Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 17
Identification of Shared Biomarkers and Immune Infiltration Signatures between Vitiligo and Hashimoto’s Thyroiditis
Authors Lu J , Song L, Luan J, Feng Y, Wang Y, Cao X, Lu Y
Received 21 November 2023
Accepted for publication 23 January 2024
Published 2 February 2024 Volume 2024:17 Pages 311—327
DOI https://doi.org/10.2147/CCID.S451080
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Jiawei Lu,1 Lebin Song,1 Jiaochen Luan,2 Yifei Feng,1 Yidan Wang,1 Xuechen Cao,1 Yan Lu1
1Department of Dermatology, The First Affiliated Hospital, Nanjing Medical University, Nanjing, People’s Republic of China; 2Department of Urology, The First Affiliated Hospital, Nanjing Medical University, Nanjing, People’s Republic of China
Correspondence: Yan Lu, Department of Dermatology, The First Affiliated Hospital, Nanjing Medical University, 300 Guangzhou Road, Nanjing, Jiangsu, 210029, People’s Republic of China, Tel +86 13913967126, Email [email protected]
Background: Vitiligo and Hashimoto’s thyroiditis (HT) are concomitant autoimmune diseases characterized by the destruction of melanocytes or thyrocytes. We aimed to explore the immunological mechanism of this comorbidity and screen their potential biomarkers.
Methods: We downloaded the microarray datasets from the GEO database. Differentially expressed genes (DEGs) and immune-related genes (IRGs) were selected. The immune-related differentially expressed genes (IRDEGs) were obtained by taking the intersection. Candidate biomarkers were elected by Cytoscape software. CIBERSORT was used to depict immune cell infiltration prospects. Correlation analysis was conducted between infiltrating cells and several indicators. The results were validated by real-time quantitative PCR (RT-qPCR).
Results: Three datasets and 60 IRDEGs were obtained in total. Pathway enrichment analysis showed that the T cell receptor signaling pathway, IL-17 signaling pathway, receptor-ligand activity, and signaling receptor activator activity were significantly enriched. We screened out four hub genes, including IFNG, STAT1, IL1B, and CXCL10. The ROC curve indicated the highest diagnostic value of CXCL10 in both vitiligo and HT. Immuno-infiltration analysis revealed significant changes in T cell subsets and macrophage subtypes, which were correlated with four hub genes, melanocyte markers, and thyroid-specific antigens. qPCR validated the hub genes in peripheral blood mononuclear cells from patients with comorbidity.
Conclusion: IFNG, STAT1, IL1B, and CXCL10, were the key IRDEGs to vitiligo and HT. These genes may participate in the comorbidity by remodeling the immune cell infiltration pattern, and cross-expressed antigens may mediate the common damage of melanocytes and thyroid tissues.
Keywords: depigmenting skin disorder, autoimmune thyroiditis, immune cell infiltration, bioinformatics analysis
Introduction
Vitiligo is a chronic pigmentary disorder characterized by the development of white macules in the skin or hair. The prevalence of vitiligo is referred to as 0.5–2% around the world and is more common in dark-skinned individuals.1 Non-segmental vitiligo, the most common clinical subtype, often bilaterally distributes in limbs or scattered over the body. The lesions evolve or progress over time, which is psychologically stigmatizing and brings a lower quality of life.
The loss of melanocytes originates from genetic susceptibility, immunologic abnormalities, oxidative stress, and neuropeptides. Multiple susceptibility loci encoding immunoregulatory proteins have been identified for generalized vitiligo.2 The endoplasmic reticulum stress-induced unfolded protein response is considered the bridge between oxidative stress and autoimmunity in vitiligo. DAMPs, PAMPs, and innate immune cells further promote the adaptive T-cell response. The infiltrated CD8+ T cells are the culprit to the destruction of melanocytes in vitiligo,3 which release type 1 cytokines, especially IFN-γ and TNF-α. The recruitment of CD8+ T cells towards the skin is mediated by the combination of CXCR3 and CXCL9/10 which are the chemokines released by keratinocytes dependent on the IFN-γ-activated JAK/STAT pathway. IFN-γ-CXCL9/10-CXCR3 makes a positive feedback loop in vitiligo. Th17/ regulatory T cells (Treg) are in a state of imbalance. FoxP3+ Treg cell and its chemokine, CCL22, are down-regulated in the lesions.
