Back to Journals » Clinical Ophthalmology » Volume 14
Immediate Reactions to Fluorescein and Indocyanine Green in Retinal Angiography: Review of Literature and Proposal for Patient’s Evaluation
Authors Meira J, Marques ML , Falcão-Reis F, Rebelo Gomes E, Carneiro Â
Received 16 October 2019
Accepted for publication 19 December 2019
Published 20 January 2020 Volume 2020:14 Pages 171—178
DOI https://doi.org/10.2147/OPTH.S234858
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Scott Fraser
Jorge Meira, 1,* Maria Luís Marques, 2,* Fernando Falcão-Reis, 1, 3 Eva Rebelo Gomes, 2 Ângela Carneiro 1, 3
1Department of Ophthalmology, Centro Hospitalar Universitário São João, Porto, Portugal; 2Department of Allergy and Clinical Immunology, Centro Hospitalar Universitário do Porto, Porto, Portugal; 3Department of Surgery and Physiology, Faculty of Medicine, University of Porto, Porto, Portugal
*These authors contributed equally to this work
Correspondence: Jorge Meira
Department of Ophthalmology of São João Hospital, Avenida Prof. Hernâni Monteiro, Porto 4202 – 451, Portugal
Tel +351225512100
Fax +351225513669
Email [email protected]
Introduction: Contrast rapid sequence angiography with fluorescein or indocyanine green (ICG) is a diagnostic procedure commonly used in ophthalmology. Adverse reactions to fluorescein and ICG are rare and may be classified as toxic, of hypersensitivity and non-specific. The evaluation and management of a patient with an adverse reaction is a challenge for the majority of ophthalmologists, as is the assessment of risk factors that may contraindicate the procedure.
Purpose: We aim to review the concepts underlying adverse reactions to fluorescein and ICG, especially those of hypersensitivity, and present a proposal or the evaluation of the patients in need to perform retinal angiography and for the treatment of immediate reactions to fluorescein and ICG.
Methods: The available literature was examined using PubMed-Medline, and using the MeSH terms “fluorescein”, “Indocyanine green”, “ophthalmic dyes”, “retinal angiography”, “adverse reactions”, and “allergic reaction”.
Conclusion: This review may help ophthalmologists to identify patients with higher risk of a hypersensitivity reaction and give them tools to recognize patients with suspected hypersensitivity that may benefit from an allergy study.
Keywords: fluorescein, indocyanine green, ophthalmic dyes, retinal angiography, adverse reactions, hypersensitivity reaction
Introduction
The reported incidence of adverse reactions to fluorescein and indocyanine green (ICG) varies according to studies and has an overall incidence that ranges from 0.05% for ICG and 5% for fluorescein.1 Although rare, they can be severe and life-threatening. Ophthalmologists should be aware of possible risks and complications associated with this procedure.2
Three major types of reactions have been described: hypersensitivity reactions, toxic reactions and non-specific reactions. A hypersensitivity reaction is defined as an immune response that is exaggerated or inappropriate against an antigen or allergen. Coombs and Gell classified hypersensitivity reactions into four types: Type I (IgE mediated), immediate hypersensitivity (typically ≤1 hr after drug administration) reactions (IHR); type II (cytotoxic); type III (immune complex) and type IV (T-cell mediated), late HR (occur >24 hrs until 7 days after drug administration). 2,3 The type I, IgE-mediated reactions are characterized by the release of histamine and other mediators from mast cells and basophiles, following IgE antibody bridging by the allergen. A rapid increase in vascular permeability as well as contraction of smooth muscles lead to symptoms such as urticaria, angioedema, bronchospasm, cardiovascular depression and, in severe cases, shock.4,5 The severity of an anaphylactic reaction can depend on the allergen dose, the entry route, and the amount of allergen-specific IgE antibody.6 If an anaphylaxis is suspected, plasma tryptase (taken approximately 1 hr after the beginning of the reaction) or measurement of urinary methyl histamine should be assessed whether important mast cell degranulation has occurred. The types II, III and IV are not addressed in this review.4
Another classification of reactions due to contrast dyes is based on severity and does not consider its physiopathology. It includes the division into mild, moderate and severe.2,7 Mild reactions are defined as transient and resolve spontaneously without treatment. Most commonly comprise symptoms such as nausea, vomiting, sneezing, inadvertent arterial injection and pruritus. Moderate adverse reactions include urticaria, angioedema, syncope, thrombophlebitis, pyrexia, local tissue necrosis, nerve palsy and medical intervention is needed. Severe reactions require intensive intervention, and the patients may have poor recovery. These reactions include laryngeal edema, bronchospasm, anaphylaxis, hypotension, shock, myocardial infarction, pulmonary edema, hemolytic anemia, cardiac arrest, tonic-clonic seizures, and death.2,7
The existence of a previous suspected hypersensitivity reaction may require an allergy work-up in order to prove its causality and a severe reaction (independently on its physiopathology) should be seen as a contra-indication.
