Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 14
Immediate nor Delayed Type Hypersensitivity Plays a Role in Late Inflammatory Reactions After Hyaluronic Acid Filler Injections
Authors Decates T, Kadouch J , Velthuis P, Rustemeyer T
Received 24 March 2021
Accepted for publication 12 May 2021
Published 31 May 2021 Volume 2021:14 Pages 581—589
DOI https://doi.org/10.2147/CCID.S312198
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jeffrey Weinberg
Video abstract presented by Tom Decates.
Views: 8676
Tom Decates,1,* Jonathan Kadouch,1,* Peter Velthuis,1 Thomas Rustemeyer2
1Department of Dermatology, Erasmus Medical Center, Rotterdam, The Netherlands; 2Department of Dermatology, Amsterdam University Medical Center, Amsterdam, The Netherlands
*These authors contributed equally to this work
Correspondence: Thomas Rustemeyer
Amsterdam University Medical Center, Dermatology of Department, Postbus 7057, 1007 MB Amsterdam, Amsterdam, The Netherlands
Email [email protected]
Purpose: The exact etiology of late inflammatory reactions (LIRs) to hyaluronic acid (HA) fillers is currently unknown. Some argue that these result from a hypersensitivity reaction, although evidence to support this is very scarce. Most reports on such reactions are not substantiated by positive skin tests. The purpose of our study was to determine whether immediate or delayed type hypersensitivity reaction follows hyaluronic acid (HA) filler injections.
Patients and Methods: Twelve patients were referred for general allergic screening (patch tests), as well as specific intradermal testing (injection of 0.1cc boluses) on the medial upper arm with a selection of several currently available hyaluronic acid (HA) fillers on the market. A positive allergic reaction was defined as erythema, firmness or swelling.
Results: During the 4 month follow-up, no reactions to any of the tested HA fillers were reported. No correlation was found between results from the general allergic screening and a history with LIRs to HA fillers.
Conclusion: The results suggest that neither type I nor type IV hypersensitivity plays a role in late inflammatory reactions (LIRs) to hyaluronic acid (HA) fillers.
Keywords: cosmetic dermatology, allergy, cosmetic, complications
Introduction
Adverse events (AEs) in filler injections treatments are often classified into two types depending on the time of onset (Table 1). Most reports of delayed-onset complications of fillers are based on permanent fillers. However, recently several reports have been published on late inflammatory reactions to hyaluronic acid fillers, with erythema, edema and nodules at and in proximity to the injected sites of the face.1–3
For possible hypotheses for such delayed-onset complications exist, namely foreign-body reactions, microbial contamination (in biofilms or otherwise), type-IV hypersensitivity reactions, or adjuvant-based reactions (Figure 1).4–28 Interestingly, all four etiological factors are believed to be capable of inducing granulomatous immune reactions.17,22,29,30 Also all filler agents used for soft-tissue augmentation are thought to elicit some degree of granulomatous inflammatory reaction following injection.20,31 To a certain point this is considered to be part of a normal physiological response to fillers.20,32
 |
Figure 1 Oversight of the different etiological hypothesis for late inflammatory reactions (LIRs) to fillers. |
A genuine granulomatous foreign body reaction (GFBR) is predominantly composed of histiocytes/macrophages and multinucleated giant cells encapsulating filler particles.20,33 The exact pathophysiology of filler-induced GFBR, or ‘filler granulomas’, has yet to be elucidated. Current insights have shown that particles larger than 5 μm require the presence of aggregated macrophages, or MNGCs, to be phagocytosed.34–36 Particles larger than 15 to 20 μm are generally not subject to true phagocytosis. Failure of effective phagocytosis leads to granuloma formation, consisting of macrophages and MNGCs, as well as a contiguous infiltrate of lymphocytes secreting pro-inflammatory cytokines (ie, tumor necrosis factor alpha (TNF-α), interferon gamma (IFN-γ) and interleukin 12 (IL-12)).29
Some authors have postulated that all granulomatous reactions to fillers are in fact type IV (delayed-type) hypersensitivity reactions.17 Evidence to support hyaluronic acid as antigen inducing delayed-type hypersensitivity reactions is very scarce, since most cases reported skin-tests were not performed. Other authors have written about the introduction of Vycross (VYC) HAs that has introduced a new variable that may be changing the immune tolerance of these substances, resulting in a higher incidence of delayed nodules than previously expected.25
We therefore believe it could be of value to further investigate the role of HA-fillers in inducing delayed-type hypersensitivity reactions by using golden-standard intradermal skin-testing in patients with late-onset inflammatory AEs to hyaluronic acid fillers.