Vitiligo is often accompanied by several autoimmune diseases, primarily thyroid diseases. A higher prevalence (12.9–24.1%) of thyroid diseases has been found in vitiligo patients.4–6As one of the most prevalent comorbid diseases of vitiligo, HT is recognized as a risk factor for developing papillary thyroid cancer and thyroid lymphoma.4 HT often occurs in immune-defected individuals with genetic susceptibility or triggers including environmental factors, oxidative stress, trace elements, and infections. Histologically, we can observe diffuse lymphocytic infiltration in lymphoid follicles and germinal centers, which leads to a transient hyperthyroid phase to hypothyroidism of the thyroid gland. HT is characterized by humoral immunity-mediated destruction of the thyroid gland, together with abnormality in innate and cellular immunity. Exogenous and endogenous dangers (PAMPs, DAMPs) may trigger the innate immune response in thyroid cells in the absence of immune cells.7 As the receptors of PAMPs and DAMPs, Toll-like receptors (TLRs)2/3/9/10 are upregulated in the immune cells in HT.8 The imbalance of Th17/Treg adds to the development and progression of HT.9
Patients with comorbidity have poorer therapeutic responses to the traditional treatment and recurrent clinical courses. A retrospective study reported a higher percentage of the involved body surface area in vitiligo patients with thyroid pathology.10 Also, patients with vitiligo and autoimmune thyroiditis have a predisposition to developing acral lesions and discolored patches of wrists.11 In the subgroup analysis, vitiligo had a stronger association with HT in males than females, and they had the strongest association in children and adolescents (aged under 20 years).12 Genetic factors13 and autoimmune etiology are primarily involved in the synchronicity of the two diseases, but the exact immunological mechanism of the comorbidity remains unclear.
The rapid development of microarray and high-throughput sequencing technology has brought a valid means of identifying hub genes and potential molecular biomarkers for multiple diseases, such as neoplastic diseases and autoimmune diseases. To our knowledge, this is the first bioinformatics-based study on the immunological mechanism of this comorbidity.
Methods
Access to GEO Datasets
Genes expression profiling of vitiligo and HT were screened using the GEO database (http://www.ncbi.nlm.nih.gov/geo). The following criteria were used to filter the datasets: (1) the gene expression profiling obtained from tissue specimens; (2) expression profiling by array or high-throughput sequencing of mRNA; (3) available raw or processed data for further analysis. Three eligible datasets were enrolled, including GSE75819, GSE138198, and GSE54958. GSE75819 series on the GPL6884 platform (Illumina HumanWG-6 v3.0 expression bead chip) was the dataset of vitiligo. GSE138198 and GSE54958 on the GPL6244 platform (Affymetrix Human Gene 1.0 ST Array) were merged as the dataset of HT.
Data Processing
Raw and series matrix files of the three microarray datasets were downloaded from the GEO database. For all raw data, the probe expression matrix was extracted and normalized with the “normalizebetweenarrays” function of the “limma” package. R package “sva” was utilized to eliminate heterogeneity of the merged dataset (GSE138198 and GSE54958) caused by different experimental batches. Then probe expression matrices were converted into gene expression matrices according to the annotation documents of corresponding platforms.
Identification of Differentially Expressed Genes (DEG) and IRGs
The Immunology Database and Analysis Portal database (ImmPort) was applied to single out the immune-related genes set. The differential analysis was performed by comparing normal tissues with pathological tissues using “limma” package in R computing environment, separately in the vitiligo and HT IRGs sets with conditioned adjusted P-value <0.05. We obtain the qualified immune-related differentially expressed genes (IRDEGs) by taking the intersection of IRDEGs in two datasets.
Function and Pathway Enrichment Analysis
GO function and KEGG pathway enrichment analyses of the co-expressed IRDEGs were performed through R package “ClusterProfiler”. Adjusted P-value <0.05 was considered significant.
PPI Network Construction and Module Analysis
Based on the STRING online tool, a biological database designed to construct a protein–protein interaction (PPI) network of targeted genes and analyze the functional interactions between proteins, the PPI network of the IRDEGs was built with a confidence score >0.4. The visualization of the network was processed by Cytoscape software (version 3.8.2). Molecular Complex Detection (MCODE) and cytoHubba are plug-in procedures of Cytoscape software. MCODE was used to explore the significant modules. We selected the hub genes by taking the intersection of the top ten genes ranked by the nine algorithms (MCC, MNC, degree, EPC, Bottleneck, closeness, radiality, betweenness, and stress) in cytoHubba.
Construction of ceRNA Network
We predicted miRNAs of the target gene and then filtered the overlapped mRNA-targeted miRNAs based on four online tools. Similarly, we acquired the target lncRNA of the core miRNA by using StarBase 3.0 (http://starbase.sysu.edu.cn/). The results were displayed after being processed by Cytoscape software.
Immune Infiltration and Correlation Analysis
CIBERSORT is a method for characterizing the immune cell composition of tissues based on their gene expression profiles. The immune cell compositions were predicted via CIBERSORT using the LM22 signature gene file with 1000 permutations, respectively in vitiligo and HT datasets. We explored the correlation of the identified hub genes with the composition of infiltrating immune cells and specific antigens using Spearman’s rank correlation analysis in R software. The results were visualized using the “ggplot2” package in R software.
Participants
In the present study, we enrolled six vitiligo patients with concomitant HT identified in the dermatology department of the first affiliated hospital of Nanjing Medical University. We also recruited six age- and sex-matched healthy controls (HCs) for comparison. Ethical approval for this study was obtained from the local Ethics Committee. Written informed consent was signed by all subjects before the study. All experiments were performed according to the Declaration of Helsinki and guidelines for Good Clinical Practice.
Peripheral Blood Mononuclear Cells (PBMC) Isolation
Peripheral venous blood was extracted into sodium-heparin tubes, and PBMC were extracted through a concentration gradient technique (Ficoll-PaqueTM Plus, GE Healthcare Life Sciences, Sweden). The cells were washed twice with PBS and centrifuged at 400g for 30 minutes at RT to isolate PBMC.
