Back to Journals » Journal of Inflammation Research » Volume 15
Identification of a circRNA/miRNA/mRNA ceRNA Network as a Cell Cycle-Related Regulator for Chronic Sinusitis with Nasal Polyps
Authors Sun Q, Liu Z, Xu X, Yang Y, Han X, Wang C, Song F, Mou Y , Li Y, Song X
Received 15 January 2022
Accepted for publication 5 April 2022
Published 23 April 2022 Volume 2022:15 Pages 2601—2615
DOI https://doi.org/10.2147/JIR.S358387
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ning Quan
Qi Sun,1,2,* Zhen Liu,1,2,* Xiangya Xu,1,* Yujuan Yang,1,2 Xiao Han,1,2 Cai Wang,1– 3 Fei Song,1,2,4 Yakui Mou,1,2 Yumei Li,1,2 Xicheng Song1,2
1Department of Otorhinolaryngology, Head and Neck Surgery, Yantai Yuhuangding Hospital, Qingdao University, Yantai, People’s Republic of China; 2Shandong Provincial Clinical Research Center for Otorhinolaryngologic Diseases, Yantai Yuhuangding Hospital, Yantai, People’s Republic of China; 3School of Clinical Medicine, Weifang Medical University, Weifang, People’s Republic of China; 4Department of Binzhou Medical University, Clinical Medical College Second, Binzhou Medical University, Yantai, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xicheng Song; Yumei Li, Department of Otolaryngology, Head and Neck Surgery, Yantai Yuhuangding Hospital, Qingdao University, Yantai, 264000, People’s Republic of China, Tel +860535-6691999, Fax +860535-6240341, Email [email protected]; [email protected]
Purpose: To explore the mechanisms by which circRNA/miRNA/mRNA competitive endogenous RNAs (ceRNA) networks regulate CRSwNP.
Methods: The expression profiles of circRNAs, miRNAs, and mRNAs from patients with CRSwNP and control subjects were acquired from the Gene Expression Omnibus database. The circRNA/miRNA/mRNA ceRNA network was constructed based on the predicted circRNA–miRNA interactions and miRNA–mRNA interactions. Hub-mRNAs were screened by protein–protein interaction network analysis and Cytoscape molecular complex detection. The expression of factors in tissue and in hsa_circ_0031594 siRNA transfection cells was verified by RT-qPCR and the association between them was revealed by Spearman correlation analysis. Receiver operating characteristic curve analysis was performed with the pROC R package.
Results: The differential expression of 5423 circRNAs, 415 miRNAs, and 3673 mRNAs was identified in CRSwNP subjects compared to control subjects. Among these, 9 circRNAs, 39 miRNAs, and 78 mRNAs were screened to construct a ceRNA network. Ultimately, a subnetwork including circRNA hsa_circ_0031594, hsa-miR-1260b, hsa-miR-6507-5p, NCAPG2, RACGAP1, CHEK1 and PRC1 was screened out. RT-qPCR validated that the expression of hsa_circ_0031594, NCAPG2, PRC1 was significantly increased, and hsa-miR-1260b and hsa-miR-6507-5p were expressed significantly less in patients with CRSwNP than in control subjects. In addition, the AUCs of hsa_circ_0031594, hsa-miR-1260b, hsa-miR-6507-5p, NCAPG2, and PRC1 to discriminate CRSwNP patients were 0.995, 0.842, 0.862, 0.765, and 0.816. Spearman correlation showed that the expression of hsa_circ_0031594 was negatively correlated with hsa-miR-1260b and hsa-miR-6507-5p, and positively correlated with NCAPG2 and PRC1. In human nasal epithelial cell (HNEpC) line, knocking down hsa_circ_0031594 could increase the expression of hsa-miR-1260b and hsa-miR-6507-5p, and reduce the expression of NCAPG2 and PRC1.
Conclusion: CeRNA networks including hsa_circ_0031594, hsa-miR-1260b, and NCAPG2, and hsa_circ_0031594, hsa-miR-6507-5p, and PRC1 may be key regulators for CRSwNP occurrence, and may be potential targets for the pathogenesis and treatment development of CRSwNP.
Keywords: circRNA, miRNA, mRNA, ceRNA network, CRSwNP
Corrigendum for this paper has been published.
Introduction
Chronic sinusitis with nasal polyps (CRSwNP) is characterized by chronic inflammation of the nasal cavity and paranasal sinus mucosa.1 Patients with CRSwNP experience a high burden of symptoms, including stuffy nose, runny nose, and loss of the sense of smell, which have negative impacts on physical and mental health. Despite advances in medical therapy and surgical intervention for CRSwNP, the recurrence rate remains high2 and the pathophysiological mechanisms underlying CRSwNP remain unclear.
Circular RNAs (circRNAs) are a type of ncRNA deriving from exon, intron, or intergenic regions, and are characterized by having a covalently closed continuous loop.3 It is widely reported that circRNA plays an important role in cell and tissue proliferation.4,5 For example, circGCN1L1 promotes cell proliferation and apoptosis by targeting miR-330-3p and TNF-α.6 CircRNAs play important roles in CRSwNP occurrence.7 Yu et al found that 1794 circRNAs were downregulated and 1081 circRNAs were upregulated in CRSwNP patients relative to control subjects.7 MicroRNAs (miRNAs) are a class of small noncoding RNAs that regulate the expression of target genes by preventing the translation or inducing the degradation of target mRNAs.8 Zhang et al identified differential miRNA expression profiles between patients with CRSsNP and those with eosinophilic CRSwNP.9 They reported that up-regulated expression of miR-125b contributes to mucosal eosinophilia in eosinophilic CRSwNP.9 Silveira et al reported that miR-205-5p was directly implicated with cell cycle regulation, and related to T2-polarity in CRSwNP.10 Liu et al found that upregulated miR-21 can inhibit activation of NF-κB P65 and increase IL-10 expression through targeting to PDCD4 in nasal epithelial cells, which could further limit the expression of pro-inflammatory cytokines in CRSwNP.11 Although many studies have indicated differential expressions of circRNAs and miRNAs between patients with CRSwNP and other patients or controls, the specific regulatory mechanisms through regulation of circRNA/miRNA/mRNA networks contributing to CRSwNP remain unclear.
