Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 15
Imidazole Propionate is Increased in Diabetes and Associated with Stool Consistency
Authors Wu B, Tan L, Wang W, Feng X, Yan D
Received 3 March 2022
Accepted for publication 17 May 2022
Published 7 June 2022 Volume 2022:15 Pages 1715—1724
DOI https://doi.org/10.2147/DMSO.S362715
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ming-Hui Zou
Bowen Wu,1 Li Tan,2 Weihua Wang,3 Xingzhong Feng,4 Dan Yan1,5,6
1Beijing Friendship Hospital, Capital Medical University, Beijing, People’s Republic of China; 2Beijing Shijitan Hospital, Capital Medical University, Beijing, People’s Republic of China; 3Center of Pharmaceutical Technology, Tsinghua University, Beijing, People’s Republic of China; 4Tsinghua University Yuquan Hospital, Beijing, People’s Republic of China; 5Beijing Institute of Clinical Pharmacy, Beijing, People’s Republic of China; 6Beijing Key Laboratory for Evaluation of Rational Drug Use, Beijing, People’s Republic of China
Correspondence: Xingzhong Feng, Tsinghua University Yuquan Hospital, No. 5 Shijingshan Road, Shijingshan District, Beijing, People’s Republic of China, Email [email protected] Dan Yan, Beijing Friendship Hospital, Capital Medical University, No. 95 Yong’an Road, Xicheng District, Beijing, People’s Republic of China, Email [email protected]
Background: Imidazole Propionate (ImP) is a new marker of Type 2 diabetes mellitus (T2DM), which can induce impaired glucose metabolism and weaken the efficacy of metformin. An extensive exploration into literature suggests that ImP may be associated with stool consistency.
Purpose: Through an in-depth study of the relationship between stool consistency, bile acids, fecal microbiota and ImP, we intend to explore the mechanism driving the ImP content difference in T2DM.
Patients Under Study and Methods: This is a single-center, prospective, cross-sectional study. Plasma ImP and stool consistency were analyzed among 96 diabetic subjects and 45 healthy subjects. All subjects were divided into the stool consistency normal (N) group and the stool consistency abnormal group, of which the abnormal group was sub-divided into the hard stool (H) group and the soft stool (S) group. After identifying the correlation between ImP and stool consistency, we analyzed the influence of bile acids and fecal microbiota on ImP in diabetic subjects.
Results: For T2DM patients, the ImP level of the abnormal stool consistency group was significantly higher than that of the normal stool consistency group (P < 0.001). Results were verified in 45 healthy subjects (P = 0.002). ImP was significantly associated with taurocholic acid (TCA) (P = 0.003) in feces, taurodeoxycholate (TDCA) (P = 0.003), glycochenodeoxycholate (GCDCA) (P = 0.021), and glycocholic acid (GCA) (P = 0.031) in plasma. The Shannon index of Group N was significantly higher than that of Group H (P = 0.041) and Group S (P = 0.003).
Conclusion: ImP was higher in diabetic patients with abnormal stool consistency than in those with normal stool consistency, which was related to the proportion of bile acids and fecal microbial structure. These findings may improve our understanding of ImP and contribute to the treatment of T2DM by improving stool consistency.
Keywords: ImP, T2DM, stool consistency, bile acids
Introduction
Type 2 diabetes mellitus (T2DM) is a metabolic disease, which is closely related to the metabolites of intestinal microbiota. Some bacterial metabolites, such as branched chain amino acids,1 secondary bile acids2 and short-chain fatty acids,3 can affect the condition of diabetes. The latest studies indicate that Imidazole Propionate (ImP), produced by intestinal microbiome through abnormal histidine metabolism,4 is a new marker of T2DM.5 ImP induces impaired glucose metabolism by activating the P38 γ -MTOR1-S6K1 signal and reduces the efficacy of metformin.6 This is an important discovery in the study of diabetes and microbiology in recent years.7,8 ImP levels in T2DM patients can vary by a hundredfold, and studies have shown significant differences of ImP levels among T2DM patients, which is related to inflammation, diet, and intestinal microflora composition.9,10
In 1972, it was discovered that ImP would increase with the onset of gastrointestinal symptoms and decrease with improvement of such symptoms.11 A recent study has discovered that for patients with irritable bowel syndrome, there is a significant increase in their ImP.12 One of the symptoms exhibited by irritable bowel syndrome is the change in stool consistency, which has a strong influence on intestinal flora.13,14 Therefore, we hypothesize that ImP differences in T2DM patients are closely related to stool consistency.