Materials and Methods
From February 2018 to July 2020 a total of 12 patients were enrolled in this prospective study. All were referred to the outpatient clini c of the Amsterdam University Medical Centre (Amsterdam, The Netherlands), for diagnostic evaluation of potential hypersensitivity reactions to hyaluronic acid (HA) fillers.
Only patients >18 years having experienced a late inflammatory response (LIR) in the face following treatment with HA fillers were included. Exclusion criteria were a still active LIR, pregnancy or intent for pregnancy, breastfeeding, any active inflammatory or infectious skin condition at the sites used for testing (arms and back), a known coagulopathy or use of NSIADs, use of steroids or other anti-inflammatory medication, alcohol and/or drug abuse, a severe or unstable (autoimmune) co-morbidity.
All included patients first underwent regular patch testing performed with the European baseline series, the regional series of cosmetic products, fragrance series and own cosmetic products according to recommendations of de Groot.37 Allergens were tested using Van der Bend test chambers (Brielle, The Netherlands) applied on the back and covered with Fixomull stretch (BSN Medical, Hamburg, Germany). Readings were performed on day (D) 2, D3 or D4, and D7 according to ESCD criteria.38
Skin prick tests were performed with 0.05mL of the series of inhalation allergens (ALK, Almere, The Netherlands) and intracutaneous tests with 0.1mL of six fillers which were tested on both inner sides of the upper arms in a randomized manner. The tested fillers were Juvederm® Volbella, Restylane® Kysse, Stylage® M, Belotero® Balance, Etermis® 2 (Figure 2). The skin prick tests and intracutaneous tests were read after 15 minutes. In addition, the intracutaneous tests were read after D2, D3 or D4, and D7. Potential late reactions were monitored after 2 and 4 weeks, and patients were instructed to contact the department in case of any later reaction. A positive allergic reaction was defined as redness, firmness, pain or swelling at the site of injection.
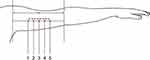 |
Figure 2 Design of intradermal testing protocol. |
All study patients provided written informed consent for the treatment procedure. The study was conducted in accordance with guidelines of the Declaration of Helsinki. The study was approved by the medical ethics committee of the Erasmus Medical Center.28
Results
A total of twelve individuals could be included, all female (aged 38–66, mean age 52). Two of which had an atopic history: one patient was known with atopic dermatitis, the other with allergies for cats, avocado and nickel. All patients had been treated with an HA filler before the LIR occurred. In 1 case a permanent filler (liquid injectable silicone) had been injected 20 years ago at the same location (lips).
The affected site concurred with the injected sites in all cases and consisted of the tear trough (n = 2), nasolabial fold (n = 1), marionet lines (n = 1), lips (n = 5), cheeks (n = 2) and full face (n = 1). The reported inflammatory symptoms consisted of erythema (n = 3), swelling/edema (n = 6), nodules (n = 6). The LIRs had been treated by the patient’s own physicians with hyaluronidase (n = 7), intralesional corticosteroids (n = 1), intralesional laser therapy (ILT, n = 2) and excision (n = 2).
Patch testing showed positive reactions in three patients. Positive allergens in the first two patients were nickel (n = 2), amerchol-101 (n =1). The third patient was positive for 4-tert-butylphenol butylcarbamate (IPBC), turpentine peroxide, methyl methacrylate and several other reactions on acrylates. No positive reactions were found in the inhalation skin prick tests, or any of the intracutaneous filler tests neither immediately or during the 4 months follow-up.
Discussion
Reviewing the potential sources for the late onset inflammatory reactions, one has to consider every factor that may change in time, both in the filler substance as in the host. These are given in Figure 1 and are systematically discussed below.
Filler Characteristics (1): Growth of Micro-Organisms
Micro-organisms seem a very plausible cause of the delayed inflammatory response in LIR’s. In particular, bacteria with low virulence, as eg, Staphylococcus epidermidis, need time to colonize the filler material. The still controversial biofilm hypothesis states that the mix of bacteria form a slime that protects them from the host immune system.5,18,22,39–41 Depending on environmental condition one or a small number of the bacterial strains will have a competitive advantage and over time will outgrow the others. At some point these strain(s) can reach numbers that provoke an inflammatory response. Indeed Decates et al found bacteria to be present in a substantial number of samples from inflamed filler sites (submitted). These were almost exclusively gram-positive bacteria with low virulence, ie, S. epidermidis, Cutibacterium acnes, and others common in skin flora.