RNA Isolation and Real-Time Quantitative PCR
Total RNA was isolated from PBMC using Trizol reagent (Invitrogen, Carlsbad, CA, U.S.A.). RNA purity was detected using Quantus Fluorometer. Firstly, total RNA was used to synthesize cDNA with reverse transcription reaction. Secondly, RT-qPCR was conducted on the QuantStudio 7(Thermo Fisher) with SYBR-Green dye (Vazyme). The primers used are shown in Table 1. Each sample was tested in triplicates and underwent a melting curve analysis. Expression levels of target genes were calculated by the 2−ΔΔCt method adjusted to the internal control GAPDH.
 |
Table 1 Primers |
Statistical Analysis
Statistical analyses were carried out based on R software. Comparisons between the disease and control group that presented normal distribution were performed with the Student’s T-test. P-value <0.05 was considered significant. ROC analysis was conducted to determine the discrimination value of the genes.
Results
Identification of Shared IRDEGs
The flowchart of this research is shown in Figure 1. Three qualified datasets (GSE138198, GSE54958, and GSE75819) were downloaded. After normalization and the removal of the batch effect (Figure 2A–D), immune-related genes were extracted from the vitiligo and HT datasets for differential analysis (Figure 3A and B). We obtained 263 IRDEGs in the vitiligo dataset and 592 IRDEGs in the merged HT dataset. Sixty IRDEGs with consistent trends were acquired by taking the intersection in two datasets (Figure 3C and D). Heatmaps showed evident discrepancies in expression levels of the 60 genes between the diseases group and the healthy control group (Figure 3E and F).
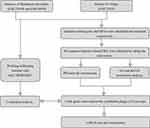 |
Figure 1 The flow chart of the research. |
Analysis of the Functional Characteristics of IRDEGs
We further processed IRDEGs for functional enrichment. Enriched KEGG pathways were primarily the T cell receptor signaling pathway, IL-17 signaling pathway, Toll-like receptor signaling pathway, proteasome, and natural killer cell-mediated cytotoxicity (Figure 4A and C). The enriched GO terms were closely related to the metabolism of cytokines (Figure 4B and D). The most prominent clusters are receptor ligand activity, signaling receptor activator activity, positive regulation of cytokine production, cytokine-mediated signaling pathway, cytokine receptor binding, etc.
Construction of PPI Network, Analysis of Modules and Hub Genes
Sixty IRDEGs were predicted by the online database STRING, and visualized on Cytoscape software (Figure 5A). Significant modules of the network were identified by plug-in MCODE, and the top three clusters were ranked based on betweenness algorithm (Figure 5B–5D). We calculated the score of the genes with nine algorithms and selected the top 10 hub genes, respectively (Figure 5E). By taking the intersection of the nine results, we picked out four hub IRDEGs, including STAT1, CXCL10, IL-1B, and IFNG. The four genes were significantly upregulated in vitiligo and HT patients compared with that in normal tissues (Figure 5F and G).
Validation of the Sensitivity and Specificity
ROC analysis of each hub gene was conducted and the gene with AUC >0.8 was considered as the potential diagnostic gene with good specificity and sensitivity. The AUC values of IFNG, IL1B, STAT1, and CXCL10 were 0.8578, 0.7956, 0.8889, and 1 in the vitiligo dataset while 0.8077, 0.8846, 0.7846, 0.8692, and 1 in the HT dataset (Figure 6A and B). Therefore, IFNG, STAT1, and CXCL10 had good sensitivity and specificity in distinguishing vitiligo or HT patients from healthy controls. Among them, CXCL10 had the highest score, and we chose it for further analysis.
Construction of ceRNA Network
miRNAs of CXCL10 were predicted in the TargetScan, miRDB, starBase, and miRWalk databases. We took the intersection and obtained one miRNA, hsa-miR-219a-2-3p (Figure 6C). Sixty-eight lncRNA of miR-219a-2-3p were acquired using the starBase database. We further constructed the ceRNA regulatory network based on the CXCL10-miRNA-lncRNA axis using Cytoscape (Figure 6D).
Evaluation of Immune Cell Infiltration
The compositions of 22 kinds of immune cells in vitiligo and HT datasets were identified by CIBERSORT and visualized on R software (Figure 7A). The infiltration bar plot of thyroid gland tissue showed upregulation of memory B cells, activated CD4+ memory T cells, follicular helper T cells, M1 macrophages, and M2 macrophages in HT patients compared with HC (Figure 7B). In the lesion of vitiligo patients, resting CD4+ memory T cells and M1 macrophages were upregulated, while Tregs, M0 macrophages, and activated dendritic cells were downregulated compared with normal skin (Figure 7C).