In this study, based on an examination of 96 T2DM subjects, we found that the ImP level of the abnormal stool consistency group was significantly higher than that of the normal stool consistency group. Results were verified in 45 healthy subjects. To explore the potential mechanism, we analyzed the factors that may affect fecal consistency: the bile acid and intestinal flora, and further looked into the relationship between these two factors and ImP.
Materials and Methods
Ethics Statement
This study has been approved by the Bioethics Committee of Beijing Friendship Hospital, Capital Medical University, with the ethical approval number of 2021-P2-140-1. The protocol used in this investigation is aligned to the principles expressed in the 1975 Declaration of Helsinki, which was revised in 2008. All participants have provided written informed consent.
Subjects
All patients and healthy individuals were recruited from Beijing Friendship Hospital, Capital Medical University. The inclusion criteria for T2DM patients were: Aged 18–75; conform to the diagnostic criteria of T2DM. The exclusion criteria were: Have a history of severe cardiovascular, respiratory, kidney, liver, gastrointestinal, blood or nervous system diseases; diabetic ketoacidosis, diabetic foot and other serious complications of diabetes; cognitive dysfunction. The inclusion criteria for healthy subjects were: Aged 18–75, without diabetes or abnormal glucose tolerance.
All patients were scored according to the Bristol stool scale (BSS), based on which, those scored 3, 4 were marked normal (N) and 1, 2, 5, 6 and 7 abnormal. Patients scored 1, 2 were classified as hard stool (H), and 5, 6 and 7 soft stool (S). According to the stool consistency, they were divided into two groups: the stool consistency normal (N) group and the stool consistency abnormal group (including Group H and Group S).
Imidazole Propionate Serum Measurements
To measure the imidazole propionate in a targeted manner, a plasma sample was extracted with 4 volumes of acetonitrile containing 5 ng/mL internal standard in a 1.5 mL polypropylene tube. After vortexing and centrifugation, 5ul of supernatant was injected into an Amide BEH column (2.1x100 mm, 1.7 mm particles; Waters), as well as acetonitrile and 10 mM ammonium acetate, which contained 10 mM ammonium acetate, 0.1% formic acid (phase A) and 10 mM using ammonium acetate. Gradient separation of 0.1% formic acid was conducted in 50% acetonitrile water (phase B). Mass spectrometry analysis was performed using Nexera X2 LC-30AD (Shimadzu Japan) and QTRAP 6500 (ABSCIEX, USA). The fragment ions 141.1/123.1 and NN dimethylphenylalanine internal standard 194/148.1 were optimized, and the imidazole propionate in the positive mode of multiple reaction monitoring was detected. The calibration curve of imidazole propionate was made in water and processed in the same way as the sample.
Bile Acids Measurements
To measure bile acids in a targeted manner, plasma samples were extracted with four times the volume of acetonitrile, and feces samples were extracted with three times the volume of methanol, containing 50 ng/mL internal standard in a 1.5 mL polypropylene tube. After vortexing and centrifugation, the supernatant was diluted with water and 10ul was injected into a C18 BEH column (2.1x100 mm, 2.5um particles; Waters, USA) and used with 0.1% formic acid in acetonitrile (phase A) and containing 0.1% formic acid in water (phase B), gradient separation. ACQUITY UPLC I-Class (Waters, USA) was used, coupled with QTRAP 4500 (ABSCIEX, USA) for mass spectrometry. The ion LCA 375.3/375.3; UDCA 391.4/391.4; HDCA 391.3/391.3; CDCA 391.3/391.3; DCA 391.3/391.3; CA 407.2/407.2; GUDCA 448.3/74.1; GCDCA 448.4/74.1; GDCA 448.3/74.2; GCA 464.3/74.1; TUDCA 498.3/80.1; TDCA 498.2/80.0; TCA 514.2/80.1 were optimized and detect bile acids were detected based on the nimodipine internal standard 417/122 by multiple reaction monitoring negative mode. The bile acid calibration curve was made in water and processed in the same way as the sample.