A patient reacting to fluorescein or to ICG (mild to moderate reactions) may be mislabeled “allergic” although the reaction experienced was non-specific or toxic in its origin. An allergy study should be considered if there is a concern in giving these contrast media dyes in future needed procedures. In this article, we aim to provide a review of the literature and propose an evaluation algorithm for suspected immediate reactions to fluorescein or to ICG. Moreover, some misconceptions regarding personal history of allergies, that are usually seen as contraindications, will also be reviewed in order to ease the evaluation of a patient in need of these procedures.
Materials and Methods
An in-depth search of the available literature was performed on PubMed using the following keywords: “fluorescein”, “indocyanine green”, “ophthalmic dyes”, “retinal angiography”, “adverse reactions”, “allergic reaction”. Additional sources were selected from the reference lists of published articles.
All publications consulted were reviewed for data regarding the management of adverse reactions to fluorescein and ICG.
We attempted to explore differences in adverse reactions with special attention for those of immediate hypersensitivity as well as discuss risk factors and propose an evaluation algorithm for patients with suspected hypersensitivity reactions.
Physical Properties and Use of Fluorescein and ICG in Retinal Angiography
Sodium fluorescein is a yellow-red, synthetic salt dye that is most commonly used to evaluate flow patterns of subterranean waters, as a cosmetic and pharmacologic color, and as a labeling agent in protein research.8 It has a molecular weight of 376.7 kDa.9 Once injected into the bloodstream, approximately 80% of the dye becomes bound to plasma proteins (particularly albumin) while the rest remains unbound.9 The dye is metabolized by both the liver and kidneys and is eliminated in the urine within 24–36 hrs of injection. Fluorescein angiography relies on the special fluorescent property of sodium fluorescein (defined as the ability of certain molecules to emit light of longer wavelength when stimulated by light of shorter wavelength). After stimulation, electrons return to the base energy level, emitting energy in the form of electromagnetic waves, which produces visible light.10–12 A stimulation source transmits light energy to the patient’s retina using either a flash/filter or a laser in the 485–500 nm range. The energy is then either reflected back by the retina as blue light, or absorbed by the sodium fluorescein and emitted back as green light. Then, a capturing device (camera) uses a green filter (520–535 nm) to selectively capture the fluorescent image onto film or a digital surface. For these properties, it is considered ideal in the study of blood-retinal barriers.