Filler Characteristics (2): Structural Changes in Chemical Composition of the Filler Substance
Degradation of cross-linked HA filler may expose trace substances of BDDE, bacterial proteins, etc. Where high-molecular-weight HA exerts a primarily anti-inflammatory effect, low molecular weight HA is proinflammatory and can serve as an endogenous danger signal activating the innate immune system. Theoretically, this independently increases the risk of delayed-onset nodules development with injection of the VYC family of fillers.42 Gradual exposure or release of these molecules could trigger the immune the system. Polyethylene glycol (PEG) as cross-linker for HA filler has shown a really high safety profile. Up to now, no granulomas or delayed inflammatory reaction have been described following HA filler cross linked with PEG.33–35
Filler Characteristics (3): Too Much Product, Faults in Choice of Filler Type or Injection Technique
Too much filler or filler with incorrect features for a specific area may lead to an immune response which would not have occurred with adequate use of the product. For example, it is known that filler nodules can appear over time by incorrect use or incorrect positioning of filler material, muscle- or gravity-induced displacement or accumulation and capsular contraction.43 Some filler should not be used in the dynamic areas of the face, others have rheological properties making them unsuitable for superficial placement. Doing so might prolong or even sustain an inflammatory healing response following implantation.43,44 Filler’s rheology can be modified by the cross-linking agent. When PEG is used instead of BDDE a longer distance between HA molecules is present, this induces a higher elasticity for HA filler cross linked with PEG.33–35
Host Characteristics: Local, Trauma
Over the years we have observed three cases of patients with local trauma, one after full face fractional CO2 laser treatment, one after 35% TCA peeling of the whole face and one after a scooter accident, that developed palpable and tender nodules on cheeks and/or nasolabial folds. These nodules were hypo-echoic and identified as HA fillers. These resolved after treatment with hyaluronidase.38
Host Characteristics: Systemic, Lifestyle Change/Concomitant Systemic Disorders
Many colleagues, as we did, have seen cases of LIR in patients after chemotherapy for various cancers. Apparently, the cancers or the effects on the immune system brought about by the chemotherapy changed filler–host interaction sufficiently to provoke a clinically significant reaction. Also, we observed LIR in a patient that lost 60 kilograms of body weight voluntarily (unpublished case Dr Velthuis).
Host Characteristics: Systemic, Altered Immune Status
Several articles describe the onset of LIRs after alterations in the immune status due to (suspected) infections or flu-like symptoms.2,5,18,22,39–41 The exact pathophysiological mechanism by which an infectious process induces an LIR is unknown, though probably the clinically non-apparent interaction between filler substance and host immune system is altered and leads to a late-onset inflammatory response. Also, recently COVID-19 vaccination has led to LIR.39
We now continue to discuss the potential causes for inflammation in the above-mentioned circumstances.
Specific Immunity
The most striking observation of this study is the absence of any response on skin testing of each of the six used HA fillers on the arms in any of the twelve patients. It must be noted that a true allergic reaction is a systemic immune response that should affect all injected sites.6,45 Since the filler aliquots were injected intradermally, and the degradation of the used HA fillers takes approximately 6–12 months, both type-I and type-IV hypersensitivity reactions are investigated by this approach. Aside from our study limitations, the results of this study suggest that a systemic immune response as etiological basis for LIRs is not probable.
Many authors have postulated that LIRs arise as a consequence of (type IV) hypersensitivity reactions. However, in a literature search on this topic we found only two studies describing true allergic diagnostics on suspected delayed type hypersensitivity reactions.46,47 Micheels investigated 8 patients with LIRs to Restylane® (Q.Med, Uppsala, Sweden) and Hylaform® (Biomatrix, Inc., Ridgefield, NJ, USA) using intradermal testing.46 Of the seven patients with positive reactions, five patients reacted positive to Restylane®, and five to Hylaform®. Histologic samples showed foreign body reactions with giant cells, however no eosinophils. The article was not clear as to how or if the LIRs were treated and if the LIRS reoccurred, something one might expect if untreated and caused by a hypersensitivity reaction. Lowe et al published in 2001 their results of dermal tests performed in five patients with LIRs suspected to be delayed type hypersensitivity reactions.47 Of the five tested patients, four tested positive. Histologic analysis again showed foreign body reactions with lymphocytes, giant cells and plasma cells, but no eosinophils. All lesions resolved with intralesional triamcinolone 3mg/mL injections, the use of intralesional hyaluronidase injections was not mentioned. In a recent study by Turkmani et al 14 patients suffered LIRs following influenza-like illness.2 The authors diagnosed the LIRs as delayed hypersensitivity reactions based on clinical presentation, however no epidermal/intradermal allergic testing was performed. Symptoms were treated with oral corticosteroids only (n = 10) of with a combination of oral corticosteroids and intralesional hyaluronidase injections (n = 4). In all cases with complete resolution. Based on these results one would expect that a true delayed-type hypersensitivity reaction would persist/reoccur after ending the corticosteroid treatment in patients not treated with hyaluronidase, making this diagnosis less likely in corticosteroid-alone cohort (n = 10). In addition, a bacterial/biofilm etiology would also not resolve after treatment with corticosteroids alone. We must conclude that at least in a number of cases of LIRs, a pathophysiological etiology other than delayed hypersensitivity must be in play.
