine-based formulations yet to be examined in the future studies against SSR asthma in order to identify and characterize their roles through therapeutic targeting against Th2 transcription factors, and cytokines. Recently stem cell-based exosomes in the nanotherapeutic formulation have been explored for treating autoimmune diseases.114 For instance, the proteomic characterization is required to explore the pharmacological mechanisms and efficacy of these molecules against SSR.
Acknowledgments
Our sincere thanks to the staff of Department of Radiation oncology, The First affiliated Hospital of Zhengzhou University. Authors thanks JSSAHER for the fellowship given to Narasimha M Beeraka, HemanthVikram PR, JSS Pharmacy college, Mysuru, India, and Narasimha M Beeraka, CEMR, Mysuru, JSSAHER, Karnataka, India.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
This study was supported by the National Natural Science Foundation of China (No. 81700729).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Menzies-Gow A, Mansur AH, Brightling CE. Clinical utility of fractional exhaled nitric oxide in severe asthma management. Eur Respir J. 2020;55(3):1901633. doi:10.1183/13993003.01633-2019
2. Klehm C, Hildebrand E, Meyers MS. Mitigating chronic diseases during archaeological fieldwork: lessons from managing asthma, diabetes, and depression. Adv Archaeol Pract. 2021;9(1):41–48. doi:10.1017/aap.2020.49
3. Dharmage S, Perret J, Custovic A. Epidemiology of asthma in children and adults. Front Pediatr. 2019;7:246. doi:10.3389/fped.2019.00246
4. Reddel HK, Busse WW, Pedersen S, et al. Should recommendations about starting inhaled corticosteroid treatment for mild asthma be based on symptom frequency: a post-hoc efficacy analysis of the START study. Lancet. 2017;389(10065):157–166. doi:10.1016/S0140-6736(16)31399-X
5. Reddel HK, Bacharier LB, Bateman ED, et al. Global Initiative for Asthma (GINA) strategy 2021–executive summary and rationale for key changes. J Allergy Clin Immunol Pract. 2021. doi:10.1183/13993003.02730-2021
6. Adcock IM, Ford PA, Bhavsar P, Ahmad T, Chung KF. Steroid resistance in asthma: mechanisms and treatment options. Curr Allergy Asthma Rep. 2008;8(2):171–178. doi:10.1007/s11882-008-0028-4
7. Marshall CL, Hasani K, Mookherjee N. Immunobiology of steroid-unresponsive severe asthma. Front Allergy. 2021;50:718267.
8. Xu Y, Liu H, Song L. Novel drug delivery systems targeting oxidative stress in chronic obstructive pulmonary disease: a review. J Nanobiotechnology. 2020;18(1):1–25. doi:10.1186/s12951-020-00703-5
9. Mishra B, Singh J. Novel drug delivery systems and significance in respiratory diseases. In: Targeting Chronic Inflammatory Lung Diseases Using Advanced Drug Delivery Systems. Elsevier; 2020:57–95.
10. SreeHarsha N, Venugopala KN, Nair AB, et al. An efficient, lung-targeted, drug-delivery system to treat asthma via microparticles. Drug Des Devel Ther. 2019;13:4389. doi:10.2147/DDDT.S216660
11. Takizawa H. Recent development of drug delivery systems for the treatment of asthma and related disorders. Recent Pat Inflamm Allergy Drug Discov. 2009;3(3):232–239. doi:10.2174/187221309789257414
12. Xue Y, Zhou Y, Bao W, et al. STAT3 and IL-6 contribute to corticosteroid resistance in an OVA and ozone-induced asthma model with neutrophil infiltration. Front Mol Biosci. 2021;8. doi:10.3389/fmolb.2021.717962
13. Thompson RJ, Sayers I, Kuokkanen K, Hall IP. Purinergic receptors in the airways: potential therapeutic targets for asthma? Front Allergy. 2021;2:16. doi:10.3389/falgy.2021.677677
14. Gounni AS, Koussih L, Atoui S, Tliba O. New insights on the role of Pentraxin3 in allergic asthma. Front Allergy. 2021;2:20.
