Back to Journals » Drug Design, Development and Therapy » Volume 13
Icariin Alleviates IL-1β-Induced Matrix Degradation By Activating The Nrf2/ARE Pathway In Human Chondrocytes
Authors Zuo S, Zou W, Wu RM, Yang J, Fan JN, Zhao XK, Li HY
Received 27 January 2019
Accepted for publication 29 October 2019
Published 21 November 2019 Volume 2019:13 Pages 3949—3961
DOI https://doi.org/10.2147/DDDT.S203094
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Manfred Ogris
Shi Zuo,1,* Wei Zou,2,3,* Rong-Min Wu,4 Jing Yang,5 Jian-Nan Fan,2 Xue-Ke Zhao,5 Hai-Yang Li1
1Department of Hepatobiliary Surgery, The Hospital Affiliated to Guizhou Medical University, Guiyang, Guizhou, People’s Republic of China; 2Department of Sports Medicine, The Hospital Affiliated to Guizhou Medical University, Guiyang, Guizhou, People’s Republic of China; 3Department of Orthopedics, The Fourth People’s Hospital of Guiyang, Guizhou, People’s Republic of China; 4Department of Ultrasonography, The Maternity Hospital of Guizhou, Guiyang, Guizhou, People’s Republic of China; 5Department of Infectious Disease, The Hospital Affiliated to Guizhou Medical University, Guiyang, Guizhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Hai-Yang Li
Department of Hepatobiliary Surgery, The Hospital Affiliated to Guizhou Medical University, No.28, Guiyi Street, Guiyang, Guizhou 550004, People’s Republic of China
Email [email protected]
Jian-Nan Fan
Department of Sports Medicine, The Hospital Affiliated to Guizhou Medical University, No.28, Guiyi Street, Guiyang, Guizhou 550004, People’s Republic of China
Email [email protected]
Objective: Osteoarthritis (OA) is characterized by progressive matrix destruction of articular cartilage. This study aimed to investigate the potential antioxidative and chondroprotective effects and underlying mechanism of Icariin (ICA) in interleukin-1 beta (IL-1β)-induced extracellular matrix (ECM) degradation of OA cartilage.
Methods: Human chondrocyte cell line HC-A was treated with different doses of ICA, and then MTT assay and PI staining were used to estimate ICA-induced chondrocyte apoptosis. Intracellular ROS and superoxide dismutase (SOD) and glutathione peroxidase (GPX) were measured after treatment by IL-1β with or without ICA. The mRNA and protein expression levels of redox transcription factor Nrf2 and the downstream effector SOD-1, SOD-2, NQO-1 and HO-1 were assayed to explore the detailed mechanism by which ICA alleviates ECM degradation. Finally, to expound the role of Nrf2 in ICA-mediated chondroprotection, we specifically depleted Nrf2 in human chondrocytes and then pretreated them with ICA followed by IL-1β.
Results: ICA had no cytotoxic effects on human chondrocytes and 10−9 M was selected as the optimum concentration. ROS induced by IL-1β could drastically activate matrix-degrading proteases and ICA could significantly rescue the matrix degradation and excess ROS generation caused by IL-1β. We observed that ICA activated the Nrf2/ARE pathway, consequently upregulating the generation of GPX and SOD. Ablation of Nrf2 abrogated the chondroprotective and antioxidative effects of ICA in IL-1β-treated chondrocytes.
Conclusion: ICA alleviates IL-1β-induced matrix degradation and eliminates ROS by activating the Nrf2/ARE pathway.
Keywords: icariin, Nrf2 signaling, ROS, human chondrocyte, ECM degradation
Introduction
Osteoarthritis (OA) is a common chronic joint disease characterized by the degradation and destruction of the mesochondrium. The main pathological processes of osteoarthritis include ageing, oxidative stress, inflammation and changes in catabolism-related gene expression.1–3 As demonstrated in several studies, inflammatory responses play a key role in the pathogenesis of OA, owing to the extracellular matrix (ECM) degradation caused by inflammatory mediators.4,5 An increasing number of studies have demonstrated that cellular inflammation and ECM degradation are crucial for the progression of OA.
