Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
IgG4-Related Disease Manifested as Cutaneous Plasmacytosis: A Case Report
Authors Wang W, Kang X, Ding Y, Mao L, Dilinuer A, Li W
Received 7 March 2023
Accepted for publication 8 July 2023
Published 2 August 2023 Volume 2023:16 Pages 1997—2004
DOI https://doi.org/10.2147/CCID.S406199
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jeffrey Weinberg
Weijia Wang,1– 3 Xiaojing Kang,1– 3 Yuan Ding,1– 3 Lidan Mao,1– 3 Abudureyimu Dilinuer,1– 3 Wenzheng Li1– 3
1Department of Dermatology and Venereology, People’s Hospital of Xinjiang Uygur Autonomous Region, Urumqi, People’s Republic of China; 2Xinjiang Clinical Research Center for Dermatologic Diseases, Urumqi, People’s Republic of China; 3Xinjiang Key Laboratory of Dermatology Research (XJYS1707), Urumqi, People’s Republic of China
Correspondence: Xiaojing Kang, Department of Dermatology and Venereology, People’s Hospital of Xinjiang Uygur Autonomous Region, No. 91 Tianchi Road, Tianshan District, Urumqi, People’s Republic of China, Email [email protected]
Background: IgG4-related disease (IgG4-RD) is a rare fibroinflammatory disease that has a high tendency to misdiagnosis in clinics.
Case Presentation: A 48-year-old man developed a rash with progressive itching 3 years ago after hormone therapy for an ocular “inflammatory pseudotumor”. The disease condition of this patient involved multiple organs which involved the skin. The patient was misdiagnosed with other diseases during the period of hospitalization, leading to poor therapeutic effects and repeated skin lesions. The dermatopathological report indicated plasma cell proliferative disorder, with IgG4/IgG exceeding 40% and abnormally elevated serum IgG4 levels. After the patient was diagnosed with IgG4-RD, a series of treatments improved skin lesions, relieved other symptoms, and decreased serum IgG4 levels.
Conclusion: IgG4-RD is a highly misdiagnosed disease that deserves the attention of physicians. The patient we reported could be considered a representative case of IgG4-RD that presents with skin lesions. For patients with suspected IgG4-RD, serum IgG4 testing should be performed, and further imaging, serological tests, and pathology examinations are needed to exclude malignancy, infection, and autoimmune diseases.
Keywords: IgG4-RD, fibro-inflammatory, dermatopathological, misdiagnosis, diagnosis, treatment
Introduction
IgG4-related disease (IgG4-RD) is a recently recognized chronic fibro-inflammatory disorder related to immunomodulation,1 typically characterized by elevated serum levels of IgG4 and substantial infiltration of IgG4-positive plasma cells in virtually every organ system such as the pancreas, biliary tract, salivary glands, lacrimal glands, lungs, kidneys, and retroperitoneum.2,3 IgG4-RD exhibits a predilection to occur in men aged above 50 years old,4 and is easily confused with malignancies, infections, and other immune-mediated disorders. Thus, the Japan College of Rheumatology formulated the first comprehensive diagnostic criteria for IgG-RD in 2011.5 Currently, systemic administration of glucocorticoid or rituximab can induce effective remission in most IgG4-RD patients, but disease relapse remains a notable clinical challenge.6 In 2013, Sato et al reported a case of IgG4-RD involving the skin7 and found that the potential initial symptom of IgG4-RD was easily detectable skin lesions. Therefore, skin manifestations are of great significance for the early detection and diagnosis of IgG4-RD. Here, we report a case of IgG4-RD with multi-organ (including the skin) involvement and complete clinical data.