15. Essilfie A-T, Horvat JC, Kim RY, et al. Macrolide therapy suppresses key features of experimental steroid-sensitive and steroid-insensitive asthma. Thorax. 2015;70(5):458–467. doi:10.1136/thoraxjnl-2014-206067
16. Vesikari T, Van Damme P, Giaquinto C, et al. European Society for Paediatric Infectious Diseases consensus recommendations for rotavirus vaccination in Europe: update 2014. Pediatr Infect Dis J. 2015;34(6):635–643. doi:10.1097/INF.0000000000000683
17. Essilfie A-T, Simpson JL, Horvat JC, et al. Haemophilus influenzae infection drives IL-17-mediated neutrophilic allergic airways disease. PLoS Pathog. 2011;7(10):e1002244. doi:10.1371/journal.ppat.1002244
18. Simpson JL, Powell H, Boyle MJ, Scott RJ, Gibson PG. Clarithromycin targets neutrophilic airway inflammation in refractory asthma. Am J Respir Crit Care Med. 2008;177(2):148–155. doi:10.1164/rccm.200707-1134OC
19. Chakraborty K, Paulraj R. Sesquiterpenoids with free-radical-scavenging properties from marine macroalga Ulva fasciataDelile. Food Chem. 2010;122(1):31–41. doi:10.1016/j.foodchem.2010.02.012
20. Shah SAA, Hassan S, Bungau S, et al. Chemically diverse and biologically active secondary metabolites from marine Phylum chlorophyta. Mar Drugs. 2020;18(10):493. doi:10.3390/md18100493
21. Manzoor Z, Koo J-E, Ali I, et al. 4-Hydroxy-2, 3-dimethyl-2-nonen-4-olide has an inhibitory effect on pro-inflammatory cytokine production in CpG-stimulated bone marrow-derived dendritic cells. Mar Drugs. 2016;14(5):88. doi:10.3390/md14050088
22. Lee H-J, Kang G-J, Yang E-J, et al. Two enone fatty acids isolated from Gracilaria verrucosa suppress the production of inflammatory mediators by down-regulating NF-κB and STAT1 activity in lipopolysaccharide-stimulated Raw 264.7 cells. Arch Pharm Res. 2009;32(3):453–462. doi:10.1007/s12272-009-1320-0
23. Kozuma S, Hirota-Takahata Y, Fukuda D, Kuraya N, Nakajima M, Ando O. Identification and biological activity of ogipeptins, novel LPS inhibitors produced by marine bacterium. J Antibiot (Tokyo). 2017;70(1):79–83. doi:10.1038/ja.2016.81
24. Chen S, Wang J, Lin X, et al. Chrysamides A–C, three dimeric nitrophenyl trans -epoxyamides produced by the deep-sea-derived fungus Penicillium chrysogenum SCSIO41001. Org Lett. 2016;18(15):3650–3653. doi:10.1021/acs.orglett.6b01699
25. Jeong S, Ku S-K, Min G, Choi H, Park DH, Bae J-S. Suppressive effects of three diketopiperazines from marine-derived bacteria on polyphosphate-mediated septic responses. Chem Biol Interact. 2016;257:61–70. doi:10.1016/j.cbi.2016.07.032
26. Ito K, Getting S, Charron C. Mode of glucocorticoid actions in airway disease. Scientific World J. 2006;6:1750–1769. doi:10.1100/tsw.2006.274
27. Ito K, Ito M, Elliott WM, et al. Decreased histone deacetylase activity in chronic obstructive pulmonary disease. N Engl J Med. 2005;352(19):1967–1976. doi:10.1056/NEJMoa041892
28. Ito K, Barnes PJ, Adcock IM. Glucocorticoid receptor recruitment of histone deacetylase 2 inhibits interleukin-1β-induced histone H4 acetylation on lysines 8 and 12. Mol Cell Biol. 2000;20(18):6891–6903. doi:10.1128/MCB.20.18.6891-6903.2000
29. Ito K, Yamamura S, Essilfie-‐Quaye S, et al. Histone deacetylase 2-‐mediated deacetylation of the glucocorticoid receptor enables NF-‐kappaB suppression. J Exp Med. 2006;203(1):7–13. doi:10.1084/jem.20050466
30. Löfgren C, Hjortsberg L, Blennow M, et al. Mechanisms of cross-resistance between nucleoside analogues and vincristine or daunorubicin in leukemic cells. Biochem Biophys Res Commun. 2004;320(3):825–832. doi:10.1016/j.bbrc.2004.06.016
31. Chen-Yu Hsu A, Starkey MR, Hanish I, et al. Targeting PI3K-p110α suppresses influenza virus infection in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2015;191(9):1012–1023. doi:10.1164/rccm.201501-0188OC
32. Wadhwa R, Dua K, Adcock IM, Horvat JC, Kim RY, Hansbro PM. Cellular mechanisms underlying steroid-resistant asthma. Eur Respir Rev. 2019;28:190096.