ICG is an amphiphilic tricarbocyanine dye with a molecular weight of 775 kDa.13 Due to its ability to form aggregates, the ICG lyophilizate has to be dissolved in water for injection. In the bloodstream, the dye is rapidly bound to proteins (98%).9 ICG is solely metabolized by the liver through a specific carrier-mediated transport system and excreted in the biliary system. The dye has a plasma half-life of 2 to 4 mins. While ICG dye gives off 4% of the fluorescence of sodium fluorescein, its maximal peak of absorption is at 790 to 805 nm and has a peak emission of 835 nm.10,14 Because both exciting and emitted light are in the near-infrared spectrum, it allows deeper penetration through the retina and the emitted light passes more easily through the retinal pigment epithelium (RPE), blood, lipid deposits, pigment and mild opacities (cataracts) to form images.15 Moreover, because the dye has a significantly greater molecular weight, and a greater proportion of molecules remains bound to proteins in the bloodstream than with fluorescein, the dye normally remains within the fenestrated walls of the choriocapillaris, unlike fluoresce in which leaks freely from these vessels. This property makes ICG an ideal technique for portraying the anatomy and hemodynamics of the choroidal vessels.
Epidemiology of Adverse Reactions to Fluorescein and ICG
Adverse reactions to intravenous fluorescein have an estimated incidence of 5%, with 0.05% considered as being severe.1 In the literature variable rates ranging from 3% to 20% have been reported.16,17 Some studies estimate that urticaria occurs in 0.5% to 1.2%1,7 and respiratory distress in 0.02% to 0.1% of the exposed patients.7,18 Yannuzzi et al in a report of the results of a national survey documented an overall frequency rate for moderate reaction of 1:63, for a severe reaction 1:1900, and for death 1:222.000.7 Beleña et al in a study including 11.260 patients (14.455 fluorescein angiographies), evaluated the incidence of positive intradermal skin test (0.2mL of sodium fluorescein 2% solution) in patients with self-reported history of dye sensitivity or any other allergies (except seasonal allergy). A positive result was obtained in 6.12% of them (12 positive tests in 196 patients). Those patients with positive tests were not allowed to have the fluorescein angiography. Among patients with negative results, none had an adverse reaction. In the group of patients that did not perform intradermal skin test the incidence of adverse reactions was 1.28% (186 patients).19
Adverse reactions to ICG are rarer. Severe reactions occurring in 0.05% to 0.07%,20,21 moderate reactions in 0.2%, and mild reactions in 0.15% of the exposed patients.20 A study conducted by Su et al evaluated the incidence of IHR to the simultaneous intravenous injection of fluorescein and ICG for fundus angiography. IHRs were observed in 28 of 396 patients with self-report drug allergy (7.2%) and in 145 of 3426 without allergy history (4.2%) (p=0.008), besides, patients with an allergy history also showed more severe reactions.22
Mechanisms of Adverse Reactions
The mechanisms involved in adverse reactions to fluorescein have not been fully elucidated, but the following have been proposed:2,7
(1) non-allergic histamine release
(2) IgE-mediated hypersensitivity (immediate reaction);
(3) complement activation;
(4) disturbances of arachidonic acid metabolism;
(5) vasovagal phenomena (bradycardia and arterial hypotension);
(6) anxiety-related medullary sympathetic discharge;
(7) direct pharmacologic toxic effect resulting in vasospastic events;
(8) effect of contamination of the drug during the manufacturing process;
(9) destruction of the vascular endothelium through Factor XII and the coagulation system;
(10) combinations of the above.
In the same way as for fluorescein, the mechanisms involved in adverse reactions to ICG are not fully understood and the reactions may actually be due to sodium iodide or to the molecule itself.2 The following mechanisms have been proposed:20
(1) non-allergic histamine release;
(2) IgE-mediated hypersensitivity (immediate reaction);
(3) complement activation (by disruption of the endothelial lining of blood vessels after the administration of IG);
(4) Release of other inflammatory mediators.