Correlation Analysis
The correlations among the hub IREDGs, infiltrated immune cells, and specific antigens were calculated by Spearman correlation rank. In HT patients, STAT1 was found positively correlated with activated CD4+ memory T cells. Activated NK cells and resting mast cells were negatively correlated with IFNG, IL1B, STAT1, and CXCL10 (Figure 8A). In vitiligo patients, STAT1 was positively associated with regulatory T cells. IL1B was found negatively correlated with CD8+ T cells and activated NK cells (Figure 8B).
 |
Figure 8 Correlation analysis between the hub immune-related differentially expressed genes and immune cells. (A) in Hashimoto’s thyroiditis dataset. (B) in vitiligo dataset. |
Melanocyte-specific antigens include TYR, TYPR1, TYPR2, LAMP1, S100A1, S100B, MITF, etc. Thyroid-specific epitopes include TSHR, TPO, and TG. The analysis showed that CD4+ memory T cells were correlated to the expression of TSHR and TPO in vitiligo (Figure 9A). In HT, the subtypes of macrophages were correlated with S100A1, S100B, TYRP1 and TYR, and the expression of TYR was correlated with plasma cells, CD4+ T cells, Th cells and macrophages (Figure 9B).
 |
Figure 9 Correlation analysis between antigens and immune cells. (A) in Hashimoto’s thyroiditis dataset. (B) in vitiligo dataset. *p < 0.05; **p < 0.01; ***p < 0.001. |
Validation of Expression by RT-qPCR
The transcriptional changes of the four hub IRDEGs were verified in the PBMC from the patients with comorbidity of vitiligo and HT by RT-qPCR. The expression levels of IFNG, IL1B, STAT1, and CXCL10 were significantly upregulated in the comorbidity group compared with the healthy group (Figure 10).
 |
Figure 10 Expression level of the hub genes. The relative expression level of the four hub genes adjusted to GAPDH. *p < 0.05; **p < 0.01; ***p < 0.001. |
Discussion
As the commonest cause of acquired disfiguring skin or hair depigmentation, vitiligo has a detrimental influence on a patient’s quality of life and mental health.14 A recent study showed the vulnerability of vitiligo patients to depression, anxiety, skin irritation, and cancer.15–17 The correlation between vitiligo and autoimmune diseases has been well-established,6 such as thyroid diseases, alopecia areata, inflammatory bowel disease, type 1 diabetes mellitus, etc. HT, a familiar cause of hypothyroidism in adults, is also the most common comorbid disease of vitiligo. The comorbidity may bring about a poorer response to the treatment and a higher recurrence rate.10
Earlier studies have found multiple vitiligo susceptibility genes encoded proteins involved in immune functions and these genes were shared with autoimmune thyroid diseases.18 Recent research implied different patterns of melanocyte-specific antigen expression in the thyroid tissue between HT and healthy people,19 which provided clues to the shared immunological basis for vitiligo and HT. The purpose of our research was to identify the key immune-related genes involved in both vitiligo and HT and investigate the correlation among the hub genes, infiltrating immune cells, and the specific antigens of melanocytes and thyrocytes. Thereby, we might find the overlapped immune process and potential molecular targets to predict and treat this comorbidity.
We extracted the immune-related genes and conducted the differential analysis of vitiligo and HT datasets. Then, we obtained 60 IRDEGs by taking the intersection. GO analysis showed the enrichment of cytokine-associated and receptor ligand-mediated pathways. The enriched KEGG pathways were closely related to T cell-mediated immunity and innate immunity, such as the T cell receptor signaling pathway, IL-17 pathway, Toll-like signaling pathway, and NK cell-mediated cytotoxicity. The results indicated the simultaneous involvement of innate and adaptive immunity.
We acquired four hub genes among the IRDEGs (IFNG, IL1B, STAT1, and CXCL10) as potential biomarkers of the comorbidity, all of which were explored significance to different extents in vitiligo and HT.
Vitiligo patients reflected an IFN-γ-specific signature accompanied by the upregulation of its downstream chemokines CXCL10 and CXCL9 in skin and blood.20 IFN-γ, a cellular immunity driver and promotor of autoreactive cytotoxic CD8+ T cells, is destructive to melanocytes in vitiligo. It was demonstrated that both natural killer cells and innate lymphoid cells can produce IFN-γ in the presence of reactive oxygen species (ROS), a shared pathogenic factor of HT and vitiligo.21 In the IFN-γ transgenic mice model, the constitutive expression of IFN-γ in the thyroid follicular cells induced disruption of thyroid architecture and function.22 It was found in NOD.H-2h4 mice that IFN-γ mediated the development of lymphocytic autoimmune thyroiditis and inhibited the hyperplasia and proliferation of thyrocytes.23
CXCR3, the common receptor of CXCL9 and CXCL10, is expressed on pathogenic T cells which are recruited by the CXCL9/10 to the target tissue and subsequently produce IFN-γ, making a positive feedback loop. Expression of CXCR3 was strongly upregulated in the melanocytes of vitiligo patients and the lymphocytes from neoplastic nodules of HT patients.24 The activation of CXCR3 isoform B was found to be an inducer of cultured melanocyte apoptosis,21 a vital form of melanocyte loss.