Non-Specific Immunity
The generation of a granulomatous foreign body reaction (GFBR) after implantation of a biomaterial, or “foreign body”, is considered a normal physiological response from the host to any foreign body.20,31 No definition currently exists that differentiates between a physiological and a pathological GFBR.20,32 Within minutes after implantation, host plasma components are absorbed on the surface of the bio-implant, after which platelets and other components of the coagulation cascade induce clot formation. Platelet adhesion/activation and the release of pro-inflammatory cytokines, chemokines and growth factors sequentially induce the acute and chronic parts of the inflammatory response.44 Damage-associated molecular patterns (DAMPs), pathogen-associated molecular patterns (PAMPs) and Alarmins present at the implantation site activate the innate immune response through pattern recognition receptors (PRRs), Toll-like receptors (TLRs) and C-type lectin on macrophages, leukocytes and dendritic cells. In the acute phase, neutrophils attempt to degrade the biomaterial through phagocytosis and the release of reactive oxygen species, proteolytic enzymes and neutrophil extracellular traps (NETs, consisting of granular proteins, chromatin DNA, elastase, histones).44 NETs are also involved in trapping pathogens and prevention of infection spread. This inflammatory phase must slowly be replaced by an anti-inflammatory phase with secretion of anti-inflammatory cytokines (ie, IL-10) and recruiting fibroblasts for effective tissue regeneration. The shift towards an anti-inflammatory healing response at the end of a “normal” foreign body response can be impeded by specific (physicochemical) characteristics of the implanted biomaterial.43,44 For example, failure of effective phagocytosis (eg, particles larger than 15 to 20 μm are generally too large for ingestion by macrophages or giant cells) leads to a chronic inflammation pathway and granuloma formation,29,34–36,44 Similarly, excessive production of NETs by neutrophils can impair healing and lead to a chronic inflammation and encapsulation.44
Adjuvant Based Filler Reaction
Some authors have postulated that fillers act as adjuvants, rather than as antigens.7,24,48 Adjuvants are defined as substances that may stimulate immune responses without having specific antigenic properties themselves.49 Adjuvants are believed to influence both the innate and adaptive immune systems by mimicking evolutionary conserved molecules (eg, PAMPs, DAMPs, Alarmins) capable of binding TLRs, causing the release of Th1 inflammatory cytokines and increasing the activity of dendritic cells (DCs), lymphocytes and tissue macrophages.49 Infection, trauma and vaccination may trigger adjuvant activity or act as adjuvants themselves.7,49–52 The risk of abnormal immune responses is believed to be increased by sequential exposure to (different) adjuvant stimuli.7,52 Shoenfeld et al introduced the name “autoimmune/inflammatory syndrome induced by adjuvants’, in short ‘ASIA’ or Schoenfeld”s syndrome, to describe the spectrum of immune-mediated systemic diseases that may be triggered by previous exposure to an adjuvant stimulus.53,54 Whether an adjuvant-induced immune response remains limited or evolves into a full-blown systemic disease (ie, ASIA) is believed to depend on specific adjuvant characteristics and the extent in which innate, adaptive and regulatory immune responses are activated.7,29,30 A genetic predisposition for the development of ASIA has also been postulated.7,29,52
Considerations
Adjuvants that have been reported to be able to cause ASIA are silicone, aluminum salts, pristane and certain infectious components.49,51,54 Interestingly, also acrylamides and hyaluronic acid compounds have been identified as potential adjuvants.7,24,29,30,55–57 HA-fillers functioning as adjuvants and causing inflammatory TLR-mediated responses through the innate immune system might explain several inconsistencies found in clinical practice and world literature on the topic of LIRs.2,5,18,39,58 For instance, several authors support a prominent or leading role for bacterial contamination or infection in the origin of LIRs.3,5 This hypothesis conflicts with the many negative cultures described in the literature, but also with all the positive results reported with anti-inflammatory (eg, corticosteroids) treatment alone. Some have reported complete resolutions of LIRs using a treatment plan mainly composed of antibiotics and without corticosteroids.3 However, the antibiotics that were used are known for their immunomodulatory effects and were used simultaneously with intralesional hyaluronidase injections that dissolve the HA filler.