CircRNAs can act as miRNA ‘sponges’ or protein scaffolds to regulate gene transcription.12 CircRNAs can regulate mRNA expression by competitively associating with miRNAs, instead of their target mRNAs, thus forming competitive endogenous RNA (ceRNA) network.6,13–15 However, whether the differential expression of circRNAs can regulate CRSwNP through forming circRNA/miRNA/mRNA ceRNA networks remains unclear. Our study explores the mechanisms through circRNA/miRNA/mRNA ceRNA networks may regulate CRSwNP pathogenesis, providing rationale for these RNAs to be considered as potential targets for the diagnosis and treatment of CRSwNP.
Materials and Methods
Microarray Data Information
The raw expression data for circRNA, miRNA, and mRNA was collected from the GEO database (http://www.ncbi.nlm.nih.gov/geo/). Genomic microarray analysis of circRNA expression (GSE169375) and miRNA expression (GSE169376) was obtained for 9 nasal mucosa samples, including samples from 6 CRSwNP patients and 3 control subjects.7 Significantly differentially expressed (DE) circRNAs between the 6 CRSwNP samples and the 3 control samples were screened by a cutoff of P < 0.05 and |log2fold change (FC)|>1.5,16,17 using the Limma R package. Significantly DEmiRNAs were identified by a cutoff of P < 0.05 using the Limma R package. MRNA expression data was obtained from nasal polyp tissue samples from 6 CRSwNP subjects and from uncinate tissue from 6 control subjects in GSE36830.18 DEmRNAs were identified with a p-value of P < 0.05 using the Limma R package. The basic information for the samples in the three GEO datasets used in this study are shown in Table 1.
 |
Table 1 Basic Information of the Three Microarray Datasets from GEO |
Prediction of circRNA–miRNA Interaction
The circBase website (http://www.circbase.org/)19 was used to evaluate the characteristics of circRNAs. The top ten DEcircRNAs (Table 2), including the top 5 up-regulated and the top 5 down-regulated DEcircRNAs, were identified based on the absolute value of log2FC. The interactions between DEmiRNAs and the top ten DEcircRNAs were predicted using the the miRanda (http://www.microrna.org)20 and the RNAhybrid (http://bibiserv.techfak.uni-bielefeld.de/rnahybrid) databases.21
 |
Table 2 Basic Characteristics of the Top Ten DEcircRNAs |
Prediction of miRNA–mRNA Interaction
The mRNAs that were potentially related to the differentially expressed miRNAs were screened based on the intersection predicted from three online miRNA target prediction tools, including TargetScan (http://www.targetscan.org/),22 miRDB (http://www.mirdb.org/),23 and miRTarBase (http://mirtarbase.mbc.nctu.edu.tw/php/index.php).24 The miRNA-related mRNAs that were not differentially expressed between CRSwNP subjects and control subjects in GSE36830 were excluded.
Construction of a circRNA–miRNA–mRNA ceRNA Network
The circRNA/miRNA/mRNA regulatory network was constructed by integrating circRNA-miRNA pairs and miRNA-mRNA pairs25 and was visualized using Cytoscape software (http://cytoscape.org/; version 3.8.0).
Functional Annotation of mRNAs in the ceRNA Network
The biological processes, cellular components, and molecular functions related to the mRNAs in the ceRNA network were analyzed by Gene Ontology (GO) (http://geneontology.org/). Signalling pathways related to the mRNAs in the ceRNA network were analyzed by Kyoto Encyclopedia of Gene and Genome (KEGG) (https://www.kegg.jp/). The results were depicted using clusterProfiler R package.
Construction of PPI Network and Identification of Hub Genes
A PPI network related to mRNAs in the ceRNA network was established by the string database (minimum required interaction score = 0.400 and hide disconnected nodes in the network) (http://string-db.org/). The resulting PPI network was visualized using Cytoscape software. Subsequently, the hub-mRNAs were evaluated using the Cytoscape Molecular Complex Detection (MCODE) (module with the following criteria: degree = 2; node score = 0.2; k-core = 2; and max. depth = 100),26 according to the topology.
Patient Inclusion and Exclusion Criteria
A total of 28 subjects including 14 patients with CRSwNP and 14 control subjects were enrolled in this study. Patient inclusion criteria: diagnosed with CRSwNP according to the European Position Paper on Rhinosinusitis and Nasal Polyps (EPOS);27 no treatment with corticosteroids, immunomodulatory agents, or antibiotics within 4 weeks before enrollment. Patients with acute infections, acetylsalicylic acid-intolerance, fungal sinusitis, immunodeficiency, coagulation disorder, or cystic fibrosis and pregnant women were excluded. Nasal polyp tissues from patients with CRSwNP and nasal mucosal tissues from control subjects were collected. This study was approved by the Institution Ethics Committee of Yantai Yuhuangding Hospital and all patients provided informed consent before enrollment. Our experiments were performed in accordance with the Declaration of Helsinki.