Analysis of the Intestinal Microbial Community
DNA was extracted from about 100 mg of the feces. Then, the V3-V4 region of 16S rRNA was amplified using the primers 338F (5’-ACTCCTACGGGAGGCAGCA-3’) and 806R (5’-GGACTACHVGGGTWTCTAAT-3’). The purified amplicons were constructed for single-end sequencing based on the IonS5TMXL sequencing platform. The reads were cut, filtered and then clustered into operational taxonomic units (OTUs) according to 97% similarity. The diversity analysis of gut microbiota included alpha and beta diversity analyses. The alpha-diversity is used to describe the richness and evenness of gut microbiota. The beta-diversity is used to analyze differences in the composition of gut microbiota. The UniFrac distances were used in Principal Coordinate Analysis (PCoA). Raw 16S rRNA data was public on NCBI with project ID SUB11371228.
Statistical Analysis
The ImP, age, blood glucose and other indicators of the enrolled subjects were represented by mean ± standard deviation. The Wilcoxon symbolic rank test was used to analyze the data of ImP, age, blood glucose and other indicators among the abnormal stool consistency group and normal stool consistency group.One-way ANOVA was used to compare the differences in ImP bile acids and Shannon index among the Group H, Group S and Group N. The cross-sectional multiple logistic regression was used to analyze the influence of blood glucose, bile acids and other factors on ImP. A P value of < 0.05 was regarded as statistically significant. Statistical analyses were performed using SPSS 25.0 and GraphPad Prism 8.0.
Results
Background Characteristics of the Subjects of Study
We recruited 96 patients with T2DM, 40 of whom had abnormal stool consistency (28 males and 12 females), including 22 in Group H and 18 in Group S, and 56 of whom had normal stool consistency (39 males and 17 females). We carried out an extensive collection of common diabetes indicators (Table 1). We measured ImP concentrations in fasting plasma samples from all of the 96 patients with T2DM. The ImP ranged from 1.25 nmol/L to 102.95 nmol/L.
 |
Table 1 Patient Characteristics According to Stool Consistency |
The Relationship Between ImP and Stool Consistency
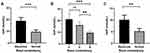
For T2DM patients, the ImP level of the abnormal stool consistency group was significantly higher than that of the normal stool consistency group (P < 0.001) (Figure 1A). Whether in Group H (P < 0.001) or Group S (P = 0.002) (Figure 1B), it was significantly higher than that in Group N. There was no significant difference between Group H and Group S (P = 0.575). In order to increase the credibility of the results, we performed multi-tests on the data, and the data of Group N and the abnormal stool consistency group were randomly and evenly divided into five parts, and four parts of each group were taken for testing each time, and a total of 25 tests were conducted. The results showed that the three tests P = 0.001 and the 22 tests P < 0.001 were consistent with the previous results. To avoid interference from other factors, the multiple regression analysis was conducted using ImP as the dependent variable and other indicators as independent variables. It was found that in addition to stool consistency, ImP was also significantly correlated with postprandial blood glucose (P = 0.028) (Table 2). In order to exclude the interference of blood glucose, we recruited another 45 healthy people, including 24 with normal stool consistency (ImP: 5.18±5.87 nmol/L) and 21 with abnormal stool consistency (ImP: 10.11±8.66 nmol/L). It was found that after the exclusion of the interference of blood glucose, ImP was still significantly correlated with stool consistency (P = 0.002) (Figure 1C).
 |
Table 2 Multiple Logistic Regression Analysis of the ImP |
ImP is Associated with Bile Acids
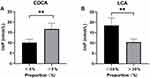
Bile acid is one of the indicators of stool consistency and easy to detect. In colon, CDCA and LCA activate TGR5 receptors on intestinal neurons,15 thus increasing the permeability and peristalsis of intestinal mucosa and affecting the stool consistency.16 It was found that LCA ≤ 30% or CDCA ≥ 5% in fecal bile acid was significantly related to abnormal stool consistency.17 We detected 13 types of bile acids in the feces of 89 diabetic patients (19 of them belong to Group H, 16 Group S and 54 Group N). The results showed that the ImP level of subjects with LCA ≤ 30% was significantly higher than that of those with LCA > 30% (P = 0.005) (Figure 2A); subjects with CDCA ≥ 5% had significantly higher ImP levels than those with CDCA < 5%(P = 0.008) (Figure 2B).