Interleukin-1 (IL-1) was the first interleukin to be identified and has served as a ground-breaking molecule with implications extending far beyond its extended family. IL-1 affects virtually all cells and organs and is a major pathogenic mediator of autoinflammatory, autoimmune, infectious and degenerative diseases. The effect of IL-1 on the central nervous system include fever and activation of the hypothalamus-pituitary-adrenal (HPA) axis. Cortisol downstream of HPA axis has a regulatory function on innate immunity and inflammation.6–10
IL-1α and IL-1β, are encoded by distinct genes, bind to the same receptor (IL-1R1) and have similar biological properties. However, distinctions do exist and impact on immunity, inflammation and cancer. The IL-1α precursor is constitutively present in epithelial layers of the entire gastrointestinal tract, lung, liver, kidney, endothelial cells, and astrocytes. Upon cell death by necrosis, as occurs in ischemic diseases such as myocardial infarction, stroke, acute renal failure and tumor necrosis, the IL-1α precursor is released. Unlike the IL-1β precursor, the IL-1α precursor is fully active and functions as an “alarmin” by rapidly initiating a cascade of inflammatory cytokines and chemokines, which accounts for sterile inflammation.11,12 Thus, IL-1α mediates the early phases of sterile inflammation. In addition to the IL-1α precursor released from necrotic cells, there is a membrane form of IL-1α present on activated monocytes. However, circulating IL-1α is rarely detected even in persons with severe infections but is contained in apoptotic bodies released from endothelial cells.13
IL-1β, a key pro-inflammatory cytokine that signals primarily through the type 1 IL-1 receptor (IL-1R1), plays an important role in OA. IL-1β is produced as a 269-amino-acid precursor that is cleaved by IL-1β-converting enzyme (ICE) to the active IL-1β form that is secreted.14 Some studies have shown that IL-1 signaling is opposed by the naturally occurring peptide IL-1 receptor antagonist, which is a therapeutic agent for the treatment of arthritis15 The IL-1β signaling cascade represents a highly conserved response to various pathological processes, including OA. Conversely, the IL-1β pathway can, in turn, aggravate OA by accelerating ECM degradation and inducing excess ROS generation and the expression of prostaglandin E2 (PGE2) and nitric oxide (NO).16,17 Hence, in this study, we used IL-1β to simulate the in vitro inflammatory response of chondrocytes to septic arthritis. In addition, we found that IL-1β-induced inhibition of pathophysiological processes, such as ECM degradation, was an effective strategy for the treatment of OA.
NF-E2-related factor 2 (Nrf2) is a sensitive redox transcription factor in the leucine zipper family, and Kelch-like ECH-associated protein 1 (Keap1) is its specific repressor. Nrf2/Keap1 signaling mediates cellular responses to oxidative stresses and a series of electrophilic xenobiotics.18–20 Under normal conditions, Nrf2 exists in the cytoplasm, combining with Kelch-like epoxy chloropropane-related protein 1 (Keap1) in the form of a dipolymer. However, in response to an oxidative stress source, the configuration of Keap1 changes, leading to the dissociation of Nrf2 in the cytoplasm. Nrf2 is then phosphorylated for transfer into the nucleus, where it combines with the antioxidant reaction element (ARE) to regulate a variety of antioxidant enzymes and protect the cell, including activation of the expression of SOD-1, SOD-2, HO-1 and NQO-118,21 A great deal of research has shown that the Nrf2/Keap1 pathway is actually responsible for defending tissues against the effects of chronic inflammation and cancer by preventing oxidative stress.19,22
Herba epimedium, a perennial herb of the berberidaceae that is rich in flavonoids and lignin compounds, has validated curative effects in the treatment of several age-related diseases including osteoporosis, sexual dysfunction and OA.23,24 Icariin (ICA) (chemical structure see Figure 1A) is the major pharmacologically active flavonol diglycoside of herba epimedium, which has been shown to promote matrix formation, including the facilitation of bone matrix generation. Additionally, the anti-inflammatory effect of ICA is known to be mediated through the suppression of NF-kappaB activity and upregulation of SIRT6 enzyme activity in vitro.25–27 Understanding of the chondroprotective effects of ICA is gradually emerging owing to research using a rat osteoarthritis model and or chondrosarcoma cells.28,29 Additionally, ICA has been shown to have an antioxidant effect in human chondrocytes, which might be associated with its chondroprotective effects.30
However, the relationship between antioxidative and chondroprotective effects induced by ICA is not clear. Moreover, the chondroprotective effect of ICA has not been explored in detail, and reports concerning the underlying mechanisms are lacking. Therefore, the present research was undertaken to verify the chondroprotective effects and underlying mechanism of ICA using IL-1β-induced human chondrocytes. Our results demonstrate that ICA inhibits IL-1β-induced matrix degradation and catabolic responses through a reduction of ROS excess generation by induction of the Nrf2/ARE pathway in human chondrocytes.