RNA Extraction and RT-qPCR
Total RNA was extracted from tissue samples using Trizol reagent (Sparkjade, China), according to the manufacturer’s instructions. Then 1 µg RNA was reverse-transcribed using AG reverse transcription kit (Accurate Biology, China). The RT-qPCR reactions were performed using SYBR Green qPCR Mix kit (Sparkjade, China) according to the manufacturer’s instructions. The extraction and amplification of miRNAs was performed according to the manufacturer’s instructions (Vazyme, China). The primers for RT-qPCR are shown in Table 3.
 |
Table 3 Primer Sequences of circRNA, miRNAs, mRNAs for RT-qPCR |
ROC Curve and Correlation Analysis
ROC curve analysis was carried out to calculate the areas under curve (AUC) and estimate the functional utility of the selected the hub gene in discriminating CRSwNP patients from normal subjects. The pROC R package was used for ROC analyses. The correlation between hsa_circ_0031594, hsa-miR-1260b, hsa-miR-6507-5p, NCAPG2 and PRC1 was assessed by Spearman correlation analysis.
Nasal Epithelial Cell Line Culture and Small Interference RNA Transfection
The human nasal epithelial cell (HNEpC) line was obtained from BeNa Culture Collection (BNCC, China). The HNEpC cells were cultured by Minimum Essential Medium containing 10% fetal bovine serum at 37°C with 5% CO2. Three small interference RNAs (siRNAs) for hsa_circ_0031594 were generated by GenePharma (GenePharma Corporation, Shanghai, China). The sequences: hsa_circ_0031594-1 siRNA, 5’-ACGCAACCAGGCAAUGGUGTT-3’; hsa_circ_0031594-2 siRNA, 5’-UUACGCAACCAGGCAAUGGTT-3’; hsa_circ_0031594-3 siRNA, 5’-ACCAGGCAAUGGUGGCUUGCU-3’. The siRNA sequences of negative control (NC) were 5’-UUCUCCGAACGUGUCACGUTT-3’; All siRNAs were transfected by Lipofectamine® 3000 transfection reagent (Thermo Fisher Scientific, America) according to the instruction. After transfection for 48 h, the expression of hsa_circ_0031594 was tested by real-time PCR analysis to assess the transfection efficiency.
Nuclear Cytoplasm Separation and Nucleic Acid Electrophoresis
3*106 HNEpC cells were collected for nuclear cytoplasm separation and RNA extraction by using the PARIS™ kit (Thermo Fisher Scientific, America) according to the manufacturer’s instructions. The RNA is reversely transcribed using HiScript Q RT SuperMix for qPCR (+gDNA wiper) (Vazyme, China). The hsa_circ_0031594 was amplified by using 2x EasyTaq PCR SuperMix+ Dye (TransGen Biotech, China) PCR reagent according to the instructions. The amplified nucleic acid was tested by agarose gel electrophoresis. The expression of hsa_circ_0031594, U6 (control expressed in the nuclear), and GAPDH (control expressed in the cytoplasm) in nuclear and cytoplasm were analyzed by PCR.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism (GraphPad Software, CA). The significance of differences between groups was tested by Student’s t-test. The relative expression of genes was calculated using the 2−ΔΔCT method. A p-value of P < 0.05 was considered as statistically significant.
Results
Identification of Differentially Expressed circRNAs, miRNAs, and mRNAs Between CRSwNP Patients and Controls
A flowchart detailing the analysis steps is shown in Figure 1. In brief, 5423 DEcircRNAs were found to be differentially expressed in patients with CRSwNP compared to controls in GSE169375. The DEcircRNAs are shown in a volcano plot (Figure 2A), and the top 50 DEcircRNAs visualized in a heatmap (Figure 2B). Additionally, 415 DEmiRNAs in patients with CRSwNP compared to controls (Figure 2C) were identified from GSE169376, and the top 50 DEmiRNAs were visualized in a heatmap (Figure 2D). Meanwhile, 3673 DEmRNAs in patients with CRSwNP compared to controls were identified from GSE36830 (Figure 2E), and the top 50 DEmRNAs were visualized in a heatmap (Figure 2F).
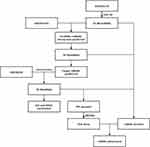 |
Figure 1 Flowchart of the study. |
Construction of the ceRNA Network
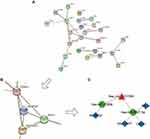
To better comprehend the role of circRNAs in regulating CRSwNP, a circRNA/miRNA/mRNA regulatory network was generated. The top ten DEcircRNAs (Table 2) were selected as initial candidates for the ceRNA network construction. Of the 415 DEmiRNAs, 39 miRNAs were predicted to be related to the top ten DEcircRNAs by the miRanda and RNAhybrid databases. Of the DEcircRNAs, hsa_circ_0092542 had no potential interaction with miRNAs, so it was excluded from subsequent analyses. We chose 78 mRNAs predicted to interact with the 39 miRNAs. Finally, the 9 DEcircRNAs, the 39 miRNAs, and the 78 mRNAs (Tables S1 and S2) were used to construct the ceRNA network (Figure 3). 62 circRNA-miRNA interaction pairs and 88 miRNA-mRNA interaction pairs were found within the ceRNA network (Figure 3).
 |
Figure 3 The ceRNA network of circRNA/miRNA/mRNA in CRSwNP. Red dots represent circRNAs. Green dots indicate miRNAs. Blue dots indicate mRNAs. |
GO and KEGG Enrichment Analyses
In order to understand the biological functions and pathways impacted by the 78 DEmRNAs in CRSwNP, GO annotation and KEGG pathway analysis were performed. GO enrichment analysis consists of biological processes, molecular function, and cellular components (Figure 4A). The 78 DEmRNAs were primarily enriched in biological processes such as neutrophil degranulation, neutrophil activation, positive regulation of cell cycle, and tube formation. The 78 DEmRNAs were mainly enriched in cellular components including fibrillar center, extrinsic component of membrane, and nuclear membrane, and were enriched in molecular functions including kinase regulator activity, transcription corepressor activity, and protein serine/threonine kinase activity (Figure 4A and Table S3). The 78 DEmRNAs in CRSwNP were significantly enriched in KEGG pathways including the MAPK signaling pathway, insulin signaling pathway, p53 signaling pathway, and the cell cycle (Figure 4B and Table S4).