33. Foster PS, Plank M, Collison A, et al. The emerging role of micro RNA s in regulating immune and inflammatory responses in the lung. Immunol Rev. 2013;253(1):198–215. doi:10.1111/imr.12058
34. Plank MW, Maltby S, Tay HL, et al. MicroRNA expression is altered in an ovalbumin-induced asthma model and targeting miR-155 with antagomirs reveals cellular specificity. PLoS One. 2015;10(12):e0144810. doi:10.1371/journal.pone.0144810
35. Lu TX, Munitz A, Rothenberg ME. MicroRNA-21 is up-regulated in allergic airway inflammation and regulates IL-12p35 expression. J Immunol. 2009;182(8):4994–5002. doi:10.4049/jimmunol.0803560
36. Cruz-Topete D, Cidlowski JA. One hormone, two actions: anti-and pro-inflammatory effects of glucocorticoids. Neuroimmunomodulation. 2015;22(1–2):20–32. doi:10.1159/000362724
37. Kwak Y, Song CH, Yi HK, et al. Involvement of PTEN in airway hyperresponsiveness and inflammation in bronchial asthma. J Clin Invest. 2003;111(7):1083–1092. doi:10.1172/JCI16440
38. Sanjeewa KA, Jayawardena TU, Lee HG, Herath KH, Jee Y, Jeon Y-J. The protective effect of Sargassum horneri against particulate matter-induced inflammation in lung tissues of an in vivo mouse asthma model. Food Funct. 2019;10(12):7995–8004. doi:10.1039/C9FO02068C
39. Chen X, Miao M, Zhou M, et al. Poly-L-arginine promotes asthma angiogenesis through induction of FGFBP1 in airway epithelial cells via activation of the mTORC1-STAT3 pathway. Cell Death Dis. 2021;12(8):1–14. doi:10.1038/s41419-021-04055-2
40. Kim RY, Horvat JC, Pinkerton JW, et al. MicroRNA-21 drives severe, steroid-insensitive experimental asthma by amplifying phosphoinositide 3-kinase–mediated suppression of histone deacetylase 2. J Allergy Clin Immunol. 2017;139(2):519–532. doi:10.1016/j.jaci.2016.04.038
41. Kim R, Pinkerton J, Essilfie A, Robertson A, Baines K, Brown A. Inhibition of NLRP3 inflammasome-mediated, interleukin-1β-dependent inflammatory responses attenuates severe, steroid-resistant experimental asthma. Am J Respir Crit Care Med. 2017;196(3):283–297. doi:10.1164/rccm.201609-1830OC
42. Pinkerton JW, Kim RY, Robertson AA, et al. Inflammasomes in the lung. Mol Immunol. 2017;86:44–55. doi:10.1016/j.molimm.2017.01.014
43. Simpson JL, Grissell TV, Douwes J, Scott RJ, Boyle MJ, Gibson PG. Innate immune activation in neutrophilic asthma and bronchiectasis. Thorax. 2007;62(3):211–218. doi:10.1136/thx.2006.061358
44. Baines KJ, Simpson JL, Wood LG, Scott RJ, Gibson PG. Transcriptional phenotypes of asthma defined by gene expression profiling of induced sputum samples. J Allergy Clin Immunol. 2011;127(1):153–160. e159. doi:10.1016/j.jaci.2010.10.024
45. Simpson JL, Phipps S, Baines KJ, Oreo KM, Gunawardhana L, Gibson PG. Elevated expression of the NLRP3 inflammasome in neutrophilic asthma. Eur Respir J. 2014;43(4):1067–1076. doi:10.1183/09031936.00105013
46. Darville T, Welter-Stahl L, Cruz C, Sater AA, Andrews CW, Ojcius DM. Effect of the purinergic receptor P2X 7 on chlamydia infection in cervical epithelial cells and vaginally infected mice. J Immunol. 2007;179(6):3707–3714. doi:10.4049/jimmunol.179.6.3707
47. Bel EH, Ortega HG, Pavord ID. Glucocorticoids and mepolizumab in eosinophilic asthma. N Engl J Med. 2014;371(13):2434. doi:10.1056/NEJMoa1403291
48. Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. NEJM. 2014;371(13):1198–1207. doi:10.1056/NEJMoa1403290