Risk Factors for Adverse Reactions to Fluorescein and ICG
Some risk factors have been associated with the occurrence of adverse reactions with fluorescein, such as diabetes (p=0.002; RR=1.80), arterial hypertension (p=0.002; RR=1.84) and atopy (p=0.001; RR=3.99).23,24 Other factors like velocity of administration, injected volume and concentration of the dye do not seem to influence the occurrence of adverse reactions.25
In patients with a history of allergic disease (anaphylaxis, asthma, allergic rhinitis or urticaria) the incidence of adverse reactions also seems to be higher.23 Some authors also include the previous history of food allergy or drug allergy,22 previous reactions to colloidal plasma substitutes and history of dye allergy.1
An important point to address is seafood and shellfish allergy which has mostly be seen as contra-indication for the administration of contrast media and some dyes, due to a possible “iodine allergy”. It has been well established that patients with true allergy to seafood and shellfish have specific IgE against proteins within the meat of the fish, and that iodine content plays no etiologic role. At this time, the idea of cross-reactivity between iodine and radiocontrast media and dyes has been discarded so, the risk for adverse reactions in these patients should be considered the same as for other food allergies.26
The presence of other comorbidities, such as urticaria, asthma (especially if severe or non-controlled) and drug allergies can be enhancers for more severe reactions as it is documented for other drug allergies.27
Clinical Presentation of Adverse Reactions
The majority of the reported adverse reactions to fluorescein and ICG are mild or moderate.1,7,20,21,23 In what concerns hypersensitivity reactions, the majority seem to be immediate type 1 (IgE mediated) reactions (see Table 1). However, most of the reported cases have not been supported by an allergy workup in order to prove the causality. Table 2 describes the classification of adverse reactions according to their severity.
 |
Table 1 Reports of Hypersensitivity Reactions to Fluorescein and ICG |
 |
Table 2 Classification of Adverse Reactions According to Their Severity2,7 |
Immediate type 1 reactions are characterized by IgE antibody bridging by the allergen with activation of mast cells and basophiles conducting to release of histamine and other mediators. A rapid increase in vascular permeability, as well as contraction of smooth muscle cells, leads to symptoms such as urticaria, angioedema, bronchospasm, cardiovascular depression and, in severe cases, shock.2 The onset of an IgE-mediated reaction is rapid (generally within minutes) and needs prompt recognition. The distinction between a hypersensitivity reaction and a toxic one can be challenging.
Proposed Evaluation of the Patient Before Angiography
In those patients who have never been exposed to fluorescein and ICG, the analysis of risk factors should be taken into account.
The ophthalmologist should ask about personal history of asthma, urticaria, food or drug allergies and if the patients needed the relief medication or need to increase doses of their controller medication in the last month (in order to check the disease control).
In patients with unstable asthma and/or acute urticaria the exam should be postponed until resolution of the symptoms, as these conditions can contribute as enhancers for a more severe reaction.
In what concerns to premedication, considering the different physiopathology and mechanisms that can be involved, there is no single treatment that prevents the onset of all possible reactions. However, it has been proved with radiocontrast media, that the use of prophylactic medication can reduce the probability of adverse reactions especially in those patients with previous mild to moderate reactions and with risk factors.28
A systematic review performed by Tramèr et al showed that with iodine contrast media, the use of antihistamines and corticosteroids decreases the risk of cutaneous and respiratory symptoms and that the combination of clemastine and cimetidine prevents angioedema.29
Considering this protective effect on the onset of cutaneous and respiratory symptoms corticosteroids and antihistamines may be administered on the follow situations aiming prevention of hypersensitivity reactions:
- In case of personal history of any allergies, diabetes mellitus or arterial hypertension, they do not contraindicate the performance of the exam and premedication with antihistamines and corticosteroids could be performed, as for example : methylprednisolone 40mg IV and cetirizine 10mg (or loratadine 10mg) PO 2 hrs before the exam and methylprednisolone 32mg PO (or prednisolone 40mg PO) 12 hrs before the exam.
- In case of previous mild suspected toxic reactions, we suggest the follow premedication: methylprednisolone 32mg PO (or prednisolone 40mg PO) and cetirizine 10mg (or loratadine 10mg) 12 and 7 hrs before the exam. 1 hr before the exam hydrocortisone 200 mg IV, clemastine 2mg diluted in 100mL of saline solution IV and ranitidine 50mg IV.