 |
Figure 4 GO and KEGG analyses of 78 DEmRNAs. (A) GO analysis. (B) KEGG pathway analysis. |
Hub Genes and ceRNA Subnetwork Screen
The PPI network was established by the string database. The PPI network of the 78 DEmRNAs contained 24 proteins and 21 interaction edges (Figure 5A). The hub interaction cluster consisted of four genes: polycomb inhibitory complex 1 (PRC1), checkpoint kinase (CHEK1), Rac GTPase Activating Protein 1 (RACGAP1), and Non-SMC lectin II complex subunit G2 (NCAPG2), as identified by the Cytoscape MCODE module (Figure 5B). By mapping the 4 hub genes into the preliminary circRNA/miRNA/mRNA network, a circRNA/miRNA/mRNA subnetwork consisting of hsa_circ_0031594, hsa-miR-1260b, hsa-miR-6507-5p, PRC1, CHEK1, RACGAP1, NCAPG2 was proposed (Figure 5C).
Verification of Expression of the ceRNA Subnetwork Factors in CRSwNP
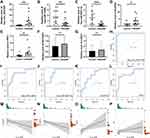
In order to verify the ceRNA subnetwork analysis, we tested the expression of ceRNA subnetwork factors in nasal polyp samples from CRSwNP patients and nasal mucosa samples from control subjects. We found that expression of hsa_circ_0031594 was significantly higher in nasal polyps than in control nasal mucosa (Figure 6A). Expression of hsa-miR-1260b and hsa-miR-6507-5p was significantly lower in nasal polyps than in control nasal mucosa (Figure 6B and C). The expression of NCAPG2 and PRC1 in nasal polyps was significantly higher than in control nasal mucosa (Figure 6D and E). However, the expression of RACGAP1 and CHEK1 was not significantly different between CRSwNP and control samples (Figure 6F and G). ROC analysis was performed to verify the correlation between gene expression and CRSwNP. The AUCs of hsa_circ_0031594, hsa-miR-1260b, hsa-miR-6507-5p, NCAPG2, and PRC1 were 0.995, 0.842, 0.862, 0.765, and 0.816, respectively (Figure 6H–L). Spearman correlation analysis results (Figure 6M–P) showed that the expression of hsa_circ_0031594 was negatively correlated with the expression of hsa-miR-1260b (Spearman correlation coefficient = −0.55, p = 0.003) and hsa-miR-6507-5p (Spearman correlation coefficient = −0.58, p = 0.001), and positively correlated with the expression of NCAPG2 (Spearman correlation coefficient = 0.60, p = 0.001), and PRC1 (Spearman correlation coefficient = 0.47, p = 0.011).
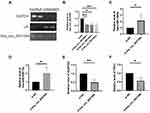
Knocking Down hsa_circ_0031594 Upregulated hsa-miR-1260b and hsa-miR-6507-5p and Downregulated NCAPG2 and PRC1
Through nuclear and cytoplasm separation, hsa_circ_0031594 was localized in cytoplasm of HNEpC cells (Figure 7A). When knocking down hsa_circ_0031594 expression by siRNAs in HNEpC cells, the expression of hsa_circ_0031594 were obviously downregulated (Figure 7B). Meanwhile, the expression levels of hsa-miR-1260b and hsa-miR-6507-5p were increased and the level of NCAPG2, and PRC1 was reduced in the hsa_circ_0031594 siRNA group than in the NC group (Figure 7C–F). These results showed knocking down hsa_circ_0031594 could upregulate hsa-miR-1260b and hsa-miR-6507-5p and downregulate NCAPG2 and PRC1.
Discussion
CRSwNP is heterogeneous disease that is associated with significant public health problems and causes heavy socioeconomic burden.28,29 Abnormal gene expression is a key factor leading to the high incidence of CRSwNP,30,31 but it is unclear how these genes are regulated. In our study, we explored the function of a circRNA/miRNA/mRNA ceRNA network in regulating CRSwNP through bioinformatics analysis and RT-qPCR verification. We found that hsa_circ_0031594/hsa-miR-1260b/NCAPG2 and hsa_circ_0031594/hsa-miR-6507-5p/PRC1 ceRNA subnetworks might be primary regulators of CRSwNP, and our study provides potential targets for the diagnosis and treatment of CRSwNP.
Research by Yu et al identified the differential expression of 2875 circRNAs and 192 miRNAs between CRSwNP subjects and control subjects.7 This indicated that circRNAs and miRNAs may play important roles in the occurrence and development of CRSwNP. However, how these circRNAs and miRNAs interact to regulate CRSwNP remained unknown. Functionally, circRNAs can act as ‘miRNA sponges’ and competitively bind miRNAs to regulate the target mRNA post-transcriptional activity,32 thus forming a ceRNA network to regulate disease occurrence or development. For example, hsa_circ_0000520/miR-556-5p/NLRP3 could mediate cell pyroptosis and inflammation to regulate ovalbumin-induced allergic rhinitis in mice.33 However, no ceRNA networks have yet been reported to regulate CRSwNP. In our study, differentially expressed genes from GSE169375, GSE169376, and GSE36830 were extracted to construct a ceRNA network in order to explore circRNA/miRNA/mRNA functions in CRSwNP. We found that the hsa_circ_0031594/ hsa-miR-1260b/ NCAPG2 and hsa_circ_0031594/ hsa-miR-6507-5p/PRC1 ceRNA subnetworks might be hub regulators of CRSwNP. We used RT-qPCR to validate the differential expression of hsa_circ_0031594, hsa-miR-1260b, hsa-miR-6507-5p, NCAPG2, and PRC1 between CRSwNP patients and control subjects. In HNEpC, knocking down hsa_circ_0031594 could increase the expression of hsa-miR-1260b and hsa-miR-6507-5p, and reduce the expression of NCAPG2 and PRC1. These data suggests that ceRNA networks play important roles in CRSwNP.