Materials And Methods
Treatment Of Human Chondrocytes With IL-1β Or ICA
Human chondrocytes-Adult (HC-A) (Sigma, TX, USA) (1×106/well of 6-well plate) were seeded in Dulbecco’s Modified Eagle’s Medium/Nutrient Mixture F-12 (DMEM/F-12) supplemented with 10% fetal calf serum (FCS), 100 units/mL penicillin, and 100 mg/mL streptomycin for 2–3 days after plating. At approximately 80% confluence, they were serum-starved overnight and then treated with different concentrations of ICA (Sigma, TX, USA) for 2 h followed by stimulation with IL-1β (1 ng/mL) (Pepro Tech, NJ, USA). Chondrocytes treated with 0.1% DMSO served as a control.
Cell Culture And MTT Assay
HC-A were cultured in a humidified incubator at 37 °C and 5% CO2 in 96-well plates. The cells (1 × 106 cells mL-1) were maintained in Dulbecco Minimal Eagle’s Medium (Gibco, Carlsbad, CA, USA) plus 10% fetal bovine serum (Australia origin, Gibco, Carlsbad, CA, USA). ICA (10−10~10−7 M, 72 h) or simple saline (control) was added to the HC-A cultures. Cell viability was assessed using a modified MTT assay. Briefly, 10 μl of MTT solution (5 mg/mL in phosphate-buffered saline) was added to the 96-well plates and incubated continually for 4 h at 37 °C. The MTT solution was then removed, and formazan dye was dissolved in dimethyl sulfoxide (Sigma-Aldrich) for 10 min by shaking. The absorbance values were measured at 570 nm using a Bio-Rad Model 450 Microplate Reader (Bio-Rad). The cell viability was indirectly determined by examining the ratio of the absorbance value of ICA-treated cells relative to the control. The final results were determined by analyzing three independent experiments. GAPDH used as control and none of treatment group affect the GAPDH.31
Estimation Of Apoptosis By Propidium Iodide (PI) Staining
Chondrocytes (1×106/well of 6-well plate) were treated with ICA (10−10~10−7 M) for 72 h, and ICA-induced chondrocyte apoptosis was estimated by PI staining. In brief, the chondrocytes were cultured, washed twice with cold PBS, and then incubated with PI for 45 min at 37 °C in the dark. All cells were counterstained with DAPI (1:1000, Invitrogen) at room temperature.
Measurement Of Intracellular Reactive Oxygen Species
Intracellular ROS were determined using the nonfluorescent dye 2′7′-dichlorodihydrofluorescein diacetate (Sigma-Aldrich), which is oxidized by ROS generated by cells into a fluorescent dye 2′,7′-dichlorofluorescin. The control group, IL-1β (1 ng/mL), and IL-1β + NAC (10 mM)-treated HC-A cells were incubated in the presence of 10 μM 2′7′-dichlorodihydrofluorescein diacetate for 20 min. The fluorescence was measured using a BD FACS Aria (Becton, Dickinson and Company, Franklin Lakes, NJ, USA).
Superoxide Dismutase And Glutathione Peroxidase (GPX) Assays
Oxidative stress was assessed by measuring the superoxide dismutase (SOD) and glutathione peroxidase (GPX) levels in the developing YSM. SOD and GPX activities were elucidated using commercial kits (Nanjing, Jiangsu Province, China). The results from the control group, IL-1β (1 ng/mL) or IL-1β + NAC (10 mM)-treated samples were analyzed based on the differences in their respective fluorescein decay curves.