Analogous to these inconsistencies, the diagnosis of delayed hypersensitivity reaction mediated LIRs has its difficulties. Many have described resolution of the LIRs without dissolving or removing the filler.1,2,59 If all LIRs were caused by delayed hypersensitivity reactions, one would expect inflammatory symptoms to reoccur after ceasing immunosuppressive/immunomodulating therapy, for as long as the filler remains in situ.
A leading role for the innate immune system, possible mediated by adjuvant characteristics of filler materials in genetically predisposed patients, might explain why similar clinical symptoms (ie, LIRs) have been associated with such diverse therapies, both positively and negatively. Also, bacterial presence would not necessarily mean they act through a biofilm or infection but may execute their (adjuvant) effect by triggering the local innate immune system. It might even be that some patients are genetically predisposed in having a “sensitive” innate immune system, explaining why some individuals develop LIRs and others not.29
Future Perspectives
To elucidate the pathophysiology of LIRs more research is necessary. LIRs are known to be invalidating for patients and challenging for physicians. When investigating LIRs authors often look for a common denominator as cause for all LIRs. However, we must keep in mind that different etiologies may give similar clinical phenomena. This is why future research should focus on the different aspects of host immune responses, such as secreted cytokines, upregulated membrane receptors and induced immune pathways. Also, with the degradation of cross-linked HA filler, this may expose trace substance of BDDE, bacterial proteins and low molecular weight HA to exert a direct pro-inflammatory reaction. Our future research will focus on this as well. Genetic predisposition for developing LIRs might be elucidated by HLA-typing. But most importantly, authors should refrain from postulating diagnoses and conclusions based on inadequate investigations.
Limitations
This study investigated hypersensitivity reactions to fillers in patients having experienced LIRs. Although we tried to include as many patients as possible, the cohort was limited due to lack of includable individuals. In addition, the diagnostic protocol would not allow all fillers available on the market to be tested, since this number is substantial.
Conclusion
In our discussion we evaluated the pathophysiology of LIRs in a broad perspective. Amongst the etiological hypotheses that exist in the scientific literature are biofilms, hypersensitivity reactions, filler characteristics, host genetic predisposition and changes in filler substance of host immune status over time. A concise but complete summary of potential causes is given in Figure 2.
Although LIRs have often been diagnosed as hypersensitivity reactions, in our study no positive reactions to the six used HA fillers were reported. Both type-I and type-IV hypersensitivity reactions were investigated by our diagnostic protocol. The results suggest that neither type I nor type IV hypersensitivity plays a role in late inflammatory reactions (LIRs) to hyaluronic acid (HA) fillers.
Ethical Approval
This study was approved by the local research ethics committee (reference number: MEC-2016-660) and was performed in accordance with the ethical standards set forth in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Funding
There is no funding to report.
Disclosure
Dr Jonathan Kadouch is a Consultant for Merz Aesthetics. All authors report no conflicts of interest in this work.
References
1. Artzi O, Loizides C, Verner I, Landau M. Resistant and Recurrent Late Reaction to Hyaluronic Acid-Based Gel. Dermatologic Surgery. 2016;42(1):31–37. doi:10.1097/DSS.0000000000000562