Neutrophils are predominant in approximately 50% of patients with CRS without nasal polyps, and also play a role in patients with severe type 2 CRS with nasal polyp disease.34 Neutrophils promote tissue remodeling and impair the epithelial barrier by producing TGF-β2 and oncostatin M.35,36 In addition, activated neutrophils can induce an accumulation of eosinophils,37 and enhance the progression of nasal polyps through a Th2 immune response,38,39 thereby establishing a more persistent and severe pathogenesis of CRSwNP. Neutrophils also induce exacerbation of type 2 immune responses by releasing neutrophil extracellular traps into extracellular space following rhinovirus infection.40 In our study, GO analysis revealed that the 78 DEmRNAs in the ceRNA network were enriched in biological processes related to neutrophil activation, indicating that circRNA/miRNA/mRNA ceRNA network may regulate CRSwNP occurrence and development by activating neutrophils. Moreover, we found through KEGG analysis that the MAPK pathway is involved in CRSwNP. This is consistent with a previous report in which TGF-β1 activated MAPK signaling pathways to promote the development of CRSwNP by initiating EMT.41 These results demonstrate that the genes involved in the ceRNA network play important roles in CRSwNP occurrence and development.
Through PPI network and Cytoscape MCODE analysis of hub genes, PRC1, RACGAP1, CHEK1, and NCAPG2 were screened out. These genes are enriched in biological processes involved in the cell cycle, according to GO analysis. NCAPG2 can promote cell proliferation by regulating G2/M42 or by activating STAT3 and NF-κB/ miR-188-3p pathways.43,44 PRC1 regulates cell proliferation and cell cycle transition through P53.45 In addition, PCR1 reinforces Wnt signaling to promote cell proliferation.46 CHEK1, a serine/threonine specific protein kinase, phosphorylates many downstream effectors to initiate cell cycle checkpoints, cell cycle arrest, DNA repair, and cell death.47 RACGAP1 promotes cell proliferation and restricts apoptosis.48,49 The depletion of RACGAP1 leads to the formation of multinucleated cells, the failure of cytokinesis, and apoptosis.50–52
Abnormal epithelial proliferation is one of the typical features of CRSwNP,53 which leads to abnormal function and remodeling of the nasal epithelial cell barrier, especially squamous cell metaplasia and secretory hyperplasia of epithelial cells.54,55 Abnormal epithelial proliferation can also change the local inflammatory environment and lead to chronic inflammation by producing the epithelial-derived cytokines IL33 and thymic stromal lymphopoietin (TSLP).54–58 These data indicates that cell cycle-associated factors play important roles in CRSwNP pathogenesis. In our study, cell cycle-related factors NCAPG2, PRC1, RACGAP1, and CHEK1 were identified as hub genes for CRSwNP. Through RT-qPCR, we verified increased expression of NCAPG2 and PRC1 in CRSwNP subjects compared to control subjects, showing that NCAPG2 and PRC1 may regulate CRSwNP pathogenesis by mediating cell cycle and cell proliferation.
In the circRNA/miRNA/mRNA subnetwork, hsa_circ_0031594 may act as a ceRNA to capture hsa-miR-1260b and positively regulate the expression of NCAPG2. In addition, hsa_circ_0031594 can also act as a hsa-miR-6507-5p sponge and positively regulate the expression of PRC1/RACGAP1/CHEK1. Hsa_circ_0031594 is upregulated in CRSwNP, which is consistent with a previous report.7 Hsa-miR-6507-5p has been reported to be upregulated in patients with achalasia, however its mechanism has not been studied.59 We found that hsa-miR-6507-5p and hsa-miR-1260b were downregulated in CRSwNP. Hsa_circ_0031594 (Circ-EGLN3) has been reported to promote renal cell carcinoma (RCC) cell proliferation and aggressiveness by enhancing the IRF7 level via sponging miR-1299,60 or by upregulating HMGXB3 via sponging miR-1224-3p.61 Hsa-miR-1260b, mediated by YY1, activates KIT signaling by targeting SOCS6 to regulate NSCLC cell proliferation and apoptosis.62 Therefore, we speculate that the circRNA/miRNA/mRNA network related to hsa_circ_0031594 is a new candidate target related to cell proliferation in CRSwNP. Verification of the differential expression by RT-qPCR revealed that the hsa_circ_0031594/ hsa-miR-1260b/ NCAPG2 axis and hsa_circ_0031594/ hsa-miR-6507-5p/ PRC1 axis may be key pathways in regulating CRSwNP, and ROC analysis results also showed that these subnetworks may have important clinical diagnostic significance. The hsa_circ_0031594/hsa-miR-6507-5p/RACGAP1/CHEK1 axis was not verified in our study, and whether this axis plays a more important role in CRSwNP requires further evaluation.
Conclusion
In conclusion, this study identified a comprehensive circRNA/miRNA/mRNA network related to CRSwNP, which included the 9 DEcircRNAs, the 39 miRNAs, and the 78 mRNAs. Furthermore, the hub-ceRNA network, which included hsa_circ_0031594, hsa-miR-1260b, and NCAPG2 axis, and hsa_circ_0031594, hsa-miR-6507-5p, and PRC1 axis was identified and verified by clinical CRSwNP samples expression analysis and in-vitro cell experiments. Our study will help to explore the mechanisms underlying the occurrence and development of CRSwNP and further develop potential treatment strategies for the disease. However, since these results are mainly based on computational biology and RT-qPCR experimental verification, further biological and molecular experiments will be needed to verify our hypothesis.
