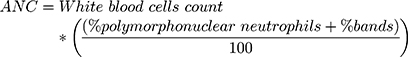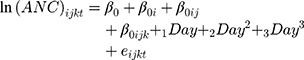Back to Journals » Pharmacogenomics and Personalized Medicine » Volume 13
IL-6 −572C>G and CARD8 304T>A Genetic Polymorphisms are Associated with the Absolute Neutrophil Count in Patients with Hematological Malignancies Under Chemotherapy: An Application of Multilevel Models to a Preliminary Pharmacogenetic Study
Authors Martinez MF , Alveal E, Soto TG, Bustamante EI , Ávila F, Bangdiwala SI, Flores I, Benavides C, Morales R , Varela NM , Quiñones LA
Received 10 May 2020
Accepted for publication 2 July 2020
Published 19 August 2020 Volume 2020:13 Pages 337—343
DOI https://doi.org/10.2147/PGPM.S261208
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Martin H Bluth
Matias F Martinez,1,2 Enzo Alveal,1 Tomas G Soto,1,3 Eva I Bustamante,4 Fernanda Ávila,5 Shrikant I Bangdiwala,6,7 Ivonne Flores,4 Claudia Benavides,4 Ricardo Morales,4 Nelson M Varela,1,2 Luis A Quiñones1,2
1Laboratory of Chemical Carcinogenesis and Pharmacogenetics (CQF), Department of Basic and Clinical Oncology (DOBC), Faculty of Medicine, University of Chile, Santiago, Chile; 2Latin American Network for the Implementation and Validation of Pharmacogenomic Clinical Guidelines (RELIVAF-CYTED), Madrid, Spain; 3Departamento De Ciencias Básicas Santiago, Facultad De Ciencias, Universidad Santo Tomás, Santiago, Chile; 4Cancer Institute Arturo López Pérez Foundation, Santiago, Chile; 5Infectology Section, Medicine Department, Clinical Hospital of the University of Chile, Santiago, Chile; 6Population Health Research Institute, McMaster University, Hamilton, ON, Canada; 7Department of Health Research Methods, Evidence, and Impact, McMaster University, Hamilton, ON, Canada
Correspondence: Luis A Quiñones; Nelson M Varela
Laboratory of Chemical Carcinogenesis and Pharmacogenetics (CQF), Department of Basic and Clinical Oncology (DBOC), Faculty of Medicine, University of Chile, Santiago, Chile
Tel +56-2-29770741
; Tel +56-2-29770743
Email [email protected]; [email protected]
Purpose: Neutropenia is a common event in patients undergoing cytotoxic chemotherapy for the treatment of a hematological malignancy. Some polymorphisms, as IL-6 − 572C>G (rs1800796), IL-1β − 31 G>A (rs1143627), and CARD8 304T>A (rs2043211), in genes related to the inflammatory process, could affect the level of absolute neutrophil count (ANC) after chemotherapy. Since an efficient inflammatory process enhances neutrophil survival, we hypothesize that these polymorphisms are associated with ANC.
Patients and Methods: We carried out a prospective cohort study in two hospitals in Santiago, Chile. The patients included were adults diagnosed with acute myeloblastic leukemia, acute lymphoblastic leukemia, or non-Hodgkin’s lymphoma, undergoing cytotoxic chemotherapy. We use a multilevel linear regression model to test our hypothesis. The best model was selected using the Akaike’s information criterion (AIC).
Results: We analyzed 1726 hemograms and ANCs from 172 hospitalizations from 32 patients. The results show that CC and CG genotypes of IL-6 − 572 C>G polymorphism are associated with higher ANCs compared with the GG genotype (Ln (ANC) ∼ 0.81 IC95% 0.02– 1.55). Similarly, TT and AT genotypes of CARD8 304T>A polymorphism were related to higher ANCs compared with AA (Ln (ANC) ∼ 0.95 IC95% 0.02– 1.82). IL-1β genetic polymorphism had no statistically significant association with ANC.
Conclusion: IL-6 rs1800796 − 572C>G and CARD8 rs2043211 304T>A polymorphisms are associated with the absolute neutrophil count in patients undergoing cytotoxic chemotherapy for treatment of hematological malignancies. Our findings might be useful to improve the safety of chemotherapy through predictive ANC models.