Case Report
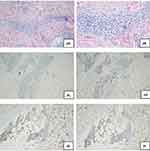
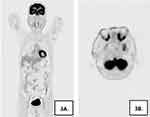
A 48-year-old male patient from the ethnic minority of Manchu in China developed rice-grain-sized red papules on his back while undergoing hormone therapy and radiotherapy for the ocular “inflammatory pseudotumor” three years ago, without obvious predisposing factors and accompanied by pruritus. Subsequently, the rashes gradually expanded and merged into patches, spreading to the head-face-neck area and torso with severer pruritus. At that time, the patient visited several hospitals and received treatment with antihistamine drugs and topical glucocorticoid ointment, but no evident improvement was observed. In the recent 2 weeks, the patient presented to our hospital complaining of increased and thickened skin lesions in the head-face-neck area and torso together with intensified pruritus. He declared good conditions and denied any history of infectious diseases, hypertension, diabetes, psychotic disorders, etc. Dermatological examination showed hypertrophic dark red plaques with clear margins and irregular shapes in his head-face-neck area and torso, some of which had merged together (Figure 1A and B). Auxiliary examination indicated eosinophils of 1.12×109/L ↑, automatically detected total IgE of 292.31 IU/mL ↑, IgG subclasses of 416900 mg/L ↑, complement C3 of 0.65g/L ↓, and complement C4 of 0.11 g/L ↓. No obvious abnormality was found in blood routine, biochemical parameters, coagulation functions, autoantibodies, lymphocyte subsets, tumor markers, and thyroid functions. The dermatopathological report revealed (neck) basket-woven hyperkeratosis, hypertrophy of the spinous layer, and mild pseudoepitheliomatous hyperplasia. Besides, obvious perivascular infiltration of clumps of lymphocytes, plasma cells, and eosinophils was also observed from the superficial to deep dermis, which involved the lipid membranes, together with mild mucin deposition in collagen spaces (Figure 2A and B). Immunohistochemical (IHC) examination was focally positive for CD138 and scatteredly positive for Ki-67, with IgG4/IgG exceeding 40% (Figure 2C–F). Given the above testing results, the patient was suspected of plasma cell proliferative disorder. Positron emission tomography-computed tomography (PET-CT) showed the extensively and uniformly thickened skin in the frontal area, face, and back, diffusely increased FDG uptake, soft tissue density nodules within subcutaneous fat spaces in the left posterior lumbar, and mild uptake of the imaging agent. Multiple lymph nodes and several enlarged ones were found near the bilateral neck (IIA), bilateral armpits, and iliac vessels of bilateral pelvic wall, with enhanced FDG metabolism (Figure 3A). Moreover, soft tissue density occupancy in the intermuscular spaces of the left retrobulbar muscle cone was observed together with improved FDG metabolism. In combination with his medical history, these manifestations were consistent with inflammatory pseudotumor changes within the eye frame (Figure 3B). His pancreas was full without elevated FDG uptake. Other than poor erythroid hyperplasia, bone marrow puncture did not demonstrate any obvious abnormalities. Based on the Diagnostic Criteria published by the Japan College of Rheumatology in 2011, the patient was diagnosed with IgG4-RD.
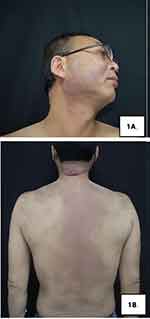 |
Figure 1 Clinical presentations at admission. (A and B) Hypertrophic dark red plaques were seen in the head-face-neck area and torso, which had partially merged together. |
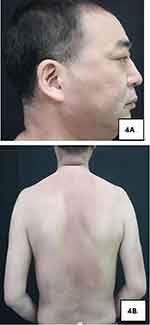
For treatment, the moisturizer was applied topically on the skin lesions and meanwhile, tacrolimus ointment was coated externally. Moreover, 12 mg of methylprednisolone tablets were taken orally every morning and evening, while 7.5 mg of methotrexate tablets were administered once a week. After two weeks of treatment, his pruritus was significantly relieved and skin lesions also resolved (Figure 4A and B). Subsequently, the dose of methylprednisolone tablets was reduced by 4 mg every two weeks, but the dose of methotrexate tablets remained unchanged. During the treatment, the patient experienced pain in his left fingertips, which was the manifestation of left-side ulnar arterial occlusion suggested by upper limb vascular ultrasound. Hence, oral aspirin enteric-coated tablets were adopted to alleviate the pain in the left fingertips. On reexaminations 3 months later, the levels of eosinophils and automatically detected total IgE returned to normal, and the level of IgG subclass 4 decreased to 1430 mg/L. Besides, upper limb vascular ultrasound indicated incomplete occlusion in the left-side ulnar artery and the potential of partial recanalization. Orbital magnetic resonance imaging (MRI) revealed no visible abnormalities in both eyeballs and eye frames.This study followed theCare Checklist (Supplementary Items File).