Abbreviations
CRSwNP, chronic sinusitis with nasal polyps; CeRNA, competitive endogenous RNA; MiRNA, microRNA; GEO, Gene Expression Omnibus; GO, Gene Ontology; KEGG, Kyoto Encyclopedia of Gene and Genome; PPI, protein–protein interaction; MCODE, molecular complex detection; ROC, receiver operating characteristic; AUC, area under curve.
Data Sharing Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethics Approval and Informed Consent
All study surgical procedures and experiment protocols were performed in accordance with standard guidelines approved by the Institution Ethics Committee of Yantai Yuhuangding Hospital.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (82071021), and the Major Scientific and Technological Innovation Project of Shandong Province (2020CXGC011302).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by grants from the National Natural Science Foundation of China (82071021), and the Major Scientific and Technological Innovation Project of Shandong Province (2020CXGC011302).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Zhang Y, Lou H, Wang Y, Li Y, Zhang L, Wang C. Comparison of corticosteroids by 3 approaches to the treatment of chronic rhinosinusitis with nasal polyps. Allergy Asthma Immunol Res. 2019;11(4):482–497. doi:10.4168/aair.2019.11.4.482
2. Chalermwatanachai T, Vilchez-Vargas R, Holtappels G, et al. Chronic rhinosinusitis with nasal polyps is characterized by dysbacteriosis of the nasal microbiota. Sci Rep. 2018;8(1):7926. doi:10.1038/s41598-018-26327-2
3. Tsai CY, Shen CY, Liu CW, et al. Aberrant non-coding RNA expression in patients with systemic lupus erythematosus: consequences for immune dysfunctions and tissue damage. Biomolecules. 2020;10(12):1641. doi:10.3390/biom10121641
4. Xu Q, Cheng D, Li G, et al. CircHIPK3 regulates pulmonary fibrosis by facilitating glycolysis in miR-30a-3p/FOXK2-dependent manner. Int J Biol Sci. 2021;17(9):2294–2307. doi:10.7150/ijbs.57915
5. Ji N, Wang Y, Gong X, Ni S, Zhang H. CircMTO1 inhibits ox-LDL-stimulated vascular smooth muscle cell proliferation and migration via regulating the miR-182-5p/RASA1 axis. Mol Med. 2021;27(1):73. doi:10.1186/s10020-021-00330-2
6. Zhu H, Hu Y, Wang C, Zhang X, He D. CircGCN1L1 promotes synoviocyte proliferation and chondrocyte apoptosis by targeting miR-330-3p and TNF-α in TMJ osteoarthritis. Cell Death Dis. 2020;11(4):284. doi:10.1038/s41419-020-2447-7
7. Yu J, Kang X, Xiong Y, Luo Q, Dai D, Ye J. Gene expression profiles of circular RNAs and MicroRNAs in chronic rhinosinusitis with nasal polyps. Front Mol Biosci. 2021;8:643504. doi:10.3389/fmolb.2021.643504
8. Ambros V. The functions of animal microRNAs. Nature. 2004;431(7006):350–355. doi:10.1038/nature02871
9. Zhang XH, Zhang YN, Li HB, et al. Overexpression of miR-125b, a novel regulator of innate immunity, in eosinophilic chronic rhinosinusitis with nasal polyps. Am J Respir Crit Care Med. 2012;185(2):140–151. doi:10.1164/rccm.201103-0456OC
10. Silveira MLC, Tamashiro E, Santos ARD, et al. miRNA-205-5p can be related to T2-polarity in chronic rhinosinusitis with nasal polyps. Rhinology. 2021;59(6):567–576. doi:10.4193/Rhin21.109
11. Liu R, Du J, Zhou J, et al. Elevated microRNA-21 is a brake of inflammation involved in the development of nasal polyps. Front Immunol. 2021;12:530488. doi:10.3389/fimmu.2021.530488
12. Lasda E, Parker R. Circular RNAs: diversity of form and function. Rna. 2014;20(12):1829–1842. doi:10.1261/rna.047126.114
13. Li B, Huang N, Wei S, et al. lncRNA TUG1 as a ceRNA promotes PM exposure-induced airway hyper-reactivity. J Hazard Mater. 2021;416:125878. doi:10.1016/j.jhazmat.2021.125878
14. Liu Y, Xue M, Du S, et al. Competitive endogenous RNA is an intrinsic component of EMT regulatory circuits and modulates EMT. Nat Commun. 2019;10(1):1637. doi:10.1038/s41467-019-09649-1
15. Qian W, Cai X, Qian Q, et al. lncRNA ZEB1-AS1 promotes pulmonary fibrosis through ZEB1-mediated epithelial-mesenchymal transition by competitively binding miR-141-3p. Cell Death Dis. 2019;10(2):129. doi:10.1038/s41419-019-1339-1
16. Chen Y, Huang X, Zhu K, et al. LIMD2 is a prognostic and predictive marker in patients with esophageal cancer based on a cerna network analysis. Front Genet. 2021;12:774432. doi:10.3389/fgene.2021.774432
17. Zhu M, Li M, Zhou W, et al. Qianggan extract improved nonalcoholic steatohepatitis by modulating lncRNA/circRNA immune ceRNA networks. BMC Complement Altern Med. 2019;19(1):156. doi:10.1186/s12906-019-2577-6
18. Stevens WW, Ocampo CJ, Berdnikovs S, et al. Cytokines in chronic rhinosinusitis. Role in eosinophilia and aspirin-exacerbated respiratory disease. Am J Respir Crit Care Med. 2015;192(6):682–694. doi:10.1164/rccm.201412-2278OC