CXCL10 and CXCL9, both combined with CXCR3, have different weights in inflammatory diseases.25,26 CXCL10 is dominant over CXCL9 in vitiligo and HT.27,28 CXCL9 recruits the immune cells while CXCL10 mediates the migration and localization of melanocyte-specific CD8+ T cells to the epidermis.20 CXCL10 is correlated with disease severity, activity, treatment response, and outcome in vitiligo.29 In the mouse model, the neutralization of CXCL10 was able to reverse the depigmentation partially. The upregulation of the level of CXCL10 was also found in the serum and tissue of HT patients30 with a higher titer in those with a more aggressive thyroid autoimmune process such as hypothyroidism or a hypoechoic ultrasonographic pattern.31 CXCL10 is absent in normal human thyrocytes but could be triggered by synergistic stimulation of IFN-γ and TNF-α in a dose-dependent pattern.32
The Janus kinase/signal transducer and activator of transcription (JAK/STAT) signaling pathway is composed of a rapid membrane-to-nucleus signaling module and a subsequent transduction cascade regulating multiple-immunology processes. IFN-γ is the primary cytokine mediator that activates the JAK/STAT1 pathway. In vitiligo, IFN-γ binds to the keratinocyte-surface receptors and recruits JAKs to transduce the signal. The phosphorylated STAT1 translocates to the nucleus and induces the expression of IFN-γ stimulated chemokines, such as CXCL9 and CXCL10,33 which recruit cytotoxic T cells to the epidermal, where melanocytes are destructed. Similarly, activated STAT1 dimers were detected in germinal macrophages and infiltrating lymphocytes in HT.34
IL-1β is one of the most prominent messengers of inflammatory signaling and was found upregulated in active vitiligo patients35,36 and HT patients.37 It drives the differentiation and activation of Th1 and Th17 cells. IL-17 is the downstream cytokine of Th17 cells. IL-17 signaling pathway was enriched in the KEGG pathway enrichment.38 IL-1β, with the help of IL-23, promotes the production of innate IL-17 from γδ T cells, which further contributes to the generation of Th17 cells.39 The pathogenic Th17 cells contribute to the secretion of IFN-γ and then add to the IFN-γ-mediated inflammatory disease.40 As the inducer of FAS expression on thyroid follicular cells, IL-1β mediates the apoptosis of thyroid follicular cells through FAS/FASL signaling. Grave’s disease is another autoimmune thyroid disease and is distinguished from HT histologically with lymphocyte infiltration but no destruction of thyrocytes. The level of IL-1β is lower in the thyroid tissue of Grave’s disease than that of HT, which implies a critical role of IL-1β in thyrocyte destruction.41
The ROC curve indicated the predominant diagnostic value of CXCL10 for both vitiligo and HT. We predicted the most essential miRNA, hsa-miR-219a-2-3p, for CXCL10, and forecasted the CXCL10-miRNA-lncRNA regulatory axis using the starBase database. MiRNA-219a-2-3p has been reported in regulating proliferation and apoptosis in different cells and diseases. The overexpression of miR-219a-2-3p can induce thyroid cancer cell cycle arrest at G0/G1 phase by targeting its downstream gene HPSE.42 miR-219a-2-3p also regulates the apoptosis of pituitary adenoma cells43 and gastric cancer cells.44 However, the regulatory role of miR-219a-2-3p in thyrocytes or melanocytes is unknown.
We used CIBERSORT to evaluate the composition of 22 kinds of immune cells of vitiligo and HT. Alteration of T cell subsets and macrophage phenotypes were prominent in both HT and vitiligo patients compared with healthy controls. Studies have found the main role of CD4+ T cells in HT. In addition to Th1 cells, recent research also revealed the enhancement of Th17 cells45 and unbalanced Th17/Treg (CD4+CD25+highFoxP3+) cells in HT patients.46 CD8+ T cells are recognized as the main infiltrated immune cells localized to the basal layer skin of vitiligo. In addition, infiltration of CD4+ T cells and the deficiency of Treg cells were also detected.47,48 This implies overlapping immune cell infiltration in this comorbidity. The polarization of M0 to M1 macrophages is induced by Th1 cytokines such as IFN-γ and TNF-α. The predominance of STAT1 activation promotes M1 macrophage polarization, too. M1 macrophages are engaged in ROS-induced organelle or tissue damage and result in cytotoxic and proinflammatory functions by secreting cytokines including IL-1β, IL-6, IL-23, CXCL9, CXCL10, etc.49
Correlation analysis indicated that hub IRDEGs were significantly associated with the shifts in immune cell populations. Melanocyte-specific antigens are expressed differently in thyroid tissues of HT patients and healthy individuals. Thyroid tissues of HT patients without vitiligo express TRP-2, LAMP1 and CD69, but do not express NKI-β, Pmel17, TRP1, HMB45 and S100.50 A variety of skin cells, such as melanocytes, keratinocytes, and fibroblasts, can express thyroid-specific epitopes (TSHR, TG, and TPO).51,52 With correlation analysis, we found the types of immune infiltrating cells, especially the subsets of T cells and macrophages, were significantly correlated with the expression of the antigens. The activated immune cells in vitiligo patients might cause secondary thyroid damage by attacking the melanocyte-specific antigens expressed in the thyroid tissue.
Patients of vitiligo and HT showed a higher expression level of the four hub genes quantified by RT-qPCR. The consistent results may provide a basis for the novel potential biomarkers or targets for the diagnosis and treatment of this comorbidity.