2. Turkmani MG, De Boulle K, Philipp-Dormston WG. Delayed hypersensitivity reaction to hyaluronic acid dermal filler following influenza-like illness. Clin Cosmet Investig Dermatol. 2019;12:277–283. doi:10.2147/CCID.S198081
3. Marusza W, Olszanski R, Sierdzinski J, et al. The impact of lifestyle upon the probability of late bacterial infection after soft-tissue filler augmentation. Infect Drug Resist. 2019;12:855–863. doi:10.2147/IDR.S200357
4. Alhede M, Er O, Eickhardt S, et al. Bacterial biofilm formation and treatment in soft tissue fillers. Pathog Dis. 2014;70(3):339–346. doi:10.1111/2049-632X.12139
5. Christensen L, Breiting V, Bjarnsholt T, et al. Bacterial infection as a likely cause of adverse reactions to polyacrylamide hydrogel fillers in cosmetic surgery. Clin Infect Dis. 2013;56(10):1438–1444. doi:10.1093/cid/cit067
6. Marusza W, Mlynarczyk G, Olszanski R, et al. Probable biofilm formation in the cheek as a complication of soft tissue filler resulting from improper endodontic treatment of tooth 16. Int J Nanomedicine. 2012;7:1441–1447. doi:10.2147/IJN.S27994
7. Alijotas-Reig J, Garcia-Gimenez V, Llurba E, Vilardell-Tarres M. Autoimmune/inflammatory syndrome (ASIA) induced by biomaterials injection other than silicone medical grade. Lupus. 2012;21(12):1326–1334. doi:10.1177/0961203312458838
8. Dayan SH, Arkins JP, Brindise R. Soft tissue fillers and biofilms. Facial Plastic Surgery. 2011;27(1):23–28. doi:10.1055/s-0030-1270415
9. Requena L, Requena C, Christensen L, Zimmermann US, Kutzner H, Cerroni L. Adverse reactions to injectable soft tissue fillers. J Am Acad Dermatol. 2011;64(1):1–34. doi:10.1016/j.jaad.2010.02.064
10. Colombo G, Caregnato P, Stifanese R, Ferrando G. Destructive granulomatous reaction to polyacrylamide lip injection: solution for a complex case. Aesthetic Plast Surg. 2011;35(4):662–665. doi:10.1007/s00266-011-9660-9
11. Rohrich RJ, Monheit G, Nguyen AT, Brown SA, Fagien S. Soft-tissue filler complications: the important role of biofilms. Plast Reconstr Surg. 2010;125(4):1250–1256. doi:10.1097/PRS.0b013e3181cb4620
12. Sadashivaiah AB, Mysore V. Biofilms: their role in dermal fillers. J Cutan Aesthet Surg. 2010;3(1):20–22. doi:10.4103/0974-2077.63257
13. Hassid VJ. Soft-tissue filler complications: the important role of biofilms. Plast Reconstr Surg. 2010;126(5):1801–1802. doi:10.1097/PRS.0b013e3181ef93b5
14. Christensen LH. Host tissue interaction, fate, and risks of degradable and nondegradable gel fillers. Dermatologic Surgery. 2009;35(Suppl 2):1612–1619. doi:10.1111/j.1524-4725.2009.01338.x
15. Akrish S, Dayan D, Taicher S, Adam I, Nagler RM. Foreign body granulomas after injection of Bio-alcamid for lip augmentation. Am J Otolaryngol. 2009;30(5):356–359. doi:10.1016/j.amjoto.2008.07.001
16. Alijotas-Reig J, Garcia-Gimenez V, Vilardell-Tarres M. Late-onset immune-mediated adverse effects after poly-L-lactic acid injection in non-HIV patients: clinical findings and long-term follow-up. Dermatology. 2009;219(4):303–308. doi:10.1159/000243804
17. Alijotas-Reig J, Garcia-Gimenez V, Miro-Mur F, Vilardell-Tarres M. Delayed immune-mediated adverse effects related to polyacrylamide dermal fillers: clinical findings, management, and follow-up. Dermatologic Surgery. 2009;35(Suppl 1):360–366. doi:10.1111/j.1524-4725.2008.01041.x
18. Bjarnsholt T, Tolker-Nielsen T, Givskov M, Janssen M, Christensen LH. Detection of bacteria by fluorescence in situ hybridization in culture-negative soft tissue filler lesions. Dermatologic Surgery. 2009;35(Suppl 2):1620–1624. doi:10.1111/j.1524-4725.2009.01313.x
19. Furmanczyk PS, Wolgamot GM, Argenyi ZB, Gilbert SC. Extensive granulomatous reaction occurring 1.5 years after DermaLive injection. Dermatologic Surgery. 2009;35(Suppl 1):385–388. doi:10.1111/j.1524-4725.2008.01044.x