19. Glažar P, Papavasileiou P, Rajewsky N. circBase: a database for circular RNAs. RNA. 2014;20(11):1666–1670. doi:10.1261/rna.043687.113
20. John B, Enright AJ, Aravin A, Tuschl T, Sander C, Marks DS. Human MicroRNA targets. PLoS Biol. 2004;2(11):e363. doi:10.1371/journal.pbio.0020363
21. Krüger J, Rehmsmeier M. RNAhybrid: microRNA target prediction easy, fast and flexible. Nucleic Acids Res. 2006;34(Web Server issue):W451–454. doi:10.1093/nar/gkl243
22. Agarwal V, Bell GW, Nam JW, Bartel DP. Predicting effective microRNA target sites in mammalian mRNAs. Elife. 2015;4. doi:10.7554/eLife.05005
23. Chen Y, Wang X. miRDB: an online database for prediction of functional microRNA targets. Nucleic Acids Res. 2020;48(D1):D127–d131. doi:10.1093/nar/gkz757
24. Huang HY, Lin YC, Li J, et al. miRTarBase 2020: updates to the experimentally validated microRNA-target interaction database. Nucleic Acids Res. 2020;48(D1):D148–D154. doi:10.1093/nar/gkz896
25. Zhao H, Chen L, Shan Y, et al. Hsa_circ_0038383-mediated competitive endogenous RNA network in recurrent implantation failure. Aging. 2021;13(4):6076–6090. doi:10.18632/aging.202590
26. Bader GD, Hogue CW. An automated method for finding molecular complexes in large protein interaction networks. BMC Bioinform. 2003;4:2. doi:10.1186/1471-2105-4-2
27. Fokkens WJ, Lund VJ, Hopkins C, et al. European position paper on rhinosinusitis and nasal polyps 2020. Rhinology. 2020;58(Suppl S29):1–464. doi:10.4193/Rhin20.401
28. McCormick JP, Thompson HM, Cho DY, Woodworth BA, Grayson JW. Phenotypes in chronic rhinosinusitis. Curr Allergy Asthma Rep. 2020;20(7):20. doi:10.1007/s11882-020-00916-6
29. Wang SB, Chen SM, Zhu KS, Zhou B, Chen L, Zou XY. Increased lipopolysaccharide content is positively correlated with glucocorticoid receptor-beta expression in chronic rhinosinusitis with nasal polyps. Immun Inflamm Dis. 2020;8(4):605–614. doi:10.1002/iid3.346
30. Peng Y, Zi XX, Tian TF, et al. Whole-transcriptome sequencing reveals heightened inflammation and defective host defence responses in chronic rhinosinusitis with nasal polyps. Eur Respir J. 2019;54(5):1900732. doi:10.1183/13993003.00732-2019
31. Böscke R, Vladar EK, Könnecke M, et al. Wnt signaling in chronic rhinosinusitis with nasal polyps. Am J Respir Cell Mol Biol. 2017;56(5):575–584. doi:10.1165/rcmb.2016-0024OC
32. Kristensen LS, Andersen MS, Stagsted LVW, Ebbesen KK, Hansen TB, Kjems J. The biogenesis, biology and characterization of circular RNAs. Nat Rev Genet. 2019;20(11):675–691. doi:10.1038/s41576-019-0158-7
33. Yu X, Wang M, Zhao H, Cao Z. Targeting a novel hsa_circ_0000520/miR-556-5p/NLRP3 pathway-mediated cell pyroptosis and inflammation attenuates ovalbumin (OVA)-induced allergic rhinitis (AR) in mice models. Inflamm Res. 2021;70(6):719–729. doi:10.1007/s00011-021-01472-z
34. Delemarre T, Bochner BS, Simon HU, Bachert C. Rethinking neutrophils and eosinophils in chronic rhinosinusitis. J Allergy Clin Immunol. 2021;148(2):327–335. doi:10.1016/j.jaci.2021.03.024
35. Shi LL, Xiong P, Zhang L, et al. Features of airway remodeling in different types of Chinese chronic rhinosinusitis are associated with inflammation patterns. Allergy. 2013;68(1):101–109. doi:10.1111/all.12064
36. Wang H, Li ZY, Jiang WX, et al. The activation and function of IL-36γ in neutrophilic inflammation in chronic rhinosinusitis. J Allergy Clin Immunol. 2018;141(5):1646–1658. doi:10.1016/j.jaci.2017.12.972
37. Gevaert E, Zhang N, Krysko O, et al. Extracellular eosinophilic traps in association with Staphylococcus aureus at the site of epithelial barrier defects in patients with severe airway inflammation. J Allergy Clin Immunol. 2017;139(6):1849–1860.e1846. doi:10.1016/j.jaci.2017.01.019
38. Ryu G, Kim DW. Th2 inflammatory responses in the development of nasal polyps and chronic rhinosinusitis. Curr Opin Allergy Clin Immunol. 2020;20(1):1–8. doi:10.1097/ACI.0000000000000588
39. Yu S, Cao C, Li Q, et al. Local IL-17 positive T cells are functionally associated with neutrophil infiltration and their development is regulated by mucosal microenvironment in nasal polyps. Inflamm Res. 2021;70(1):139–149. doi:10.1007/s00011-020-01424-z
40. Toussaint M, Jackson DJ, Swieboda D, et al. Host DNA released by NETosis promotes rhinovirus-induced type-2 allergic asthma exacerbation. Nat Med. 2017;23(6):681–691. doi:10.1038/nm.4332
41. Yang HW, Lee SA, Shin JM, Park IH, Lee HM. Glucocorticoids ameliorate TGF-β1-mediated epithelial-to-mesenchymal transition of airway epithelium through MAPK and Snail/Slug signaling pathways. Sci Rep. 2017;7(1):3486. doi:10.1038/s41598-017-02358-z