Limitation
Despite the meaningful results of this study, there were certain limitations. In addition to the relatively small sample size of this study, we have only verified the genes at the transcriptome level. Animal and cell experiments are needed to identify the predicted mRNA-miRNA-lncRNA axis, function of the altered immune cells, and enriched signaling pathways in this study.
Conclusions
In summary, we selected four hub genes, IFNG, STAT1, IL1B and CXCL10, which might be profoundly involved in the pathogenesis of both vitiligo and HT (Figure 11). The IRDEGs could participate in the comorbidity by reshaping innate and adaptive immunology. The simultaneous damage to the skin and thyroid might be attributed to the alteration of the T cell subtypes, macrophage polarization, and the cross-expressed antigens. Large-sample studies and experimental research are needed to further explore the exact role of these key molecules and their possible clinical values.
Ethics Approval and Consent to Participate
The experiment of the study was approved by the Medical Ethics Committee of the first affiliated hospital of Nanjing Medical University (Ethics approval number: 2022-SR-583). Written informed consent was obtained from all participants.
Funding
This work was supported by the National Natural Science Foundation of China (No. 82273549).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Ezzedine K, Lim HW, Suzuki T, et al. Revised classification/nomenclature of vitiligo and related issues: the Vitiligo Global Issues Consensus Conference. Pigm Cell Mel Res. 2012;25(3):E1–E13. doi:10.1111/j.1755-148X.2012.00997.x
2. Jin Y, Birlea SA, Fain PR, et al. Genome-wide association analyses identify 13 new susceptibility loci for generalized vitiligo. Nat Genet. 2012;44(6):676–680. doi:10.1038/ng.2272
3. Steitz J, Wenzel J, Gaffal E, Tuting T. Initiation and regulation of CD8+T cells recognizing melanocytic antigens in the epidermis: implications for the pathophysiology of vitiligo. Eur J Cell Biol. 2004;83(11–12):797–803. doi:10.1078/0171-9335-00423
4. Kakourou T, Kanaka-Gantenbein C, Papadopoulou A, Kaloumenou E, Chrousos GP. Increased prevalence of chronic autoimmune (Hashimoto’s) thyroiditis in children and adolescents with vitiligo. J Am Acad Dermatol. 2005;53(2):220–223. doi:10.1016/j.jaad.2005.03.032
5. Gey A, Diallo A, Seneschal J, et al. Autoimmune thyroid disease in vitiligo: multivariate analysis indicates intricate pathomechanisms. Br J Dermatol. 2013;168(4):756–761. doi:10.1111/bjd.12166
6. Gill L, Zarbo A, Isedeh P, Jacobsen G, Lim HW, Hamzavi I. Comorbid autoimmune diseases in patients with vitiligo: a cross-sectional study. J Am Acad Dermatol. 2016;74(2):295–302. doi:10.1016/j.jaad.2015.08.063
7. Kawashima A, Yamazaki K, Hara T, et al. Demonstration of innate immune responses in the thyroid gland: potential to sense danger and a possible trigger for autoimmune reactions. Thyroid. 2013;23(4):477–487. doi:10.1089/thy.2011.0480
8. Klatka M, Polak A, Mertowska P, et al. The Role of Toll-like Receptor 2 (TLR2) in the development and progression of Hashimoto’s Disease (HD): a case study on female patients in Poland. Int J Mol Sci. 2023;24(6):5344. doi:10.3390/ijms24065344
9. Nagayama Y, Horie I, Saitoh O, Nakahara M, Abiru N. CD4+CD25+ naturally occurring regulatory T cells and not lymphopenia play a role in the pathogenesis of iodide-induced autoimmune thyroiditis in NOD-H2h4 mice. J Autoimmun. 2007;29(2–3):195–202. doi:10.1016/j.jaut.2007.07.008
10. van Geel N, Speeckaert M, Brochez L, Lambert J, Speeckaert R. Clinical profile of generalized vitiligo patients with associated autoimmune/autoinflammatory diseases. J Eur Acad Dermatol Venereol. 2014;28(6):741–746. doi:10.1111/jdv.12169
11. Chivu AM, Balasescu E, Pandia LD, et al. Vitiligo-thyroid disease association: when, in whom, and why should it be suspected? A systematic review. J Pers Med. 2022;12(12):2048. doi:10.3390/jpm12122048
12. Bae JM, Lee JH, Yun JS, Han B, Han TY. Vitiligo and overt thyroid diseases: a nationwide population-based study in Korea. J Am Acad Dermatol. 2017;76(5):871–878. doi:10.1016/j.jaad.2016.12.034
13. Narita T, Oiso N, Fukai K, Kabashima K, Kawada A, Suzuki T. Generalized vitiligo and associated autoimmune diseases in Japanese patients and their families. Allergol Int. 2011;60(4):505–508. doi:10.2332/allergolint.11-OA-0303
14. Pandve HT. Vitiligo: is it just a dermatological disorder? Indian J Dermatol. 2008;53(1):40–41. doi:10.4103/0019-5154.39745
15. Patel S, Rauf A, Khan H, Meher BR, Hassan SSU. A holistic review on the autoimmune disease vitiligo with emphasis on the causal factors. Biomed Pharmacother. 2017;92:501–508. doi:10.1016/j.biopha.2017.05.095