RNA Isolation And qPCR Analysis
Total RNA was isolated from chondrocytes (1×106/well in a 6-well plate) using a TRIzol kit (Invitrogen, USA) according to the manufacturer’s instructions. These chondrocytes were pretreated with NAC (10 mM) or ICA (10−9 M) for 2 h, followed by treatment with IL-1β (1 ng/mL) for 24 h. First-strand cDNA synthesis and the SYBR® Green qPCR assay was performed using a PrimeScript™ RT reagent kit (Takara, Japan). Reverse transcription and amplification reactions were performed in Bio-Rad S1000™ (Bio-Rad, USA) and ABI 7000 thermal cyclers, respectively. Analysis of an invariant endogenous control gene, GAPDH, was performed in parallel to confirm that equal amounts of RNA were used in each reaction. The ratio between the intensity of fluorescently stained bands corresponding to the genes and GAPDH was calculated to quantify the transcript levels of those genes. The RT-qPCR results are representative of three independent experiments. The amplicon sequenced done by Sanger sequencing. GAPDH used as control and none of treatment group affect the GAPDH.
Morphometry Of Chondrogenic Matrix Production By Cultured Cells
Proteoglycan synthesis of cultured cells was evaluated by Alcian blue staining as previously described.32 In short, the cultured cells were fixed in 95% ethanol and then stained with 1% Alcian blue dye (pH 1.0) overnight at room temperature. The stained cartilage nodules that were formed in the presence of 10−9 M ICA or IL-1β were photographed and counted using an inverted microscope (Nikon Eclipse Ti-U, Japan). After treatment, the cultures were examined at 72 h. The average size (area) of the chondrogenic nodules was digitized as the total stain intensity/nodule number. The final results were acquired by analyzing 3 independent experiments.
Glycosaminoglycan (GAG) Assay
After treatment for 72 h, the agarose cell specimens were dissolved and centrifuged at 300 g for 10 mins at 4 °C, which provided a pellet containing cells with their cell-associated matrix. To determine the GAG amounts, the pellets were digested for 12 hrs at 55 °C in papain buffer (200 μg/mL papain in 50 mmol/L EDTA, 5 mmol/L L-cysteine, pH 3.0) (Sigma Chemical, USA). GAG was quantified using the dimethylmethylene blue assay.33 The metachromatic reaction of GAG with dimethylmethylene blue was monitored using a spectrophotometer, and the A540/A595 ratio was used to determine the amount of GAG using chondroitin sulfate C (Sigma Chemical, USA) as a standard.
Western Immunoblotting
After treatment with DMSO or IL-1β 1 ng/mL for 24 h, or pretreatment with ICA (10−9 M) for 2 h followed by IL-1β (1 ng/mL) for 24 h, chondrocytes were harvested and proteins isolated using an extraction kit (Beyotime Institute of Biotechnology, Jiangsu, China), separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis, and transferred onto polyvinylidene fluoride membranes (Bio-Rad, USA). Membranes were blocked in 5% bull serum albumin (Sigma, TX, USA) for 2 h and then incubated with primary antibodies overnight at 4 °C. Blots were then incubated with horseradish peroxidase-conjugated secondary antibody, followed by washing with TBST (Bio-Rad, USA). Blots were developed using Luminata Western HRP substrate (EMS Millipore, USA), and antibody reactive proteins were visualized by chemiluminescence and imaged using the Pxigel imaging system (Syngene, Frederick, MD).
siRNA-Mediated Depletion Of Nrf2 Expression Using Nucleofection
Human chondrocytes were transfected with 100 nM Nrf2 siRNA (SMART pool: ON-TARGET plus NFE2L2 siRNA Dharmacon, Lafayette, CO, USA) or MISSION® siRNA Universal Negative Controls (Sigma Aldrich, St. Louis, MO) using the P3 Primary Cell 4D-Nucleofector™ X Kit on 4D-Nucleofector equipment (Lonza, Walkersville, MD) following the instructions provided by the manufacturers. Transfected cells were plated in 6-well plates. Forty-eight hours after transfection, the cells were serum-starved overnight, followed by treatment with ICA (10−9 M) for 2 h, and then stimulation with IL-1β (1 ng/mL) in serum-free medium for 24 h. Gene expression levels were measured by Western blotting.