Keywords: pharmacogenetics, neutropenia, hematological neoplasms, leukemia, lymphoma, interleukin-6, CARD8 protein
Introduction
The primary treatment of hematological malignancies (HM) is cytotoxic chemotherapy, whose goal is to suppress the bone marrow to make the cancer cells disappear or to avoid the formation of new ones. Severe neutropenia, defined as an absolute neutrophil count (ANC) lower than 500 cells/mm3,1 is common in these patients (30% prevalence) and makes them more susceptible to life-threatening infections (11% of mortality).2,3 The earlier neutropenia starts, and the longer it lasts, the higher the risk.1,4,5 Currently, it is not possible to predict the value of ANC, so we cannot know the start or length of neutropenia.
Chemotherapy involves inflammation due to cell destruction; neutrophils maturation, release, and survival depend on an efficient response.6,7 Some proteins involved in the inflammatory response, as Interleukin 6 (IL-6), Interleukin 1β (IL-1β), and caspase recruitment domain family, member 8 (CARD8), had polymorphic variants that can explain the interindividual variability of chemotherapy.
IL-6 participates in the regulation of hematopoiesis, inflammation, and immune response,8,9 and stimulates the production and mobilization of neutrophils in the bone marrow.10,11 Moreover, CARD8 is a crucial component in the regulation of nuclear signaling and the activation of IL-1β12 The reduced expression of IL-1β and IL-1R and the structural alteration of CARD attenuate the immune system through poor neutrophil recruitment.13
An essential problem in pharmacogenetic studies, particularly in developing countries, is to reach an appropriate sample size for robust results. However, there are statistical methods that allow making preliminary and well-controlled analyses, whether enough information from every patient is available. Multilevel models (MM) allow us to analyze repeated measures and grouped data in a reduced sample of patients.
We hypothesize that genetic polymorphisms on IL-6, IL-1β, and CARD8 are associated with the ANC value in patients with HM receiving cytotoxic drugs using an MM to control by correlation and confounding variables.
Patients and Methods
We carried out a prospective cohort study from October 2017 to November 2018 at the Oncologic Hospital “Fundación Arturo Lopez Pérez” (FALP) and the Clinical Hospital of the University of Chile (HCUCH) in Santiago, Chile.
We included adult patients diagnosed with acute myeloblastic leukemia, acute lymphoblastic leukemia or non-Hodgkin’s lymphoma, and had neutropenia after cytotoxic chemotherapy. They were enrolled before the first chemotherapy cycle and followed up during everyone. We excluded patients regularly taking immunosuppressive medication, pregnant women, and patients with a diagnosis of immunodeficiency.
ANC was treated as a continuous variable, and it was obtained from hemograms directly when the laboratory provided it. When not provided, it was calculated using the following formula:14
All patients signed a written informed consent and an agreement to participate in this study. The study was carried out following the strict ethical procedures recommended by the Ethics Committee of the Clinical Hospital of the University of Chile (approval received on July 18th, 2018) and the Eastern Metropolitan Health Service (approval received on July 4th, 2017), following the procedures suggested in the Declaration of Helsinki, with Chilean Laws 20.120, 20.584 and 19.628 and with the guidelines of the Good Clinical Practices.
Genotyping Analysis
DNA was isolated from peripheral blood samples using the High Pure PCR Template Preparation Kit (Catalog Number, 11,796,828,001; Roche Diagnostics GmbH, Mannheim, Germany).
IL-6 rs1800796, IL-1β rs1143627, and CARD8 rs2043211 polymorphism were analyzed using TaqMan® SNP Genotyping Assay (Catalog number, 4,362,691; Thermo Fisher Scientific, Waltham, MA, United States) in a Stratagene Mx30d00p real-time PCR system (Agilent Technologies, Santa Clara, CA, United States). Every sample was analyzed in triplicate to ensure reliability (Laboratory Protocol dx.doi.org/10.17504/protocols.io.bfuujnww).
Statistical Analysis
We use a multilevel linear regression model, which allowed us to account for intraindividual variability regarding the chemotherapy scheme and cycle and to use correlated outcome data. The unit of observation was the ANC, not the patient.