20. Lemperle G, Gauthier-Hazan N, Wolters M, Eisemann-Klein M, Zimmermann U, Duffy DM. Foreign body granulomas after all injectable dermal fillers: part 1. Possible causes. Plast Reconstr Surg. 2009;123(6):1842–1863. doi:10.1097/PRS.0b013e31818236d7
21. Christensen L. Normal and pathologic tissue reactions to soft tissue gel fillers. Dermatologic Surgery. 2007;33(Suppl 2):S168–175. doi:10.1111/j.1524-4725.2007.33357.x
22. Christensen L, Breiting V, Janssen M, Vuust J, Hogdall E. Adverse reactions to injectable soft tissue permanent fillers. Aesthetic Plast Surg. 2005;29(1):34–48. doi:10.1007/s00266-004-0113-6
23. Alijotas-Reig J, Garcia-Gimenez V, Miro-Mur F, Vilardell-Tarres M. Delayed immune-mediated adverse effects of polyalkylimide dermal fillers: clinical findings and long-term follow-up. Arch Dermatol. 2008;144(5):637–642. doi:10.1001/archderm.144.5.637
24. Alijotas-Reig J, Garcia-Gimenez V. Delayed immune-mediated adverse effects related to hyaluronic acid and acrylic hydrogel dermal fillers: clinical findings, long-term follow-up and review of the literature. J Eur Acad Dermatol Venereology. 2008;22(2):150–161. doi:10.1111/j.1468-3083.2007.02354.x
25. Gonzalez-Vela MC, Armesto S, Gonzalez-Lopez MA, Fernandez-Llaca JH, Val-Bernal JF. Perioral granulomatous reaction to Dermalive. Dermatologic Surgery. 2008;34(7):986–988. doi:10.1097/00042728-200807000-00024
26. Narins RS, Beer K. Liquid injectable silicone: a review of its history, immunology, technical considerations, complications, and potential. Plast Reconstr Surg. 2006;118(3 Suppl):77S–84S. doi:10.1097/01.prs.0000234919.25096.67
27. Zimmermann US, Clerici TJ. The histological aspects of fillers complications. Semin Cutan Med Surg. 2004;23(4):241–250. doi:10.1016/j.sder.2004.09.004
28. Lombardi T, Samson J, Plantier F, Husson C, Kuffer R. Orofacial granulomas after injection of cosmetic fillers. Histopathologic and clinical study of 11 cases. J Oral Pathol Med. 2004;33(2):115–120. doi:10.1111/j.1600-0714.2004.00194.x
29. Alijotas-Reig J, Fernandez-Figueras MT, Puig L. Late-onset inflammatory adverse reactions related to soft tissue filler injections. Clin Rev Allergy Immunol. 2013;45(1):97–108. doi:10.1007/s12016-012-8348-5
30. Alijotas-Reig J, Fernandez-Figueras MT, Puig L. Inflammatory, immune-mediated adverse reactions related to soft tissue dermal fillers. Semin Arthritis Rheum. 2013;43(2):241–258. doi:10.1016/j.semarthrit.2013.02.001
31. Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol. 2008;20(2):86–100. doi:10.1016/j.smim.2007.11.004
32. Lowe NJ, Maxwell CA, Patnaik R. Adverse reactions to dermal fillers: review. Dermatologic Surgery. 2005;31(11 Pt 2):1616–1625. doi:10.2310/6350.2005.31250
33. Duranti F, Salti G, Bovani B, Calandra M, Rosati ML. Injectable hyaluronic acid gel for soft tissue augmentation. A clinical and histological study. Dermatologic Surgery. 1998;24(12):1317–1325. doi:10.1111/j.1524-4725.1998.tb00007.x
34. Morhenn VB, Lemperle G, Gallo RL. Phagocytosis of different particulate dermal filler substances by human macrophages and skin cells. Dermatologic Surgery. 2002;28(6):484–490. doi:10.1046/j.1524-4725.2002.01273.x
35. Lemperle G, Morhenn VB, Pestonjamasp V, Gallo RL. Migration Studies and Histology of Injectable Microspheres of Different Sizes in Mice. Plast Reconstr Surg. 2004;113(5):1380–1390. doi:10.1097/01.PRS.0000112764.22839.7A
36. Tomazic-Jezic VJ, Merritt K, Umbreit TH. Significance of the type and the size of biomaterial particles on phagocytosis and tissue distribution. J Biomed Mater Res. 2001;55(4):523–529. doi:10.1002/1097-4636(20010615)55:4<523::AID-JBM1045>3.0.CO;2-G
37. de Groot AC. Patch Testing.