Statistical Analyses
The values are presented as the mean±SD, and statistically significant differences between the experimental groups and controls were analyzed by one-way ANOVA followed by post hoc analyses using the Tukey’s test. Unless otherwise noted, each experiment was repeated three times using three independent samples. P<0.05 was considered statistically significant.
Results
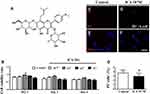
ICA At 10−9 M Exerts No Cytotoxic Effect On The Viability Of Human Chondrocytes
Icariin (chemical structure see Figure 1A) (Sigma, TX, USA), the major pharmacologically active flavonol diglycoside of Herba Epimedii, has potential chondroprotective effects, as previously reported.28,34 To confirm the chondrogenic phenotype of a monolayer culture of human chondrocytes-adult (HC-A) (Sigma-Aldrich, USA) treated with ICA, we examined the viability of the chondrocytes using the MTT assay. Compared with the untreated control, ICA treatment (10−10~10−7 M, 72 h) had no effect on chondrocyte viability at day 1, day 2 or day 3 (Figure 1B). Furthermore, the absence of a cytotoxic effect of ICA was confirmed by PI staining at 24 h (Figure 1C–F), and no increases in apoptosis or necrosis were observed in chondrocytes incubated with ICA (10−9 M) for 24 h compared with untreated control chondrocytes (Figure 1G).
IL-1β-Induced ROS Production Plays An Important Role In The Activation Of Genes Or Proteases Related To Matrix Degradation
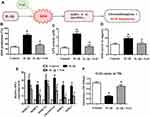
Several studies have demonstrated that ROS production is the crucial pathophysiological change during inflammation progression in articular cartilage.35,36 In our study, the intracellular ROS production assay demonstrated that IL-1β could significantly increase ROS generation after treatment for 24 h, which was clearly inhibited by NAC (Figure 2B). These chondrocytes were pretreated with NAC (10 mM) for 2 h followed by IL-1β (1 ng/mL) for 24 h or IL-1β alone. The results obtained for GPX and SOD activity further reinforced this mechanism (Figure 2C and D).
N-acetylcysteine (NAC), an admittedly efficacious scavenger of ROS, has been utilized to demonstrate whether ROS production is necessary for the activation of genes related to ECM degradation and GAG disintegration.37,38 Therefore, to investigate whether IL-1β-induced ROS generation was the main cause of the activation of matrix-degrading proteases in chondrocytes, we performed qRT-PCR to examine the expression of genes related to matrix degradation.
Our results showed that IL-1β-stimulated human chondrocytes presented a significant upregulation of the mRNA expression of MMP-3, −9, −13 and ADAMTS-4, whereas treatment with NAC clearly reversed this effect, especially for the expression of MMP-13 and ADAMTS-4 (Figure 2E). These chondrocytes were pretreated with NAC (10 mM) for 2 h followed by IL-1β (1 ng/mL) for 24 h or with IL-1β alone. In addition, the results of the GAG assay further supported that the reduction of ROS could rescue the synthesis of GAG (Figure 2F). The chondrocytes used in the GAG assay were pretreated with NAC (10 mM) for 2 h followed by IL-1β (1 ng/mL) for 24 h or IL-1β alone, and then cultured in a humidified incubator at 37 °C and 5% CO2 for an additional 48 h. Taken together, these results suggested that the activation of genes or proteases related to matrix degradation is principally caused by IL-1β-induced ROS instead of a direct effect of IL-1β.
ICA Can Significantly Reduce The Matrix Degradation Caused By IL-1β
ICA (chemical structure shown in Figure 1A), the major pharmacologically active flavonol diglycoside of Herba Epimedii as shown in some studies, has certain chondroprotective effects;30,34 however, the specific underlying mechanism is still unknown.