Discussion
Since IgG4-RD often mimics other diseases, comprehensive diagnosis criteria are required to improve the clinical recognition of the disease. The comprehensive diagnostic criteria for IgG4-RD formulated by the Japan College of Rheumatology in 2011 include the following items:5 (1) serum IgG4 level > 1350 mg/L; (2) on clinical examinations, characteristic diffuse/localized enlargement or mass formation is found in one or more organs; (3) histopathological changes show significant infiltration and fibrosis of plasma cells and lymphocytes, with IgG4+ plasma cells/IgG+ plasma cells >40% in tissues and IgG4+ plasma cells > 10 under high magnification. Patients who meet the above three criteria can be diagnosed with IgG4-RD. In this case, the patient presented with visibly elevated serum IgG4 levels and autoimmune inflammation involving four organs and tissues, including skin changes, ocular inflammatory pseudotumor, full pancreas, and ulnar arterial occlusion. A comprehensive diagnosis was made based on clinical histology, imaging, serology, appearance of involved organs, and his response to treatment. Although the disease is notorious for affecting multiple organs and sites, patients with simultaneous involvement of more than 3 organs are still rare. Furthermore, the commonly involved sites of this disease are glands and ductal tissues, while IgG4-related arterial lesions and skin changes are also rarely reported.8
The first case of IgG4-RD involving the skin was reported by Japanese scholars in 2009, after which similar cases were described successively. Currently, IgG4-related skin lesions according to the development mechanisms can be manifested as:9,10 (1) Mikulicz disease, cutaneous plasmacytosis, pseudolymphoma, and angiolymphoid hyperplasia accompanied by eosinophilia and Kimura disease, which are induced directly by the infiltration of IgG4+ plasma cells; (2) secondary skin lesions caused by IgG4-RD, such as psoriasis-like skin lesions, non-specific maculopapular rashes and erythematous lesions, urticaria vasculitis, and digital ischemia. The clinical and pathological manifestations of this case reported were consistent with those of cutaneous plasmacytosis, but the ratio of IgG4+ plasma cells in his lesional skin tissues met the diagnostic criteria of IgG4-RD. The skin lesions involved in this disease may be induced by plasma cell infiltration or IgG4-mediated inflammation,10 but the specific mechanism is still ambiguous yet.
In addition to the elevated serum IgG4 levels, the number of eosinophils and the level of automatically detected total IgE were also markedly higher than the normal ranges in this case. It is reported that11 30% of IgG4-RD patients exhibit eosinophilia, but the number of eosinophils can be reduced following drug treatment, which is identical to the conditions of the patient in our case. The findings suggested a correlation between the increase of eosinophils and the onset of IgG4-RD. A study on adaptive immunity and IgG4-RD12 indicated that activated IgG4+ B cells and plasmablasts could indirectly activate CD4+ T cells to induce the onset of IgG4-RD. Th2 cells could mediate hypersensitivity to generate IgE, suggesting the vital role of Th2 cells in the development of IgG4-RD. Furthermore, Akiyama et al13 discovered that as the number of Th2 cells increased, the serum levels of IL-4 and IgG4 as well as the number of involved organs also went up, implying a certain correlation between Th2 cells and the onset of IgG4-RD. Th2 cells can secrete IL-4, Il-5, and IL-13 to promote the activated B cells, thereby generating IgG4 and IgE, expediting the conversion of IgG to IgE, and facilitating the growth, activation, and differentiation of eosinophils.14
IgG4 boasts unique biological properties different from other three human IgG subtypes (IgG1, IgG2, and IgG3), including the exchange of Fab arms that limits their ability to generate immune complexes, the minimum ability to activate Fc receptors, and interactions with the Fc portions of other IgG subclasses.15,16 All these features facilitate the immune deactivation of IgG4. For this reason, IgG4 is considered to play an anti-inflammatory role in various disorders, but it may accelerate vascular structural injury when acting on the blood vessels.17 Despite that, the etiology of IgG4-related vasculitis is still unclear. Patients with IgG4-RD have a high tendency to develop IgG4-related large vessel vasculitis, which can involve the thoracic aorta, abdominal aorta, mesenteric artery, and splenic artery,18,19 as well as the major branches of the aorta and other blood vessels. In this context, digital ischemia is induced, followed by the occurrence of Raynaud’s phenomenon and digital gangrene.20
Glucocorticoid is the first-line treatment regimen for IgG4-RD put forward by Japanese scholars and has been widely adopted clinically. The dose is set at 30–40 mg/d initially, with a decrease of 5–10 mg/d every 1–2 weeks for 2–4 weeks to 2.5–5 mg/d, which should be maintained for less than 3 years.21 Glucocorticoid monotherapy can reach a remission rate of 97%, but it is also associated with a high relapse rate. In terms of refractory and recurrent cases, immunosuppressive agents can be administered additionally to improve efficacy and reduce hormone use,22 including methotrexate, azathioprine, mycophenolate mofetil, 6-mercaptopurine, and cyclophosphamide.23–25 For patients who are resistant to hormone therapy and intolerant to adverse events, rituximab is an important alternative treatment that can reduce the concentrations of IgG4 and plasmablasts in peripheral blood by targeting B cells.26 In the present case, the patient was systematically given oral glucocorticoid combined with the immunosuppressive agent methotrexate, and achieved satisfactory therapeutic effects.