- In those who experienced moderate toxic reactions, our recommendation is that the risk-benefit ratio should be weighted and if the information of performing the exam is an asset, our recommendation is to perform it with pre-medication (see mild suspected toxic reactions) and with the presence of a trained intensive physician.
In those patients who have already performed a retinal angiography, the previous history of a severe reaction is the main contraindication for a new exam. In what concern patients with mild-moderate reactions in which a hypersensitivity mechanism is suspected, an allergy study is advisable and posterior decisions should be taken according to results.
How to Treat an Acute Reaction
There are no clear recommendations published concerning the treatment of reactions to fluorescein or ICG. In lack of those, the recommendations of the American College of Radiology regarding the treatment of acute hypersensitivity reactions to radiocontrast media can be considered and are summarized in Table 3.30
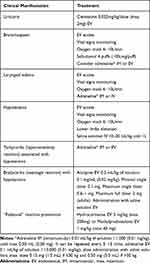 |
Table 3 Acute Treatment of Hypersensitivity Reactions |
Allergy Work-Up in a Suspected Hypersensitivity Reaction
If the clinical presentation suggests a hypersensitivity reaction and if future procedures with fluorescein are of main importance, the evaluation at an allergy clinic should be considered.
According to current knowledge, there is no evidence of a cross-reactivity between fluorescein and ICG, and in case of a suspected hypersensitivity reaction to fluorescein, the switch to ICG or any other comparable procedure should be considered as the main option.
Once the pathogenesis of IHR to fluorescein is not yet fully understood, there is no clear consensus among investigators regarding how to confirm hypersensitivity to this dye. However, skin tests have been used in the following concentrations: skin prick test with fluorescein at 20% and intradermal tests at 1:1000, 1:100 and 1:10 dilutions. Intravenous provocation tests have also been described.5,31
If an IgE-mediated mechanism is confirmed, with positive skin test results, and if there are no satisfactory alternatives, a fluorescein desensitization protocol could be considered.6,32
No evidence exists at this time in what concerns concentrations for an allergy workup for suspected hypersensitivity reactions to ICG.
Conclusion
Overall, retinal angiography is considered a safe procedure with a low incidence of adverse reactions. With this review, we aim to simplify the decision of ophthalmologists in performing or not retinal angiography with a contrast dye in a patient with a personal history of allergies.
Over the last years, there has been a growing use of a complementary diagnostic examination that allows visualization and analysis of the retinal vasculature without the need for contrast injection, optical coherence tomography angiography (OCT-A). Although still considered a second-line examination once it is not part of the diagnostic protocols, it can be used as an alternative to angiography, in situations of contraindication or intolerance to dyes.
The proposed approach is based on few limited studies that have been performed with fluorescein and ICG, as well as current knowledge of drug hypersensitivity reactions (mainly related to radiocontrast media). Prospective-randomized controlled studies are needed in order to evaluate the real incidence and prevalence of hypersensitivity reactions (once the majority of studies report the adverse reactions without a complementary allergy study), as well as case-control studies to evaluate the real risk of the current suspected risk factors (drug allergy, food allergy and respiratory allergy).
Author Contributions
Jorge Meira and Maria Luís Marques contributed equally to this work and should be considered as equal first authors. All authors contributed towards data analysis, drafting and critically revising the paper, gave final approval of the version to be published, and agreed to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Kwiterovich KA, Maguire MG, Murphy RP, et al. Frequency of adverse systemic reactions after fluorescein angiography. Results of a prospective study. Ophthalmology. 1991;98(7):1139–1142. doi:10.1016/S0161-6420(91)32165-1
2. Brockow K, Sánchez-Borges M. Hypersensitivity to contrast media and dyes. Immunol Allergy Clin North Am. 2014;34(3):547–564, viii. doi:10.1016/j.iac.2014.04.002
3. Pichler WJ. Delayed drug hypersensitivity reactions. Ann Intern Med. 2003;139(8):683–693. doi:10.7326/0003-4819-139-8-200310210-00012
4. Torres MJ, Mayorga C, Blanca M. Nonimmediate allergic reactions induced by drugs: pathogenesis and diagnostic tests. J Investig Allergol Clin Immunol. 2007;42(2):80–90.