Next, we analyzed the associations between 13 types of bile acids in feces and ImP. Surprisingly, the multiple regression analysis showed that only TCA was significantly associated with ImP (P = 0.003). Next, we detected 13 types of bile acids in the plasma of 96 diabetic patients and found that ImP was significantly correlated with TDCA (P = 0.003), GCDCA (P = 0.021) and GCA (P = 0.031) (Table 3).
 |
Table 3 Multiple Logistic Regression Analysis of the ImP and Bile Acids |
Analysis of the Fecal Microflora
In order to reduce intra-group differences, fecal microbes of 10 people in each group of H, S and N were detected. The ImP levels in Group H (20.45±10.13 nmol/L) and Group S (20.48±9.50 nmol/L) were significantly higher than those in Group N (3.83±1.74 nmol/L) (P < 0.001). The results showed that the Shannon index of Group N was significantly higher than that of Group H (P = 0.041) and Group S (P = 0.003) (Figure 3A), and there was no significant difference between Group H and Group S (P = 0.45). These three groups were separated into different clusters in the UniFrac PCoA (Figure 3B).
At the phylum level (Figure 3C), there were significant differences between Group N and Group H in Firmicutes (P = 0.049) and Actinobacteria (P = 0.01), while there were significant differences between Group H and Group S in Actinobacteria (P = 0.019). At the genus level (Figure 3D), there were significant differences between Group N and Group H in Bifidobacterium (P = 0.001) and Blautia (P =0.008), while there were significant differences between Group H and Group S in Bifidobacterium (P = 0.019). There was no significant difference between Group N and Group S at the level of genus or phylum.
Discussion
In this study, 96 diabetic subjects were evaluated and a strong association was found between ImP and stool consistency. Results were verified in 45 healthy subjects. Studies have established that ImP is not only a marker of T2DM, but also a marker of other diseases with abnormal stool consistency, such as gastrointestinal diseases. As a classic example, the incidence of lung cancer among men is higher than that among women, not only because men are more prone to lung cancer for their gene expression, but also because men smoke more often than women do. Smoking is a very important risk factor for lung cancer. In addition, ImP can modulate fetal neuro development in mice,18 suggesting that ImP may be closely related to the nervous system. Moreover, after a patient’s oral administration of antibiotics to inhibit the growth of intestinal bacteria, the ImP of plasma will rise instead,19 suggesting that ImP may have other ways of production. Our understanding of ImP may be still in the initial stage, and there are still many unknown areas for further exploration.
In order to clarify the mechanism behind the correlation between ImP and stool consistency, we conducted a study over bile acids. Bile acids are synthesized from cholesterol in the liver,20 stored in the gallbladder and discharged through the common bile duct into the duodenum; 95% of the bile acids are reabsorbed back into the blood at the end of the ileum. In the colon, CDCA and LCA activate TGR5 receptors on enteric neurons to stimulate the intestine, increasing intestinal mucosal permeability and peristalsis, thus affecting the consistency of stool. Bile acid is closely related to diarrhea,21 and it can also affect constipation.22 Subjects with soft stool excretion had high levels of primary bile acids (especially CDCA), while those with hard stool excretion had high levels of secondary bile acids (especially LCA).17
We found that there was a significant difference between Group H and Group S concerning the proportion of bile acids in CA, CDCA, and LCA. The proportion of bile acids of Group N was in the middle of the Group H and Group S and had no significant difference from that of Group S. Only LCA was significantly different between Group N and Group H. Too many or too few bile acids often have similar effects. For example, too much LCA can inhibit the growth of Clostridioides difficile (Clostridioides difficile is related to weight loss and the improvement of glucose tolerance)23 and aggravate T2DM, while too little LCA fails to inhibit the growth of E. coli, tends to induce intestinal inflammation,24 and also can aggravate T2DM. In addition, DCA also has the dual effects of aggravating and mitigating intestinal inflammation.25,26
Multiple regression showed that ImP was positively correlated with TCA in feces. TCA is an important factor affecting energy metabolism27 and intestinal flora, and plays an important role in the development of intestinal flora.28 TCA is also a great booster for the growth of Giardia Lamblia, which can cause diarrhea.29 ImP was negatively correlated with GCA in blood. This may be because GCA is positively correlated with body weight and decreases with weight loss,30 while ImP is negatively correlated with body weight (P =0.175). Despite a limited number of patients under study, previous studies established that ImP levels in obese T2DM subjects were lower than those in normal T2DM subjects, which was consistent with our findings. Low levels of ImP favor metformin in its potency, which is consistent with metformin’s efficacy in treating Type 2 diabetes with obesity.31 However, the relationship between ImP and obesity still needs further investigation.