38. Johansen JDAK, Agner K. European Society of Contact Dermatitis guideline for diagnostic patch testing recommendations on best practice. Contact Dermatitis. 2015;73:195–221. doi:10.1111/cod.12432
39. Kadouch JA, Kadouch DJ, Fortuin S, van Rozelaar L, Karim RB, Hoekzema R. Delayed-onset complications of facial soft tissue augmentation with permanent fillers in 85 patients. Dermatologic Surgery. 2013;39(10):1474–1485. doi:10.1111/dsu.12313
40. Netsvyetayeva I, Marusza W, Olszanski R, et al. Skin bacterial flora as a potential risk factor predisposing to late bacterial infection after cross-linked hyaluronic acid gel augmentation. Infect Drug Resist. 2018;11:213–222. doi:10.2147/IDR.S154328
41. Saththianathan M, Johani K, Taylor A, et al. The Role of Bacterial Biofilm in Adverse Soft-Tissue Filler Reactions: a Combined Laboratory and Clinical Study. Plast Reconstr Surg. 2017;139(3):613–621. doi:10.1097/PRS.0000000000003067
42. Kim JE, Sykes JM. Hyaluronic acid fillers: history and overview. Facial Plastic Surgery. 2011;27(6):523–528. doi:10.1055/s-0031-1298785
43. Kadouch JA. Calcium hydroxylapatite: a review on safety and complications. J Cosmet Dermatol. 2017;16(2):152–161. doi:10.1111/jocd.12326
44. Mariani E, Lisignoli G, Borzi RM, Pulsatelli L. Biomaterials: foreign Bodies or Tuners for the Immune Response? Int J Mol Sci. 2019;20(3):636. doi:10.3390/ijms20030636
45. DeLorenzi C. Complications of injectable fillers, part I. Aesthetic Surgery j. 2013;33(4):561–575. doi:10.1177/1090820X13484492
46. Micheels P. Human Anti-Hyaluronic Acid Antibodies: is it possible? Dermatologic Surgery. 2001;27(2):185–191. doi:10.1046/j.1524-4725.2001.00248.x
47. Lowe NJ, Maxwell CA, Lowe P, Duick MG, Shah K. Hyaluronic acid skin fillers: adverse reactions and skin testing. J Am Acad Dermatol. 2001;45(6):930–933. doi:10.1067/mjd.2001.117381
48. Alijotas-Reig J, Esteve-Valverde E, Gil-Aliberas N, Garcia-Gimenez V. Autoimmune/inflammatory syndrome induced by adjuvants-ASIA-related to biomaterials: analysis of 45 cases and comprehensive review of the literature. Immunol Res. 2018;66(1):120–140. doi:10.1007/s12026-017-8980-5
49. Israeli E, Agmon-Levin N, Blank M, Shoenfeld Y. Adjuvants and autoimmunity. Lupus. 2009;18(13):1217–1225. doi:10.1177/0961203309345724
50. Schijns VE. Immunological concepts of vaccine adjuvant activity. Curr Opin Immunol. 2000;12(4):456–463. doi:10.1016/S0952-7915(00)00120-5
51. Miro-Mur F, Hindie M, Kandhaya-Pillai R, Tobajas V, Schwartz S, Alijotas-Reig J. Medical-grade silicone induces release of proinflammatory cytokines in peripheral blood mononuclear cells without activating T cells. J Biomed Mater Res B Appl Biomater. 2009;90(2):510–520. doi:10.1002/jbm.b.31312
52. Jara LJ, Medina G, Gomez-Banuelos E, Saavedra MA, Vera-Lastra O. Still’s disease, lupus-like syndrome, and silicone breast implants. A case of ‘ASIA’ (Shoenfeld’s syndrome). Lupus. 2012;21(2):140–145. doi:10.1177/0961203311430970
53. Shoenfeld Y, Agmon-Levin N. ‘ASIA’ - autoimmune/inflammatory syndrome induced by adjuvants. J Autoimmun. 2011;36(1):4–8. doi:10.1016/j.jaut.2010.07.003
54. Vera-Lastra O, Medina G, Cruz-Dominguez Mdel P, Jara LJ, Shoenfeld Y. Autoimmune/inflammatory syndrome induced by adjuvants (Shoenfeld’s syndrome): clinical and immunological spectrum. Expert Rev Clin Immunol. 2013;9(4):361–373. doi:10.1586/eci.13.2
55. Alijotas-Reig J. Recurrent urticarial vasculitis related to nonanimal hyaluronic acid skin filler injection. Dermatologic Surgery. 2008;35:1–4. doi:10.1111/j.1524-4725.2008.34375.x
56. Christensen LHB, Vuust VB. Adverse reactions following injection with permanent facial filler, polyacrylamide hydrogel (Aquamid): causes and treatment. Eur J Plast Surg. 2006;28:464–471. doi:10.1007/s00238-005-0005-2
57. Cukler JB, Spindler RA, Spindler JS, Lorenzo S, Trentham C. Association between bovine collagen dermal implants and a dermatomyositis or a polymyositis-like M30467 syndrome. Ann Intern Med. 1993;118:920–928. doi:10.7326/0003-4819-118-12-199306150-00002
58. Lemperle G, Nicolau P, Scheiermann N. Is there any evidence for biofilms in dermal fillers? Plast Reconstr Surg. 2011;128(2):84e–85e. doi:10.1097/PRS.0b013e31821ef19b
59. Philipp-Dormston WG, Goodman GJ, De Boulle K, et al. Global Approaches to the Prevention and Management of Delayed-onset Adverse Reactions with Hyaluronic Acid-based Fillers. Plastic Reconstructive Surg Global Open. 2020;8(4):e2730. doi:10.1097/GOX.0000000000002730
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