42. Zhan P, Xi GM, Zhang B, et al. NCAPG2 promotes tumour proliferation by regulating G2/M phase and associates with poor prognosis in lung adenocarcinoma. J Cell Mol Med. 2017;21(4):665–676. doi:10.1111/jcmm.13010
43. Meng F, Zhang S, Song R, et al. NCAPG2 overexpression promotes hepatocellular carcinoma proliferation and metastasis through activating the STAT3 and NF-κB/miR-188-3p pathways. EBioMedicine. 2019;44:237–249. doi:10.1016/j.ebiom.2019.05.053
44. Wu J, Li L, Jiang G, Zhan H, Zhu X, Yang W. NCAPG2 facilitates glioblastoma cells’ malignancy and xenograft tumor growth via HBO1 activation by phosphorylation. Cell Tissue Res. 2021;383(2):693–706. doi:10.1007/s00441-020-03281-y
45. Wu F, Shi X, Zhang R, et al. Regulation of proliferation and cell cycle by protein regulator of cytokinesis 1 in oral squamous cell carcinoma. Cell Death Dis. 2018;9(5):564. doi:10.1038/s41419-018-0618-6
46. Chen J, Rajasekaran M, Xia H, et al. The microtubule-associated protein PRC1 promotes early recurrence of hepatocellular carcinoma in association with the Wnt/β-catenin signalling pathway. Gut. 2016;65(9):1522–1534. doi:10.1136/gutjnl-2015-310625
47. Zhang Y, Hunter T. Roles of Chk1 in cell biology and cancer therapy. Int J Cancer. 2014;134(5):1013–1023. doi:10.1002/ijc.28226
48. Wang C, Wang W, Liu Y, Yong M, Yang Y, Zhou H. Rac GTPase activating protein 1 promotes oncogenic progression of epithelial ovarian cancer. Cancer Sci. 2018;109(1):84–93. doi:10.1111/cas.13434
49. Mi S, Lin M, Brouwer-Visser J, et al. RNA-seq identification of RACGAP1 as a metastatic driver in uterine carcinosarcoma. Clin Cancer Res. 2016;22(18):4676–4686. doi:10.1158/1078-0432.CCR-15-2116
50. Niiya F, Xie X, Lee KS, Inoue H, Miki T. Inhibition of cyclin-dependent kinase 1 induces cytokinesis without chromosome segregation in an ECT2 and MgcRacGAP-dependent manner. J Biol Chem. 2005;280(43):36502–36509. doi:10.1074/jbc.M508007200
51. Kamijo K, Ohara N, Abe M, et al. Dissecting the role of Rho-mediated signaling in contractile ring formation. Mol Biol Cell. 2006;17(1):43–55. doi:10.1091/mbc.e05-06-0569
52. Yang XM, Cao XY, He P, et al. Overexpression of Rac GTPase activating protein 1 contributes to proliferation of cancer cells by reducing hippo signaling to promote cytokinesis. Gastroenterology. 2018;155(4):1233–1249.e1222. doi:10.1053/j.gastro.2018.07.010
53. Deng H, Li M, Zheng R, et al. YAP promotes cell proliferation and epithelium-derived cytokine expression via NF-κB pathway in nasal polyps. J Asthma Allergy. 2021;14:839–850. doi:10.2147/JAA.S315707
54. Meng J, Zhou P, Liu Y, et al. The development of nasal polyp disease involves early nasal mucosal inflammation and remodelling. PLoS One. 2013;8(12):e82373. doi:10.1371/journal.pone.0082373
55. Okada N, Nakayama T, Asaka D, et al. Distinct gene expression profiles and regulation networks of nasal polyps in eosinophilic and non-eosinophilic chronic rhinosinusitis. Int Forum Allergy Rhinol. 2018;8(5):592–604. doi:10.1002/alr.22083
56. Ishinaga H, Kitano M, Toda M, et al. Interleukin-33 induces mucin gene expression and goblet cell hyperplasia in human nasal epithelial cells. Cytokine. 2017;90:60–65. doi:10.1016/j.cyto.2016.10.010
57. Liao B, Cao PP, Zeng M, et al. Interaction of thymic stromal lymphopoietin, IL-33, and their receptors in epithelial cells in eosinophilic chronic rhinosinusitis with nasal polyps. Allergy. 2015;70(9):1169–1180. doi:10.1111/all.12667
58. Nagarkar DR, Poposki JA, Tan BK, et al. Thymic stromal lymphopoietin activity is increased in nasal polyps of patients with chronic rhinosinusitis. J Allergy Clin Immunol. 2013;132(3):593–600.e512. doi:10.1016/j.jaci.2013.04.005
59. Gholipour M, Mikaeli J, Mowla SJ, et al. Identification of differentially expressed microRNAs in primary esophageal achalasia by next-generation sequencing. Turk J Biol. 2021;45(3):262–274. doi:10.3906/biy-2101-61
60. Lin L, Cai J. Circular RNA circ-EGLN3 promotes renal cell carcinoma proliferation and aggressiveness via miR-1299-mediated IRF7 activation. J Cell Biochem. 2020;121(11):4377–4385. doi:10.1002/jcb.29620
61. Zhang G, Wang J, Tan W, et al. Circular RNA EGLN3 silencing represses renal cell carcinoma progression through the miR-1224-3p/HMGXB3 axis. Acta Histochem. 2021;123(6):151752. doi:10.1016/j.acthis.2021.151752
62. Xia Y, Wei K, Yang FM, et al. miR-1260b, mediated by YY1, activates KIT signaling by targeting SOCS6 to regulate cell proliferation and apoptosis in NSCLC. Cell Death Dis. 2019;10(2):112. doi:10.1038/s41419-019-1390-y
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.