The Shannon index of Group N was significantly higher than that of Group H and S, suggesting that the change in the microbiome diversity was closely related to ImP.32 The Bifidobacterium in Group H was significantly higher than that in Group N and S, which might be a result of the higher content of CDCA in the feces of Group N and S. Under normal circumstances, CDCA changes from microbiome dehydroxylation to LCA, and an increase in CDCA indicates rapid peristalsis of the colon or insufficient dehydroxylation time due to changes in the microbiome, which leads to the reduction of Bifidobacterium. Although Bifidobacterium has been regarded as a beneficial bacterium, studies have indeed shown a positive correlation between Bifidobacterium and abdominal pain, discomfort and bloating.33
ImP is related to diet, especially the intake of high protein foods such as cheese, milk or eggs.10 A high-protein diet can reduce HbA1c levels in T2DM patients but increase the frequency of gastrointestinal symptoms such as diarrhea and constipation with abnormal stool consistency.34 According to our study, abnormal stool consistency can increase ImP, which explains why ImP is positively correlated with the intake of cheese, milk and eggs.
ImP can reduce the curative effect of metformin, and our study has shown that abnormal stool consistency can lead to an increase in ImP. The main side effects brought about by metformin include diarrhea, abdominal distension, nausea, constipation and other gastrointestinal symptoms with abnormal stool consistency. That is to say, after the intake of metformin by T2DM patients, the side effects arising from abnormal stool consistency will probably lead to an increase in ImP, thus reducing the curative effect of metformin. Supplementation of short-chain fatty acids can reduce the side effects of metformin and therefore, improve its curative effect,3 which is consistent with our guess. It suggests that treating gastrointestinal symptoms may be one of the approaches to improving the efficacy of metformin.
Our study has a few limitations. First, the sample size was small, with only 96 T2DM subjects and 45 healthy subjects; and the sample coverage is limited, as only Chinese participants were included. Second, there are more than 50 kinds of bile acids, but we only detected 13 kinds of typical bile acids. It is possible that some bile acids excluded from our detection are low in content, but they have a great influence on the stool consistency. Moreover, human feces can only reflect the changes in the content of bile acids in the colon, mainly the secondary bile acids (DCA and LCA) generated by the metabolism of intestinal flora, and nearly 95% of the bile acids are reabsorbed into the blood in the ileum. The content of these bile acids is not clear, and it cannot reflect the changes in the bile acids excreted by the liver and the small intestine. Stool consistency is also subject to many other factors, such as intestinal nerves, intestinal permeability and so on. Due to limited conditions, those factors were not included in our analysis.
Conclusions
The present study has clearly shown the relationship between the ImP level and the stool consistency, which has to do with the proportion of bile acids and changes in the fecal microbial structure. These findings may help improve the treatment of T2DM by improving the stool consistency.
Abbreviations
ImP, imidazole propionate; T2DM, type 2 diabetes; TC, total cholesterol; BMI, body mass index; HDL, high-density lipoprotein cholesterol; TG, triglycerides; LDL, low-density lipoprotein cholesterol; CA, cholic acid; CDCA, chenodeoxycholic acid; DCA, deoxycholic acid; LCA, lithocholic acid; TUDCA, tauroursodeoxycholic acid; HDCA, hyodeoxycholic acid; GUDCA, glycoursodeoxycholic acid; GDCA, glycodeoxycholic acid; TDCA, taurodeoxycholate; UDCA, ursodeoxycholic acid; GCDCA, glycochenodeoxycholate; GCA, glycocholic acid; TCA, taurocholic acid.