16. Ahluwalia J, Correa-Selm LM, Rao BK. Vitiligo: not simply a skin disease. Skinmed. 2017;15(2):125–127.
17. Kussainova A, Kassym L, Akhmetova A, et al. Associations between serum levels of brain-derived neurotrophic factor, corticotropin releasing hormone and mental distress in vitiligo patients. Sci Rep. 2022;12(1):7260. doi:10.1038/s41598-022-11028-8
18. Spritz RA. Shared genetic relationships underlying generalized vitiligo and autoimmune thyroid disease. Thyroid. 2010;20(7):745–754. doi:10.1089/thy.2010.1643
19. Spritz RA, Gowan K, Bennett DC, Fain PR. Novel vitiligo susceptibility loci on chromosomes 7 (AIS2) and 8 (AIS3), confirmation of SLEV1 on chromosome 17, and their roles in an autoimmune diathesis. Am J Hum Genet. 2004;74(1):188–191. doi:10.1086/381134
20. Rashighi M, Agarwal P, Richmond JM, et al. CXCL10 is critical for the progression and maintenance of depigmentation in a mouse model of vitiligo. Sci, trans med. 2014;6(223):223ra223. doi:10.1126/scitranslmed.3007811
21. Tulic MK, Cavazza E, Cheli Y, et al. Innate lymphocyte-induced CXCR3B-mediated melanocyte apoptosis is a potential initiator of T-cell autoreactivity in vitiligo. Nat Commun. 2019;10(1):2178. doi:10.1038/s41467-019-09963-8
22. Caturegli P, Hejazi M, Suzuki K, et al. Hypothyroidism in transgenic mice expressing IFN-gamma in the thyroid. Proc Natl Acad Sci USA. 2000;97(4):1719–1724. doi:10.1073/pnas.020522597
23. Yu S, Sharp GC, Braley-Mullen H. Thyrocytes responding to IFN-gamma are essential for development of lymphocytic spontaneous autoimmune thyroiditis and inhibition of thyrocyte hyperplasia. J Iimmunol. 2006;176(2):1259–1265. doi:10.4049/jimmunol.176.2.1259
24. Jiskra J, Antosova M, Kratky J, et al. CXCR3, CCR5, and CRTH2 chemokine receptor expression in lymphocytes infiltrating thyroid nodules with coincident hashimoto’s thyroiditis obtained by fine needle aspiration biopsy. J Immunol Res. 2016;2016:2743614. doi:10.1155/2016/2743614
25. Menke J, Zeller GC, Kikawada E, et al. CXCL9, but not CXCL10, promotes CXCR3-dependent immune-mediated kidney disease. J Am Soc Nephrol. 2008;19(6):1177–1189. doi:10.1681/ASN.2007111179
26. Hyun JG, Lee G, Brown JB, et al. Anti-interferon-inducible chemokine, CXCL10, reduces colitis by impairing T helper-1 induction and recruitment in mice. Inflamm Bowel Dis. 2005;11(9):799–805. doi:10.1097/01.MIB.0000178263.34099.89
27. Hojman L, Cabrera R, Karsulovic C, Tempio F, Perez C, Lopez M. The role of CXCL10 and IL-18 as markers of repigmentation response in nonsegmental vitiligo treated with narrowband UVB phototherapy: a prospective cohort study. J Invest Dermatol. 2021;141(7):1833–1836 e1831. doi:10.1016/j.jid.2020.12.021
28. Fallahi P, Ferrari SM, Elia G, et al. Novel therapies for thyroid autoimmune diseases. Expert Rev Clin Pharmacol. 2016;9(6):853–861. doi:10.1586/17512433.2016.1157468
29. El-Domyati M, El-Din WH, Rezk AF, et al. Systemic CXCL10 is a predictive biomarker of vitiligo lesional skin infiltration, PUVA, NB-UVB and corticosteroid treatment response and outcome. Arch Dermatol Res. 2022;314(3):275–284. doi:10.1007/s00403-021-02228-9
30. Stoica RA, Dragana N, Ancuceanu R, et al. Interleukin-8, CXCL10, CXCL11 and their role in insulin resistance in adult females with subclinical hypothyroidism and prediabetes. J Clin Transl Endocrinol. 2022;28:100299. doi:10.1016/j.jcte.2022.100299
31. Antonelli A, Ferrari SM, Corrado A, Di Domenicantonio A, Fallahi P. Autoimmune thyroid disorders. Autoimmunity Rev. 2015;14(2):174–180. doi:10.1016/j.autrev.2014.10.016
32. Antonelli A, Rotondi M, Ferrari SM, et al. Interferon-gamma-inducible alpha-chemokine CXCL10 involvement in Graves’ ophthalmopathy: modulation by peroxisome proliferator-activated receptor-gamma agonists. J Clin Endocrinol Metab. 2006;91(2):614–620. doi:10.1210/jc.2005-1689
33. Richmond JM, Bangari DS, Essien KI, et al. Keratinocyte-derived chemokines orchestrate T-cell positioning in the epidermis during vitiligo and may serve as biomarkers of disease. J Invest Dermatol. 2017;137(2):350–358. doi:10.1016/j.jid.2016.09.016