49. Starkey MR, McKenzie AN, Belz GT, Hansbro PM. Pulmonary group 2 innate lymphoid cells: surprises and challenges. Mucosal Immunol. 2019;12(2):299–311. doi:10.1038/s41385-018-0130-4
50. Robinson D, Humbert M, Buhl R, et al. Revisiting T ype 2‐high and T ype 2‐low airway inflammation in asthma: current knowledge and therapeutic implications. Clin Exp Allergy. 2017;47(2):161–175. doi:10.1111/cea.12880
51. Mansuri S, Kesharwani P, Jain K, Tekade RK, Jain N. Mucoadhesion: a promising approach in drug delivery system. React Funct Polym. 2016;100:151–172. doi:10.1016/j.reactfunctpolym.2016.01.011
52. Amore E, Ferraro M, Manca ML, et al. Mucoadhesive solid lipid microparticles for controlled release of a corticosteroid in the chronic obstructive pulmonary disease treatment. Nanomedicine. 2017;12(19):2287–2302. doi:10.2217/nnm-2017-0072
53. Liu T, Han M, Tian F, Cun D, Rantanen J, Yang M. Budesonide nanocrystal-loaded hyaluronic acid microparticles for inhalation: in vitro and in vivo evaluation. Carbohydr Polym. 2018;181:1143–1152. doi:10.1016/j.carbpol.2017.11.018
54. Omlor AJ, Nguyen J, Bals R, Dinh QT. Nanotechnology in respiratory medicine. Respir Res. 2015;16(1):1–9. doi:10.1186/s12931-015-0223-5
55. Villegas L, Stidham T, Nozik-Grayck E. Oxidative stress and therapeutic development in lung diseases. J Respir Pulm Med 2014;4(04). doi:10.4172/2161-105X.1000194
56. Castellani S, Trapani A, Spagnoletta A, et al. Nanoparticle delivery of grape seed-derived proanthocyanidins to airway epithelial cells dampens oxidative stress and inflammation. J Transl Med. 2018;16(1):1–15. doi:10.1186/s12967-018-1509-4
57. Carvalho FO, Silva ÉR, Nunes PS, et al. Effects of the solid lipid nanoparticle of carvacrol on rodents with lung injury from smoke inhalation. Naunyn-Schmiedeberg’s Arch Pharmacol. 2020;393(3):445–455. doi:10.1007/s00210-019-01731-1
58. Müller R, Radtke M, Wissing S. Nanostructured lipid matrices for improved microencapsulation of drugs. Int J Pharm. 2002;242(1–2):121–128. doi:10.1016/S0378-5173(02)00180-1
59. Mehnert W, Mäder K. Solid lipid nanoparticles: production, characterization and applications. Adv Drug Deliv Rev. 2012;64:83–101. doi:10.1016/j.addr.2012.09.021
60. Thanki K, Zeng X, Justesen S, et al. Engineering of small interfering RNA-loaded lipidoid-poly (DL-lactic-co-glycolic acid) hybrid nanoparticles for highly efficient and safe gene silencing: a quality by design-based approach. Eur J Pharm Biopharm. 2017;120:22–33. doi:10.1016/j.ejpb.2017.07.014
61. Akinc A, Zumbuehl A, Goldberg M, et al. A combinatorial library of lipid-like materials for delivery of RNAi therapeutics. Nat Biotechnol. 2008;26(5):561–569. doi:10.1038/nbt1402
62. Chikuma K, Arima K, Asaba Y, et al. The potential of lipid-polymer nanoparticles as epigenetic and ROS control approaches for COPD. Free Radic Res. 2020;54(11–12):829–840. doi:10.1080/10715762.2019.1696965
63. Wang S, Yang X, Zhou L, Li J, Chen H. 2D nanostructures beyond graphene: preparation, biocompatibility and biodegradation behaviors. J Mater Chem B. 2020;8(15):2974–2989. doi:10.1039/C9TB02845E
64. Passi M, Kumar V, Packirisamy G. Theranostic nanozyme: silk fibroin based multifunctional nanocomposites to combat oxidative stress. Mater Sci Eng C. 2020;107:110255. doi:10.1016/j.msec.2019.110255
65. Boland S, Guadagnini R, Baeza-Squiban A, Hussain S, Marano F. Nanoparticles used in medical applications for the lung: hopes for nanomedicine and fears for nanotoxicity. Proc J Phy. 2011;2011:012031.