Conclusion
Since the pathogenesis of IgG4-RD has not been thoroughly clarified yet, clinical physicians should enhance their understanding of this disease and be alert to the misdiagnosis and missed diagnosis of IgG4-RD. For patients with suspected IgG4-RD, serum IgG4 detection should be performed and further imaging and serological tests are needed to exclude malignant tumors, infections, and autoimmune diseases. In addition, pathological examinations shall be performed if necessary. All in all, IgG4-RD should be diagnosed and treated timely to avoid irreversible fibrotic changes in involved organs.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Ethics Approval and Informed Consent
This study was conducted in accordance with the amended Declaration of Helsinki. This study was approved by the ethics committee of People’s Hospital of Xinjiang Uygur Autonomous Region. Informed consent was provided for all the patients included in the study.
Consent for Publish
Written informed consent has been obtained from the patient for the case details and images to be published.
Acknowledgments
We would like to acknowledge the reviewers for their helpful comments on this paper.
Author Contributions
All authors made a significant contribution to the work reported, whether in the study conception, design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; agreed on the journal for article submission; and agreed to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
The author(s) declare(s) that there is no conflict of interest.
References
1. Stone JH, Chan JK, Deshpande V, et al. Igg4-related disease. Int J Rheumatol. 2015;2013(6):539–551.
2. Katz G, Stone JH. Clinical perspectives on IgG4-related disease and its classification. Annu Rev Med. 2022;73:545–562. doi:10.1146/annurev-med-050219-034449
3. Kamisawa T, Zen Y, Pillai S, Stone JH. IgG4-related disease. Lancet. 2015;385(9976):1460–1471. doi:10.1016/S0140-6736(14)60720-0
4. Wallace Z, Zhang Y, Perugino C, et al. Clinical phenotypes of igg4-related disease: an analysis of two international cross-sectional cohorts. Ann Rheum Dis. 2019;78(3):406–412. doi:10.1136/annrheumdis-2018-214603
5. Umehara H, Okazaki K, Masaki Y, et al. Comprehensive diagnostic criteria for igg4-related disease (igg4-rd), 2011. Mod Rheumatol. 2012;22(1):21–30. doi:10.3109/s10165-011-0571-z
6. Abraham M, Khosroshahi A. Diagnostic and treatment workup for igg4-related disease. Expert Rev Clin Immunol. 2017;13(9):867–875. doi:10.1080/1744666X.2017.1354698
7. Sato Y, Takeuchi M, Takata K, et al. Clinicopathologic analysis of igg4-related skin disease. Mod Pathol. 2013;26(4):523–532. doi:10.1038/modpathol.2012.196
8. Kiyama M, Kaneko Y, Takeuchi T. Characteristics and prognosis of igg4-related periaortitis/periarteritis: a systematic literature review. Autoimmun Rev. 2019;18(9):102354. doi:10.1016/j.autrev.2019.102354
9. Hamaguchi Y, Ohyama M. Tidying up the diversity of igg4‐related skin disease. Br J Dermatol. 2014;171(5):929. doi:10.1111/bjd.13394
10. Tokura Y, Yagi H, Yanaguchi H, Majima Y, Hashizume H. Igg4-related skin disease. Br J Dermatol. 2014;171(5):959–967. doi:10.1111/bjd.13296
11. Wallace ZS, Hamid M, Mahajan VS, et al. Predictors of disease relapse in igg4-related disease following rituximab. Rheumatology. 2016;55(6):1000–1008. doi:10.1093/rheumatology/kev438