Acknowledgments
We would like to thank all participants in the present study. This work was supported by Capital Clinical Characteristic Application Research and Achievement Promotion Project (Z161100000516076) and the National Natural Science Foundation of China (82130112). The authors declare no competing financial interests.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Pedersen HK, Gudmundsdottir V, Nielsen HB, et al. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature. 2016;535(7612):376–381. doi:10.1038/nature18646
2. Jia W, Wei M, Rajani C, Zheng X. Targeting the alternative bile acid synthetic pathway for metabolic diseases. Protein Cell. 2020. doi:10.1007/s13238-020-00804-9
3. Burton JH, Johnson M, Johnson J, Hsia DS, Greenway FL, Heiman ML. Addition of a gastrointestinal microbiome modulator to metformin improves metformin tolerance and fasting glucose levels. J Diabetes Sci Technol. 2015;9(4):808–814. doi:10.1177/1932296815577425
4. Bogachev AV, Bertsova YV, Bloch DA, Verkhovsky MI. Urocanate reductase: identification of a novel anaerobic respiratory pathway in Shewanella oneidensis MR-1. Mol Microbiol. 2012;86(6):1452–1463. doi:10.1111/mmi.12067
5. Koh A, Molinaro A, Stahlman M, et al. Microbially produced imidazole propionate impairs insulin signaling through mTORC1. Cell. 2018;175(4):947–61 e17. doi:10.1016/j.cell.2018.09.055
6. Koh A, Manneras-Holm L, Yunn NO, et al. Microbial imidazole propionate affects responses to metformin through p38gamma-dependent inhibitory AMPK phosphorylation. Cell Metab. 2020;32(4):643–53e4. doi:10.1016/j.cmet.2020.07.012
7. Cani PD. Microbiota and metabolites in metabolic diseases. Nat Rev Endocrinol. 2019;15(2):69–70. doi:10.1038/s41574-018-0143-9
8. Fan Y, Pedersen O. Gut microbiota in human metabolic health and disease. Nat Rev Microbiol. 2021;19(1):55–71. doi:10.1038/s41579-020-0433-9
9. Wu H, Tremaroli V, Schmidt C, et al. The gut microbiota in prediabetes and diabetes: a population-based cross-sectional study. Cell Metab. 2020;32(3):379–90 e3. doi:10.1016/j.cmet.2020.06.011
10. Molinaro A, Bel Lassen P, Henricsson M, et al. Imidazole propionate is increased in diabetes and associated with dietary patterns and altered microbial ecology. Nat Commun. 2020;11(1):5881. doi:10.1038/s41467-020-19589-w
11. Van der Heiden C, Wadman SK, de Bree PK, Wauters EA. Increased urinary imidazolepropionic acid, N-acetylhistamine and other imid azole compounds in patients with intestinal disorders. Clin Chim. 1972;39(1):201–214. doi:10.1016/0009-8981(72)90317-8
12. Yamamoto M, Pinto-Sanchez MI, Bercik P, Britz-McKibbin P. Metabolomics reveals elevated urinary excretion of collagen degradation and epithelial cell turnover products in irritable bowel syndrome patients. Metabolomics. 2019;15(6):82. doi:10.1007/s11306-019-1543-0
13. Vandeputte D, Falony G, Vieira-Silva S, Tito RY, Joossens M, Raes J. Stool consistency is strongly associated with gut microbiota richness and composition, enterotypes and bacterial growth rates. Gut. 2016;65(1):57–62. doi:10.1136/gutjnl-2015-309618
14. Yap CX, Henders AK, Alvares GA, et al. Autism-related dietary preferences mediate autism-gut microbiome associations. Cell. 2021;184(24):5916–31 e17. doi:10.1016/j.cell.2021.10.015
15. Bunnett NW. Neuro-humoral signalling by bile acids and the TGR5 receptor in the gastrointestinal tract. J Physiol. 2014;592(14):2943–2950. doi:10.1113/jphysiol.2014.271155
16. Alemi F, Poole DP, Chiu J, et al. The receptor TGR5 mediates the prokinetic actions of intestinal bile acids and is required for normal defecation in mice. Gastroenterology. 2013;144(1):145–154. doi:10.1053/j.gastro.2012.09.055