66. Liu W, Hu T, Zhou L, et al. Nrf2 protects against oxidative stress induced by SiO 2 nanoparticles. Nanomedicine. 2017;12(19):2303–2318. doi:10.2217/nnm-2017-0046
67. Willis L, Hayes D, Mansour HM. Therapeutic liposomal dry powder inhalation aerosols for targeted lung delivery. Lung. 2012;190(3):251–262. doi:10.1007/s00408-011-9360-x
68. Allen TM. Liposomal drug formulations. Drugs. 1998;56(5):747–756. doi:10.2165/00003495-199856050-00001
69. Pinheiro M, Lúcio M, Lima JL, Reis S. Liposomes as drug delivery systems for the treatment of TB. Nanomedicine. 2011;6(8):1413–1428. doi:10.2217/nnm.11.122
70. Turrens JF, Crapo JD, Freeman B. Protection against oxygen toxicity by intravenous injection of liposome-entrapped catalase and superoxide dismutase. J Clin Invest. 1984;73(1):87–95. doi:10.1172/JCI111210
71. Suntres ZE, Shek PN. Prevention of phorbol myristate acetate-induced acute lung injury by α-tocopherol liposomes. J Drug Target. 1995;3(3):201–208. doi:10.3109/10611869509015946
72. Barnard ML, Baker RR, Matalon S. Mitigation of oxidant injury to lung microvasculature by intratracheal instillation of antioxidant enzymes. Am J Physiol Lung Cell Mol Physiol. 1993;265(4):L340–L345. doi:10.1152/ajplung.1993.265.4.L340
73. Raghu G, Amatto VC, Behr J, Stowasser S. Comorbidities in idiopathic pulmonary fibrosis patients: a systematic literature review. Eur Respir J. 2015;46(4):1113–1130. doi:10.1183/13993003.02316-2014
74. Konduri KS, Nandedkar S, Düzgünes N, et al. Efficacy of liposomal budesonide in experimental asthma. J Allergy Clin Immunol. 2003;111(2):321–327. doi:10.1067/mai.2003.104
75. Honmane S, Hajare A, More H, Osmani RAM, Salunkhe S. Lung delivery of nanoliposomal salbutamol sulfate dry powder inhalation for facilitated asthma therapy. J Liposome Res. 2019;29(4):332–342. doi:10.1080/08982104.2018.1531022
76. Saari S, Vidgren M, Herrala J, Turjanmaa V, Koskinen M, Nieminen M. Possibilities of formoterol to enhance the peripheral lung deposition of the inhaled liposome corticosteroids. Respir Med. 2002;96(12):999–1005. doi:10.1053/rmed.2002.1393
77. Elhissi A, Islam M, Arafat B, Taylor M, Ahmed W. Development and characterisation of freeze-dried liposomes containing two anti-asthma drugs. Micro Nano Lett. 2010;5(3):184–188. doi:10.1049/mnl.2010.0032
78. Maret M, Ruffie C, Periquet B, et al. Liposomal retinoic acids modulate asthma manifestations in mice. J Nutr. 2007;137(12):2730–2736. doi:10.1093/jn/137.12.2730
79. Alberca-Custodio RW, Faustino LD, Gomes E, et al. Allergen-specific immunotherapy with liposome containing CpG-ODN in murine model of asthma relies on MyD88 signaling in dendritic cells. Front Immunol. 2020;11:692. doi:10.3389/fimmu.2020.00692
80. O’riordan TG, Waldrep JC, Abraham WM, et al. Delivery of nebulized budesonide liposomes to the respiratory tract of allergic sheep. J Aerosol Med. 1997;10(2):117–128. doi:10.1089/jam.1997.10.117
81. Dekhuijzen PR, Batsiou M, Bjermer L, et al. Incidence of oral thrush in patients with COPD prescribed inhaled corticosteroids: effect of drug, dose, and device. Respir Med. 2016;120:54–63. doi:10.1016/j.rmed.2016.09.015
82. Hoesel LM, Flierl MA, Niederbichler AD, et al. Ability of antioxidant liposomes to prevent acute and progressive pulmonary injury. Antioxid Redox Signal. 2008;10(5):963–972. doi:10.1089/ars.2007.1878
83. Manconi M, Manca ML, Valenti D, et al. Chitosan and hyaluronan coated liposomes for pulmonary administration of curcumin. Int J Pharm. 2017;525(1):203–210. doi:10.1016/j.ijpharm.2017.04.044