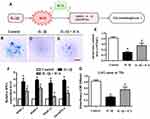
The chondrocyte micromass culture model has been widely used to simulate cartilaginous ECM formation. In micromass cultures, chondrogenesis is initiated when the chondrocytes begin to condense and aggregate to form large nodules.35,36,39 These nodules appear morphologically like cartilage and will begin to produce an extracellular matrix (ECM) after several days of culture.40 Thus, this model was employed to investigate whether ICA could reduce the matrix degradation caused by IL-1β, as identified by Alcian blue staining.
In our study, the chondrocytes were treated with DMSO, or IL-1β 1 ng/mL for 24 h, or pretreated with ICA (10−9 M) for 2 h followed by IL-1β (1 ng/mL) for 24 h, and then cultured in a humidified incubator at 37 °C and 5% CO2 in 6-well plates for an additional 48 h and stained with Alcian blue. In the presence of IL-1β (1 ng/mL), a dramatic reduction in nodule formation was observed in the cultures (Figure 3B and C), implying that IL-1β could inhibit ECM secretion and chondrogenesis. In contrast, ICA reversed the inhibitory effect of IL-1β on ECM secretion (Figure 3D). We also quantitatively determined the extent of ECM formation by measuring the area of Alcian blue staining after exposure to IL-1β or ICA for 72 h (Figure 3E), which further revealed that ICA could exert a chondroprotective effect by reducing the ECM reduction caused by IL-1β.
To further assess whether this phenomenon was involved in the change in matrix-degrading related genes, we performed quantitative PCR. The results demonstrated that IL-1β stimulation of human chondrocytes led to a significant upregulation of the mRNA expression of MMP-3, MMP-9, MMP-13 and ADAMTS-4, whereas treatment with ICA, conversely, exerted a suppressive effect on the expression of all these enzymes (Figure 3F). GAG synthesis was significantly inhibited when chondrocytes were treated with IL-1β, and the inhibitory of IL-1β on GAG synthesis was reversed when chondrocytes were pretreated with ICA for 2 h before addition of IL-1β (Figure 3G).
Taken together these results demonstrated that ICA could be an effective inhibitory agent for the suppression of matrix-degrading proteases in IL-1β-induced chondrocytes.
ICA Activates The Redox Sensitive Transcription Factor Nrf2 In Human Chondrocytes
As mentioned previously, although the chondroprotective effect of ICA has been affirmed, the detailed mechanism by which ICA alleviates ECM degradation is still unknown.
Since ROS is the main contributing factor to ECM degradation under IL-1β induction as proved (Figure 2A), and ICA can obviously suppress the expression of matrix-degrading proteases (Figure 3A), we hypothesized that treatment with ICA might reduce ECM degradation by means of alleviating oxidative stress and eliminating ROS.
As reported in a large body of literature, Nrf2 is a crucial redox transcription factor that functions in restraining oxidative stress.41–44 To examine whether ICA achieves its chondroprotective function by activating the Nrf2/ARE pathway and eliminating ROS, we examined the expression of the Nrf2/ARE pathway at both mRNA and protein levels.
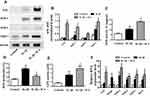
First, we measured the activation of the Nrf2/ARE pathway in human chondrocytes by detecting protein synthesis by Western blotting. The chondrocytes were treated with DMSO or IL-1β 1 ng/mL for 24 h or pretreated with ICA (10−9 M) for 2 h followed by IL-1β (1 ng/mL) for 24 h and then harvested and subjected to protein isolation. A significant upregulation of Nrf2 and its downstream proteases was detected after ICA treatment (Figure 4A and B). In addition, ICA could eliminate a considerable amount of ROS (Figure 4D). Furthermore, it dramatically promoted SOD and GPX viability, which may have led to the reduced ROS levels (Figure 4C and E).