34. Staab J, Barth PJ, Meyer T. Cell-type-specific expression of STAT transcription factors in tissue samples from patients with lymphocytic thyroiditis. Endocr Pathol. 2012;23(3):141–150. doi:10.1007/s12022-012-9204-0
35. Tomaszewska K, Kozlowska M, Kaszuba A, Lesiak A, Narbutt J, Zalewska-Janowska A. Increased serum levels of IFN-gamma, IL-1beta, and IL-6 in patients with alopecia areata and nonsegmental vitiligo. Oxid Med Cell Longev. 2020;2020:5693572. doi:10.1155/2020/5693572
36. Bhardwaj S, Rani S, Srivastava N, Kumar R, Parsad D. Increased systemic and epidermal levels of IL-17A and IL-1beta promotes progression of non-segmental vitiligo. Cytokine. 2017;91:153–161. doi:10.1016/j.cyto.2016.12.014
37. Zhang QY, Ye XP, Zhou Z, et al. Lymphocyte infiltration and thyrocyte destruction are driven by stromal and immune cell components in Hashimoto’s thyroiditis. Nat Commun. 2022;13(1):775. doi:10.1038/s41467-022-28120-2
38. Yang CA, Chiang BL. Inflammasomes and human autoimmunity: a comprehensive review. J Autoimmun. 2015;61:1–8. doi:10.1016/j.jaut.2015.05.001
39. Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KH. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31(2):331–341. doi:10.1016/j.immuni.2009.08.001
40. Belpaire A, van Geel N, Speeckaert R. From IL-17 to IFN-gamma in inflammatory skin disorders: is transdifferentiation a potential treatment target? Front Immunol. 2022;13:932265. doi:10.3389/fimmu.2022.932265
41. Zake T, Skuja S, Kalere I, Konrade I, Groma V. Upregulated tissue expression of T helper (Th) 17 pathogenic interleukin (IL)-23 and IL-1beta in Hashimoto’s thyroiditis but not in Graves’ disease. Endocr J. 2019;66(5):423–430. doi:10.1507/endocrj.EJ18-0396
42. Yang C, Zhang S, Chang X, Huang Y, Cui D, Liu Z. MicroRNA-219a-2-3p modulates the proliferation of thyroid cancer cells via the HPSE/cyclin D1 pathway. Exp Ther Med. 2021;21(6):659. doi:10.3892/etm.2021.10091
43. Wang Y, Zhao J, Zhang C, Wang P, Huang C, Peng H. MiR-219a-2-3p suppresses cell proliferation and promotes apoptosis by targeting MDM2/p53 in pituitary adenomas cells. Biosci Biotechnol Biochem. 2020;84(5):911–918. doi:10.1080/09168451.2020.1715780
44. Yang Z, Dong X, Pu M, et al. LBX2-AS1/miR-219a-2-3p/FUS/LBX2 positive feedback loop contributes to the proliferation of gastric cancer. Gastric Cancer. 2020;23(3):449–463. doi:10.1007/s10120-019-01019-6
45. Li D, Cai W, Gu R, et al. Th17 cell plays a role in the pathogenesis of Hashimoto’s thyroiditis in patients. Clin Immunol. 2013;149(3):411–420. doi:10.1016/j.clim.2013.10.001
46. Liu Y, Tang X, Tian J, et al. Th17/Treg cells imbalance and GITRL profile in patients with Hashimoto’s thyroiditis. Int J Mol Sci. 2014;15(12):21674–21686. doi:10.3390/ijms151221674
47. Mukhatayev Z, Ostapchuk YO, Fang D, Le Poole IC. Engineered antigen-specific regulatory T cells for autoimmune skin conditions. Autoimmunity Rev. 2021;20(3):102761. doi:10.1016/j.autrev.2021.102761
48. Abdallah M, Lotfi R, Othman W, Galal R. Assessment of tissue FoxP3+, CD4+ and CD8+ T-cells in active and stable nonsegmental vitiligo. Int J Dermatol. 2014;53(8):940–946. doi:10.1111/ijd.12160
49. Shapouri-Moghaddam A, Mohammadian S, Vazini H, et al. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol. 2018;233(9):6425–6440. doi:10.1002/jcp.26429
50. Gong Q, Li X, Gong Q, Zhu W, Song G, Lu Y. Hashimoto’s thyroiditis could be secondary to vitiligo: the possibility of antigen crossover and oxidative stress between the two diseases. Arch Dermatol Res. 2016;308(4):277–281. doi:10.1007/s00403-016-1641-z
51. Slominski A, Wortsman J, Kohn L, et al. Expression of hypothalamic-pituitary-thyroid axis related genes in the human skin. J Invest Dermatol. 2002;119(6):1449–1455. doi:10.1046/j.1523-1747.2002.19617.x
52. Cianfarani F, Baldini E, Cavalli A, et al. TSH receptor and thyroid-specific gene expression in human skin. J Invest Dermatol. 2010;130(1):93–101. doi:10.1038/jid.2009.180
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.