84. Barjaktarevic IZ, Arredondo AF, Cooper CB. Positioning new pharmacotherapies for COPD. Int J Chron Obstruct Pulmon Dis. 2015;10:1427. doi:10.2147/COPD.S83758
85. Paranjpe M, Müller-Goymann CC. Nanoparticle-mediated pulmonary drug delivery: a review. Int J Mol Sci. 2014;15(4):5852–5873. doi:10.3390/ijms15045852
86. Hood ED, Chorny M, Greineder CF, Alferiev IS, Levy RJ, Muzykantov VR. Endothelial targeting of nanocarriers loaded with antioxidant enzymes for protection against vascular oxidative stress and inflammation. Biomaterials. 2014;35(11):3708–3715. doi:10.1016/j.biomaterials.2014.01.023
87. Ourique AF, Dos Santos Chaves P, Souto GD, Pohlmann AR, Guterres SS, Beck RCR. Redispersible liposomal-N-acetylcysteine powder for pulmonary administration: development, in vitro characterization and antioxidant activity. Eur J Pharm Sci. 2014;65:174–182. doi:10.1016/j.ejps.2014.09.017
88. Külkamp IC, Rabelo BD, Berlitz SJ, et al. Nanoencapsulation improves the in vitro antioxidant activity of lipoic acid. J Biomed Nanotechnol. 2011;7(4):598–607. doi:10.1166/jbn.2011.1318
89. Mohamed A, Pekoz AY, Ross K, Hutcheon GA, Saleem IY. Pulmonary delivery of Nanocomposite Microparticles (NCMPs) incorporating miR-146a for treatment of COPD. Int J Pharm. 2019;569:118524. doi:10.1016/j.ijpharm.2019.118524
90. El-Sherbiny IM, Smyth HD. Controlled release pulmonary administration of curcumin using swellable biocompatible microparticles. Mol Pharm. 2012;9(2):269–280. doi:10.1021/mp200351y
91. Ding Y, Jiang Z, Saha K, et al. Gold nanoparticles for nucleic acid delivery. Mol Ther. 2014;22(6):1075–1083. doi:10.1038/mt.2014.30
92. Geiser M, Quaile O, Wenk A, et al. Cellular uptake and localization of inhaled gold nanoparticles in lungs of mice with chronic obstructive pulmonary disease. Part Fibre Toxicol. 2013;10(1):1–10. doi:10.1186/1743-8977-10-19
93. Gil D, Rodriguez J, Ward B, Vertegel A, Ivanov V, Reukov V. Antioxidant activity of SOD and catalase conjugated with nanocrystalline ceria. Bioengineering. 2017;4(4):18. doi:10.3390/bioengineering4010018
94. Oyarzun-Ampuero F, Brea J, Loza M, Torres D, Alonso M. Chitosan–hyaluronic acid nanoparticles loaded with heparin for the treatment of asthma. Int J Pharm. 2009;381(2):122–129. doi:10.1016/j.ijpharm.2009.04.009
95. Matsuo Y, Ishihara T, Ishizaki J, Miyamoto K-I, Higaki M, Yamashita N. Effect of betamethasone phosphate loaded polymeric nanoparticles on a murine asthma model. Cell Immunol. 2009;260(1):33–38. doi:10.1016/j.cellimm.2009.07.004
96. Wang W, Zhu R, Xie Q, et al. Enhanced bioavailability and efficiency of curcumin for the treatment of asthma by its formulation in solid lipid nanoparticles. Int J Nanomedicine. 2012;7:3667. doi:10.2147/IJN.S30428
97. Kim DE, Lee Y, Kim M, Lee S, Jon S, Lee S-H. Bilirubin nanoparticles ameliorate allergic lung inflammation in a mouse model of asthma. Biomaterials. 2017;140:37–44. doi:10.1016/j.biomaterials.2017.06.014
98. Chen Y-D, Liang Z-Y, Cen -Y-Y, et al. Development of oral dispersible tablets containing prednisolone nanoparticles for the management of pediatric asthma. Drug Des Devel Ther. 2015;9:5815. doi:10.2147/DDDT.S86075
99. Wang K, Feng Y, Li S, et al. Oral delivery of bavachinin-loaded PEG-PLGA nanoparticles for asthma treatment in a murine model. J Biomed Nanotechnol. 2018;14(10):1806–1815. doi:10.1166/jbn.2018.2618