17. Vijayvargiya P, Camilleri M, Burton D, Busciglio I, Lueke A, Donato LJ. Bile and fat excretion are biomarkers of clinically significant diarrhoea and constipation in irritable bowel syndrome. Aliment Pharmacol Ther. 2019;49(6):744–758. doi:10.1111/apt.15106
18. Vuong HE, Pronovost GN, Williams DW, et al. The maternal microbiome modulates fetal neurodevelopment in mice. Nature. 2020;586(7828):281–286. doi:10.1038/s41586-020-2745-3
19. Tanes C, Bittinger K, Gao Y, et al. Role of dietary fiber in the recovery of the human gut microbiome and its metabolome. Cell Host Microbe. 2021;29(3):394–407 e5. doi:10.1016/j.chom.2020.12.012
20. Chen PB, Black AS, Sobel AL, et al. Directed remodeling of the mouse gut microbiome inhibits the development of atherosclerosis. Nat Biotechnol. 2020;38(11):1288–1297. doi:10.1038/s41587-020-0549-5
21. Camilleri M, Vijayvargiya P. The role of bile acids in chronic diarrhea. Am J Gastroenterol. 2020;115(10):1596–1603. doi:10.14309/ajg.0000000000000696
22. Misawa N, Higurashi T, Takatsu T, et al. The benefit of elobixibat in chronic constipation is associated with faecal deoxycholic acid but not effects of altered microbiota. Aliment Pharmacol Ther. 2020;52(5):821–828. doi:10.1111/apt.15950
23. Von Schwartzenberg RJ, Bisanz JE, Lyalina S, et al. Caloric restriction disrupts the microbiota and colonization resistance. Nature. 2021;595(7866):272–277. doi:10.1038/s41586-021-03663-4
24. Tian Y, Gui W, Koo I, et al. The microbiome modulating activity of bile acids. Gut Microbes. 2020;11(4):979–996. doi:10.1080/19490976.2020.1732268
25. Wang L, Gong Z, Zhang X, et al. Gut microbial bile acid metabolite skews macrophage polarization and contributes to high-fat diet-induced colonic inflammation. Gut Microbes. 2020;12(1):1–20. doi:10.1080/19490976.2020.1819155
26. Wang S, Martins R, Sullivan MC, et al. Diet-induced remission in chronic enteropathy is associated with altered microbial community structure and synthesis of secondary bile acids. Microbiome. 2019;7(1). doi:10.1186/s40168-019-0740-4
27. Worthmann A, John C, Ruhlemann MC, et al. Cold-induced conversion of cholesterol to bile acids in mice shapes the gut microbiome and promotes adaptive thermogenesis. Nat Med. 2017;23(7):839–849. doi:10.1038/nm.4357
28. Van Best N, Rolle-Kampczyk U, Schaap FG, et al. Bile acids drive the newborn’s gut microbiota maturation. Nat Commun. 2020;11(1):3692. doi:10.1038/s41467-020-17183-8
29. Hornef ARKHAWNBM, Hassani K, Walker A. Disturbed gut microbiota and bile homeostasis in Giardia -infected mice contributes to metabolic dysregulation and growth impairment. Sci Transl Med. 2020;12(565):7019. doi:10.1126/scitranslmed.aay7019
30. Hibberd AA, Yde CC, Ziegler ML, et al. Probiotic or synbiotic alters the gut microbiota and metabolism in a randomised controlled trial of weight management in overweight adults. Benef Microbes. 2019;10(2):121–135. doi:10.3920/BM2018.0028
31. Allada R, Bass J, Longo DL. Circadian mechanisms in medicine. N Engl J Med. 2021;384(6):550–561. doi:10.1056/NEJMra1802337
32. Wilmanski T, Rappaport N, Earls JC, et al. Blood metabolome predicts gut microbiome alpha-diversity in humans. Nat Biotechnol. 2019;37(10):1217–1228. doi:10.1038/s41587-019-0233-9
33. Ahmed F, Brewington C, Chang KJ, Moreno CC, Gollub M, Yee J. Letter to the editor regarding: multicentre, prospective, randomised study comparing the diagnostic yield of colon capsule endoscopy versus CT colonography in a screening population (the TOPAZ study). Gut. 2022;71(1):214–215. doi:10.1136/gutjnl-2021-324396
34. Gram-Kampmann EM, Hansen CD, Hugger MB, et al. Effects of a 6-month, low-carbohydrate diet on glycaemic control, body composition, and cardiovascular risk factors in patients with type 2 diabetes: an open-label randomized controlled trial. Diabetes Obes Metab. 2022;24(4):693–703. doi:10.1111/dom.14633
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.