5. López-Sáez MP, Ordoqui E, Tornero P, et al. Fluorescein-induced allergic reaction. Ann Allergy Asthma Immunol. 1998;81(5):428–430. doi:10.1016/S1081-1206(10)63140-7
6. Nucera E, Schiavino D, Merendino E, et al. Successful fluorescein desensitization. Allergy. 2003;58(5):458. doi:10.1034/j.1398-9995.2003.00130.x
7. Yannuzzi LA, Rohrer KT, Tindel LJ, et al. Fluorescein angiography complication survey. Ophthalmology. 1986;93(5):611–617. doi:10.1016/S0161-6420(86)33697-2
8. Bernardes R, Serranho P, Lobo C. Digital ocular fundus imaging: a review. OPH. 2011;226(4):161–181. doi:10.1159/000329597
9. Ciardella AP, Prall FR, Borodoker N, Cunningham ET. Imaging techniques for posterior uveitis. Curr Opin Ophthalmol. 2004;15(6):519–530. doi:10.1097/01.icu.0000144386.05116.c5
10. Bennett TJ, Barry CJ. Ophthalmic imaging today: an ophthalmic photographer’s viewpoint - a review. Clin Exp Ophthalmol. 2009;37(1):2–13. doi:10.1111/j.1442-9071.2008.01812.x
11. Gess AJ, Fung AE, Rodriguez JG. Imaging in neovascular age-related macular degeneration. Semin Ophthalmol. 2011;26(3):225–233. doi:10.3109/08820538.2011.582533
12. Yannuzzi LA, Ober MD, Slakter JS, et al. Ophthalmic fundus imaging: today and beyond. Am J Ophthalmol. 2004;137(3):511–524. doi:10.1016/j.ajo.2003.12.035
13. Desmettre T, Devoisselle JM, Mordon S. Fluorescence properties and metabolic features of indocyanine green (ICG) as related to angiography. Surv Ophthalmol. 2000;45(1):15–27. doi:10.1016/S0039-6257(00)00123-5
14. Dzurinko VL, Gurwood AS, Price JR. Intravenous and indocyanine green angiography. Optometry. 2004;75(12):743–755. doi:10.1016/S1529-1839(04)70234-1
15. [No authors listed].Indocyanine green angiography. American Academy of Ophthalmology. Ophthalmology. 1998;105(8):1564–1569. doi:10.1016/S0161-6420(98)98048-4
16. Patz A, Finkelstein D, Fine SL, Murphy RP. The role of fluorescein angiography in national collaborative studies. Ophthalmology. 1986;93(11):1466–1470. doi:10.1016/S0161-6420(86)33558-9
17. Singerman LJ. Fluorescein angiography. Practical role in the office management of macular diseases. Ophthalmology. 1986;93(9):1209–1215. doi:10.1016/S0161-6420(86)33607-8
18. Karhunen U, Raitta C, Kala R. Adverse reactions to fluorescein angiography. Acta Ophthalmol (Copenh). 1986;64(3):282–286. doi:10.1111/j.1755-3768.1986.tb06919.x
19. Beleña JM, Núñez M, Rodríguez M. Adverse reactions due to fluorescein during retinal angiography. JSM Ophthalmol. 2013;1:1004.