Our MM had four levels15 (Table 1), 1) ”Patient,” the upper level, where genetic variables were assessed; 2) “Scheme” with eight possibilities, depending on the treatment; 3) “cycle” level and 4) “time” level, where the daily ANC and the administration of Granulocyte Colony-Stimulating Factor (G-CSF) were measured. Moreover, we included two interactions: 1) G-CSF/time and 2) administration of G-CSF/hospital.
 |
Table 1 Multilevel Diagram |
To improve the fit, we made a logarithmic transformation of the dependent variable. The “0” ANC values were replaced by “1” due to mathematical incompatibility. As this variable has a polynomic behavior, we added the variables Day2 and Day3 to the model (other models in Supplementary Table 1). The base model to add other explanatory variables is:
where 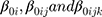 are random intercept effects for “patient,” “scheme within patient,” and “cycle within scheme within patient,” respectively.
are random intercept effects for “patient,” “scheme within patient,” and “cycle within scheme within patient,” respectively.
The best model was selected using Akaike’s Information Criterion (AIC), and variables were added to the model depending on their statistical significance in univariate analysis. The diagnosis was analyzed using the “cell means model.” Other categorical variables were analyzed using a reference category.
Analyses were performed using the software R (v3.6.1) and RStudio (v2.1.5001) with the package “lme4”16
Results
We analyzed 1726 ANCs from 32 patients. The most common scheme was “Hyper CVAD” (38% of patients in Phase I, and 50% of patients in Phase II). Table 2 shows the ANC values in the sample. Allele frequencies of the studied polymorphisms are shown in Supplementary Table 2.
 |
Table 2 Absolute Neutrophil Count by Genotype and Therapeutic/Morbid Characteristics |
Figure 1 shows genetic and non-genetic associated factors to ANC (univariate analysis in Supplementary Table 3). For IL6 −572C>G polymorphism, genotypes CC and CG are associated with higher ANCs compared with the GG genotype (Ln(ANC)~0.81 IC95% 0.02–1.55). Similarly, for CARD8 304T>A polymorphism, genotypes TT and AT were related to higher ANCs compared with AA (Ln(ANC)~0.95 IC95% 0.02–1.82). IL-1β had no statistically significant association with ANC.
About non-genetic associated factors, we found that, besides time, the HCUCH was related to higher levels of ANC after chemotherapy compared to FALP, but when the patient received G-CSF at HCUCH had lower ANC than FALP. The interaction between time and the use of G-CSF was associated positively with the ANC.
The Ln(ANC) values correlation overtime was strongly determined by the scheme and rather than by the cycle, with an Intraclass Correlation Coefficient (ICC) for the scheme of 0.4 and not much more due to cycle (see Supplementary Table 1 which shows ICCs).
Discussion
ANC monitoring could improve anticancer treatment by predicting toxicity. Previous predictive models have not included genetic factors, but drug-specific variables.17 Previous studies have included some drug-related genes and the use of oral mucosal neutrophil count as a way to predict the onset and resolution of neutropenia;18 in this study, we use drug-independent polymorphisms and non-genetic factors as possible explanations for ANC variability in patients undergoing chemotherapy for HM.
We found that patients carrying C allele of IL-6 −572 C>G polymorphism had higher ANC levels during chemotherapy, according to other studies,19,20 supporting the idea that G allele causes a change in the regulatory region that implies a lower expression of the gene and poor neutrophils’ survival.19 Also, this polymorphism was previously related to a lower level of plasmatic cytokines, supporting our results.19
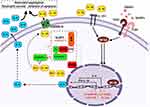
CARD8 304T>A polymorphism causes cysteine to change to a stop codon (p.C10X).21 Thus, the formed protein is not fully functional, which decreases the activation of IL-1β; Figure 2 shows the relationship between the proteins and the polymorphisms.22 Therefore, the lower activity of pro-inflammatory proteins caused by the AA genotype in this study could lead to early neutrophil apoptosis and, therefore, to lower levels of ANC after chemotherapy. Nevertheless, the association with plasmatic level cytokines has not been described.