100. Chan Y, Ng SW, Chellappan DK, et al. Celastrol-loaded liquid crystalline nanoparticles as an anti-inflammatory intervention for the treatment of asthma. Int J Poly Mater Poly Biomater. 2021;70(11):754–763. doi:10.1080/00914037.2020.1765350
101. Yong DOC, Saker SR, Wadhwa R, et al. Preparation, characterization and in-vitro efficacy of quercetin loaded liquid crystalline nanoparticles for the treatment of asthma. J Drug Deliv Sci Technol. 2019;54:101297. doi:10.1016/j.jddst.2019.101297
102. Chakraborty S, Ehsan I, Mukherjee B, et al. Therapeutic potential of andrographolide-loaded nanoparticles on a murine asthma model. Nanomedicine. 2019;20:102006. doi:10.1016/j.nano.2019.04.009
103. Lee D, Shirley SA, Lockey RF, Mohapatra SS. Thiolated chitosan nanoparticles enhance anti-inflammatory effects of intranasally delivered theophylline. Respir Res. 2006;7:112. doi:10.1186/1465-9921-7-112
104. Wang D, Nasab EM, Athari SS. Study effect of Baicalein encapsulated/loaded Chitosan-nanoparticle on allergic Asthma pathology in mouse model. Saudi J Biol Sci. 2021;28(8):4311–4317. doi:10.1016/j.sjbs.2021.04.009
105. Chattopadhyay P, Pathak MP, Patowary P, et al. Synthesized atropine nanoparticles ameliorate airway hyperreactivity and remodeling in a murine model of chronic asthma. J Drug Deliv Sci Technol. 2020;56:101507. doi:10.1016/j.jddst.2020.101507
106. Mehta P, Kadam S, Pawar A, Bothiraja C. Dendrimers for pulmonary delivery: current perspectives and future challenges. New J Chem. 2019;43(22):8396–8409. doi:10.1039/C9NJ01591D
107. Ryan GM, Kaminskas LM, Kelly BD, Owen DJ, McIntosh MP, Porter CJ. Pulmonary administration of PEGylated polylysine dendrimers: absorption from the lung versus retention within the lung is highly size-dependent. Mol Pharm. 2013;10(8):2986–2995. doi:10.1021/mp400091n
108. Conti DS, Brewer D, Grashik J, Avasarala S, da Rocha SR. Poly (amidoamine) dendrimer nanocarriers and their aerosol formulations for siRNA delivery to the lung epithelium. Mol Pharm. 2014;11(6):1808–1822. doi:10.1021/mp4006358
109. Bohr A, Tsapis N, Foged C, Andreana I, Yang M, Fattal E. Treatment of acute lung inflammation by pulmonary delivery of anti-TNF-α siRNA with PAMAM dendrimers in a murine model. Eur J Pharm Biopharm. 2020;156:114–120. doi:10.1016/j.ejpb.2020.08.009
110. Muralidharan P, Hayes D, Black SM, Mansour HM. Microparticulate/nanoparticulate powders of a novel Nrf2 activator and an aerosol performance enhancer for pulmonary delivery targeting the lung Nrf2/Keap-1 pathway. Mol Syst Des Eng. 2016;1(1):48–65. doi:10.1039/C5ME00004A
111. Trotta V, Lee W-H, Loo C-Y, Young PM, Traini D, Scalia S. Co-spray dried resveratrol and budesonide inhalation formulation for reducing inflammation and oxidative stress in rat alveolar macrophages. Eur J Pharm Sci. 2016;86:20–28. doi:10.1016/j.ejps.2016.02.018
112. Liang Z, Ni R, Zhou J, Mao S. Recent advances in controlled pulmonary drug delivery. Drug Discov Today. 2015;20(3):380–389. doi:10.1016/j.drudis.2014.09.020
113. Wakaskar RR. General overview of lipid–polymer hybrid nanoparticles, dendrimers, micelles, liposomes, spongosomes and cubosomes. J Drug Target. 2018;26(4):311–318. doi:10.1080/1061186X.2017.1367006
114. Riazifar M, Mohammadi MR, Pone EJ, et al. Stem cell-derived exosomes as nanotherapeutics for autoimmune and neurodegenerative disorders. ACS nano. 2019;13:6670–6688.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.