20. Hope-Ross M, Yannuzzi LA, Gragoudas ES, et al. Adverse reactions due to indocyanine green. Ophthalmology. 1994;101(3):529–533. doi:10.1016/S0161-6420(94)31303-0
21. Obana A, Miki T, Hayashi K, et al. Survey of complications of indocyanine green angiography in Japan. Am J Ophthalmol. 1994;118(6):749–753. doi:10.1016/S0002-9394(14)72554-1
22. Su Z, Ye P, Teng Y, Zhang L, Shu X. Adverse reaction in patients with drug allergy history after simultaneous intravenous fundus fluorescein angiography and indocyanine green angiography. J Ocul Pharmacol Ther. 2012;28(4):410–413. doi:10.1089/jop.2011.0221
23. Lira RPC, Oliveira CLDA, Marques MVRB, Silva AR, Pessoa CD. Adverse reactions of fluorescein angiography: a prospective study. Arq Bras Oftalmol. 2007;70(4):615–618. doi:10.1590/S0004-27492007000400011
24. Leila L. Adverse effects of fluorescein angiography. Acta Ophthalmol Scand. 2006;84(6):720–721. doi:10.1111/j.1600-0420.2006.00776.x
25. Kalogeromitros DC, Makris MP, Aggelides XS, et al. Allergy skin testing in predicting adverse reactions to fluorescein: a prospective clinical study. Acta Ophthalmol. 2011;89(5):480–483. doi:10.1111/j.1755-3768.2009.01722.x
26. Huang S-W. Seafood and iodine: an analysis of a medical myth. Allergy Asthma Proc. 2005;26(6):468–469.
27. Demoly P, Adkinson NF, Brockow K, et al. International consensus on drug allergy. Allergy. 2014;69(4):420–437. doi:10.1111/all.12350
28. Liccardi G, Lobefalo G, Di Florio E, et al. Strategies for the prevention of asthmatic, anaphylactic and anaphylactoid reactions during the administration of anesthetics and/or contrast media. J Investig Allergol Clin Immunol. 2008;18(1):1–11.
29. Tramèr MR, von Elm E, Loubeyre P, Hauser C. Pharmacological prevention of serious anaphylactic reactions due to iodinated contrast media: systematic review. BMJ. 2006;333(7570):675. doi:10.1136/bmj.38905.634132.AE
30. Ellis JH, Davenport MS, Dillman JR, et al. ACR manual on contrast media v10.1. Reston, USA: American College of Radiology; 2015
31. Pérez-Rodríguez E, Matheu V, Sánchez Machín I, García Robaina JC, la Torre Morín FD. Reacciones adversas durante la administración de fluoresceína endovenosa. Arch Soc Esp Oftalmol. 2005;80(8):441–442. doi:10.4321/s0365-66912005000800003
32. Knowles SR, Weber EA, Berbrayer CS. Allergic reaction to fluorescein dye: successful one day desensitization. Can J Ophthalmol. 2007;42:329–330. doi:10.3129/can.j.ophthalmol.i07-028
33. Trindade‐Porto C, Alonso‐Llamazares A, et al. Fluorescein‐induced adverse reaction. Allergy. 1999;54(11):1230.
34. Mayama M, Hirayama K, Nakano H, et al. Psoriasiform drug eruption induced by fluorescein sodium used for fluorescein angiography. Br J Dermatol. 1999;140(5):982–984.
35. Fineschi V, Monasterolo G, Rosi R, Turillazzzi E. Fatal anaphylactic shock during a fluorescein angiography. Forensic Sci Int. 1999;100(1–2):137–142.
36. Stein MR, Parker CW. Reactions following intravenous fluorescein. Am J Ophthalmol. 1971;72:861–868.
37. Duarte Ferreira R, Viana F, Duarte F, Barbosa M. Hipersensibilidade à fluoresceína - Revisão a propósito de um caso clínico [Fluorescein hypersensitivity - A case report review]. Revista Portuguesa de Imunoalergologia. 2015;23:231–235. Portuguese.
38. Olsen TW, Lim JI, Capone A Jr, Myles RA, Gilman JP. Anaphylactic shock following indocyanine green angiography. Arch Ophthalmol. 1996;114(1):97.
39. Wolf S, Arend O, Schulte K, Reim M. Severe anaphylactic reaction after indocyanine green fluorescence angiography. Am J Ophthalmol. 1992;114(5):638–639.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