The time is one of the most important explanatory variables on the behavior of ANC; linear time (Day) has a negative coefficient, which means, in the first days after chemotherapy starts, the ANC decreases. Then, with the passing of days, quadratic time (Day2) becomes more critical, making the ANC rise again (positive coefficient); cubic time (Day3) finally makes the ANC decrease to normal levels. This polynomial fit is the best model to explain the curve of neutrophils after chemotherapy administration (Supplementary Table 1).
The interaction of time with the G-CSF administration had a positive association, indicating that, while time increases, the medication’s effect is more significant due to the effect reaches its maximum some days after the first dose.23,24 Besides, the negative association between hospitals and G-CSF administration may be explained because, at HCUCH, G-CSF was used at the beginning of the neutropenia period, whereas at FALP, toward the end of the period; hence, it was more common to have lower levels of ANC during medication administration at HCUCH than at FALP.25
Multilevel modeling allows us to work with correlated data and meets the assumption of linear regression, generating a proper way to obtain more reliable results. However, despite our analysis, the main limitation of this study is the small sample size, because it does not allow carry out combinatorial analyses; the low number of patients examined could mask potential associations, especially for low-frequency polymorphisms. Thus, our results should be used only to help to demonstrate the association between ANC and polymorphisms, not the effect in the outcome. Moreover, future studies should consider the effect of other actors in the inflammatory response and also focus on a specific chemotherapy scheme or diagnosis.
In the future, our results could be used to generate or improve predictive models to enhance the safety of chemotherapy for HM. Prediction of the ANC lower than 500 cells/mm3 could help to improve antimicrobial prophylaxis, preventive isolation, and early discharge, decreasing the morbidity of patients and improving their quality of life.
Conclusion
In conclusion, IL-6 −572 C>G polymorphism was associated with a lower neutrophil count, similarly to CARD8 304T>A polymorphism. Non-genetic factors associated were the administration of G-CSF, the hospital, and the time. The multilevel analysis allows us to manage correlated data and to have more reliable results.
Acknowledgments
We acknowledge to oncologic hematology department of the Hospital Clinico de la Universidad de Chile, mainly to Rebeca Aguayo.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Klastersky J, De Naurois J, Rolston K, et al. Management of febrile neutropaenia: ESMO clinical practice guidelines. Ann Oncol. 2016;27(suppl_5):v111–v118. doi:10.1093/annonc/mdw325
2. Yılmaz Ş, Ören H, Demircioğlu F, I˙ Rken G. Assessment of febrile neutropenia episodes in children with acute leukemia treated with BFM protocols. Pediatr Hematol Oncol. 2008;25(3):195–204. doi:10.1080/08880010801938231
3. Castagnola E, Fontana V, Caviglia I, et al. A prospective study on the epidemiology of febrile episodes during chemotherapy-induced neutropenia in children with cancer or after hemopoietic stem cell transplantation. Clin Infect Dis. 2007;45(10):1296–1304. doi:10.1086/522533
4. Lehrnbecher T, Robinson P, Fisher B, et al. Guideline for the management of fever and neutropenia in children with cancer and hematopoietic stem-cell transplantation recipients: 2017 update. J Clin Oncol. 2017;35(18):2082–2094. doi:10.1200/JCO.2016.71.7017
5. Taplitz RA, Kennedy EB, Bow EJ, et al. Outpatient management of fever and neutropenia in adults treated for malignancy: American Society of Clinical Oncology and Infectious Diseases Society of America clinical practice guideline update. J Clin Oncol. 2018;36(14):1443–1453. doi:10.1200/JCO.2017.77.6211