12. Della-Torre E, Lanzillotta M, Doglioni C. Immunology of igg4‐related disease. Clin Exp Immunol. 2015;181(2):191–206. doi:10.1111/cei.12641
13. Akiyama M, Yasuoka H, Yamaoka K, et al. Enhanced IgG4 production by follicular helper 2 T cells and the involvement of follicular helper 1 T cells in the pathogenesis of IgG4-related disease. Arthritis Res Ther. 2016;18(1):167. doi:10.1186/s13075-016-1064-4
14. Lin W, Lu S, Chen H, et al. Clinical characteristics of immunoglobulin g4-related disease: a prospective study of 118 Chinese patients. Rheumatology. 2015;54(11):1982–1990. doi:10.1093/rheumatology/kev203
15. Mizushima I, Kasashima S, Fujinaga Y, Kawano M, Ishizaka N. IgG4-related periaortitis/periarteritis: an under-recognized condition that is potentially life-threatening. Mod Rheumatol. 2019;29(2):240–250. doi:10.1080/14397595.2018.1546367
16. Davies AM, Sutton BJ. Human IgG4: a structural perspective. Immunol Rev. 2015;268(1):139–159. doi:10.1111/imr.12349
17. Xu J, Bettendorf B, D’Oria M, Sharafuddin MJ. Multidisciplinary diagnosis and management of inflammatory aortic aneurysms. J Vasc Surg. 2022;78:SS0741–3. doi:10.1016/j.jvs.2022.12.024
18. Inoue D, Zen Y, Abo H, et al. Immunoglobulin g4-related periaortitis and periarteritis: CT findings in 17 patients. Radiology. 2011;261(2):625–633. doi:10.1148/radiol.11102250
19. Stone JR. Aortitis, periaortitis, and retroperitoneal fibrosis, as manifestations of igg4-related systemic disease.Current Opinion in Rheumatology. Current Opinion in Rheumatology. 2011;23(1):88–94. doi:10.1097/BOR.0b013e3283412f7c
20. Ikawa T, Kasuya A, Hirakawa S, Tokura Y. Raynaud phenomenon, digital gangrene and hypergammaglobulinaemic purpura occurring in a patient with igg4‐related disease. Br J Dermatol. 2011;165(6):1364–1366. doi:10.1111/j.1365-2133.2011.10609.x
21. Masamune A, Nishimori I, Kikuta K, et al. Randomised controlled trial of long-term maintenance corticosteroid therapy in patients with autoimmune pancreatitis. Gut. 2017;66(3):487–494. doi:10.1136/gutjnl-2016-312049
22. Omar D, Chen Y, Cong Y, Dong L. Glucocorticoids and steroid sparing medications monotherapies or in combination for IgG4-RD: a systematic review and network meta-analysis. Rheumatology. 2020;59(4):718–726. doi:10.1093/rheumatology/kez380
23. Yunyun F, Yu P, Panpan Z, et al. Efficacy and safety of low dose mycophenolate mofetil treatment for immunoglobulin G4‐related disease: a randomized clinical trial. Rheumatology(Oxford). 2019;58(1):
24. Wang L, Zhang P, Wang M, et al. Failure of remission induction by glucocorticoids alone or in combination with immunosuppressive agents in IgG4‐related disease: a prospective study of 215 patients. Arthritis Res Ther. 2018;20(1):65. doi:10.1186/s13075-018-1567-2
25. Zhang P, Gong Y, Liu Z, et al. Efficacy and safety of iguratimod plus corticosteroid as bridge therapy in treating mild IgG4‐related diseases: a prospective clinical trial. Int J Rheum Dis. 2019;22(8):
26. Carruthers MN, Topazian MD, Khosroshahi A, et al. Rituximab for IgG4‐related disease: a prospective, open‐label trial. Ann Rheum Dis. 2015;74(6):
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.