6. van der Most RG, Currie AJ, Robinson B, Lake RA. Decoding dangerous death: how cytotoxic chemotherapy invokes inflammation, immunity or nothing at all. Cell Death Differ. 2008;15(1):13. doi:10.1038/sj.cdd.4402255
7. Vyas D, Laput G, Vyas AK. Chemotherapy-enhanced inflammation may lead to the failure of therapy and metastasis. Onco Targets Ther. 2014;7:1015. doi:10.2147/OTT.S60114
8. Rose-John S, Winthrop K, Calabrese L. The role of IL-6 in host defence against infections: immunobiology and clinical implications. Nat Rev Rheumatol. 2017;13(7):399. doi:10.1038/nrrheum.2017.83
9. Wilkinson AN, Gartlan KH, Kelly G, et al. Granulocytes are unresponsive to IL-6 due to an absence of gp130. J Immunol. 2018;200(10):3547–3555. doi:10.4049/jimmunol.1701191
10. Chiba Y, Mizoguchi I, Hasegawa H, et al. Regulation of myelopoiesis by proinflammatory cytokines in infectious diseases. Cell Mol Life Sci. 2018;75(8):1363–1376. doi:10.1007/s00018-017-2724-5
11. Panayides A, Ioakeimidou A, Karamouzos V, et al. -572 G/C single nucleotide polymorphism of interleukin-6 and sepsis predisposition in chronic renal disease. Eur J Clin Microbiol Infect Dis. 2015;34(12):2439–2446. doi:10.1007/s10096-015-2500-0
12. Groslambert M, Py BF. Spotlight on the NLRP3 inflammasome pathway. J Inflamm Res. 2018;11:359. doi:10.2147/JIR.S141220
13. Miller LS, Pietras EM, Uricchio LH, et al. Inflammasome-mediated production of IL-1β is required for neutrophil recruitment against Staphylococcus aureus in vivo. J Immunol. 2007;179(10):6933–6942. doi:10.4049/jimmunol.179.10.6933
14. Al-Gwaiz LA, Babay HH. The diagnostic value of absolute neutrophil count, band count and morphologic changes of neutrophils in predicting bacterial infections. Med Princ Pract. 2007;16(5):344–347. doi:10.1159/000104806
15. Bangdiwala SI. The multilevel diagram. Int J Inj Contr Saf Promot. 2012;19(4):388–390. doi:10.1080/17457300.2012.734040
16. Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. arXiv Preprint arXiv. 2014.
17. Friberg LE, Henningsson A, Maas H, Nguyen L, Karlsson MO. Model of chemotherapy-induced myelosuppression with parameter consistency across drugs. J Clin Oncol. 2002;20(24):4713–4721. doi:10.1200/JCO.2002.02.140
18. Akpek G, Knight RD, Wright DG. Use of oral mucosal neutrophil counts to detect the onset and resolution of profound neutropenia following high‐dose myelosuppressive chemotherapy. Am J Hematol. 2003;72(1):13–19. doi:10.1002/ajh.10250
19. Sharma A, Singh K, Biswas A, et al. Impact of interleukin 6 promoter polymorphisms (− 174 G> C,− 572 G> C and− 597 G> A) on plasma IL-6 levels and their influence on the development of DVT: a study from India. Hematology. 2018;23(10):833–838. doi:10.1080/10245332.2018.1483546
20. Lok LS, Farahi N, Juss JK, et al. Effects of tocilizumab on neutrophil function and kinetics. Eur J Clin Invest. 2017;47(10):736–745. doi:10.1111/eci.12799
21. Paramel G, Sirsjö A, Fransén K. Role of genetic alterations in the NLRP3 and CARD8 genes in health and disease. Mediators Inflamm. 2015;2015:1–10. doi:10.1155/2015/846782
22. Pathan N, Marusawa H, Krajewska M, et al. TUCAN, an antiapoptotic caspase-associated recruitment domain family protein overexpressed in cancer. J Biol Chem. 2001;276(34):32220–32229. doi:10.1074/jbc.M100433200
23. Melhem M, Delor I, Pérez Ruixo JJ, et al. Pharmacokinetic–pharmacodynamic modelling of neutrophil response to G‐CSF in healthy subjects and patients with chemotherapy‐induced neutropenia. Br J Clin Pharmacol. 2018;84(5):911–925. doi:10.1111/bcp.13504
24. Craig M, Humphries AR, Nekka F, Bélair J, Li J, Mackey MC. Neutrophil dynamics during concurrent chemotherapy and G-CSF administration: mathematical modelling guides dose optimisation to minimise neutropenia. J Theor Biol. 2015;385:77–89. doi:10.1016/j.jtbi.2015.08.015
25. Ludwig H, Bokemeyer C, Aapro M, et al. Chemotherapy-induced neutropenia/febrile neutropenia prophylaxis with biosimilar filgrastim in solid tumors versus hematological malignancies: MONITOR-GCSF study. Future Oncol. 2019;15(8):897–907. doi:10.2217/fon-2018-0814
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.