Finally, to further substantiate the role of ICA in the Nrf2 pathway at mRNA level, RT-qPCR was performed to assess Nrf2 and the downstream expression of Nrf2 effectors in human chondrocytes treated with DMSO or IL-1β 1 ng/mL for 24 h or pretreated with ICA (10−9 M) for 2 h followed by IL-1β (1 ng/mL) for 24 h. The results indicated that treatment with ICA could considerably induce the mRNA expression of SOD-1, SOD-2, NQO-1 and their downstream target gene HO-1 in human chondrocytes, the downregulation of Keap1 also indicated that ICA could inhibit formation of the Nrf2/Keap1 dipolymer (Figure 4F).
Corroborating these data, we can assume that ICA can activate Nrf2/ARE pathway and, consequently, eliminate ROS, likely eventually alleviating the ECM degradation.
Primer sequence done by Sanger sequencing are mentioned below.
GAPDH (202bp)
F:5- TGTTGCCATCAATGACCCCTT-3
R:5- CTCCACGACGTACTCAGCG-3
IL1β (148bp)
F: TCAAACCTCTGGAGGAAGTGC
Genetic Ablation Of Nrf2 Abrogates The Chondroprotective And Antioxidative Effects Of ICA In IL-1β-Treated Chondrocytes
Although we showed that ICA could activate the Nrf2/ARE pathway and reduce ROS production to alleviate matrix degradation, whether the Nrf2/ARE pathway played a prominent role in the function of ICA as an inhibitor of ECM degradation was still unknown.
To expound the role of Nrf2 in ICA-mediated chondroprotection, we specifically depleted Nrf2 in human chondrocytes and then pretreated them with ICA for 2 h followed by IL-1β (1 ng/mL) for 24 h (Figure 5A and B). As demonstrated by the Western blotting results, siRNA-mediated depletion of Nrf2 significantly abrogated the ICA-induced suppression of MMP-13 (Figure 5A and D), and the GAG assay result further confirmed that the chondroprotective effect of ICA could be abrogated following depletion of the Nrf2 gene (Figure 5F).
In addition, the reduction of IL-1β-induced ROS caused by ICA also disappeared following depletion of the Nrf2 gene (Figure 5E). Moreover, ICA-induced promotion of NQO-1 protein was also abrogated when Nrf2 was depleted (Figure 5A and C). Finally, the GPX and SOD activity results further substantiated the dramatic abrogation of the antioxidative effect of ICA following Nrf2 gene depletion (Figure 5G and H). Taken together, these results demonstrate that the Nrf2 pathway plays a key role in the ICA-mediated chondroprotective and antioxidative effects.
Discussion
Under physiological conditions, articular cartilage is in a state of homeostasis, consisting of chondrocytes and ECM, which is a strictly avascular environment with a low oxygen supply.45,46 However, oxidative stress can be triggered by various adverse factors, like inflammation, causing tremendous production of ROS. During pathological conditions, oxygen tension in synovial fluid is subject to fluctuation as a consequence of ischemia-reperfusion, pathological acceleration of tissue metabolism and sustained abnormal strains on the joint.47,48 The excess ROS can then lead to clear destruction of the cartilage matrix via several intracellular pathways, including cytokine receptors, receptor tyrosine kinases, receptor serine/threonine kinases and G protein coupled receptors. In addition, ROS have been shown to activate mitogen-regulated kinase (MAPK) pathways in several systems, including extracellular signal-regulated kinase (ERK)1/2, Jun-NH2-terminal kinase and P38 MAPK cascades.40,49 Ultimately, various intracellular signaling events result in matrix degradation by mediating the activation of latent collagenase, which is involved in the upregulation of the matrix metalloproteinases (MMPs) family.50,51
However, the cause of the increased expression of MMPs and ADAMTS-4 resulting from inflammation or subsequent ROS production necessitated further study. Hence, we investigated the effects of ROS induced by IL-1β on ECM degradation and found, using NAC (an orthodox ROS scavenger), that the expression of MMPs and ADAMTS-4 mainly arose from ROS rather than a direct effect of IL-1β (Figure 2).
ICA, a kind of plant-derived agent, has received considerable attention for its potential to serve as a substitute therapy for the treatment of OA. ICA exhibits antioxidative properties in various diseases,52–54 including diabetes and osteoarthritis. These findings suggest that ICA may have therapeutic potential for the promotion of ECM synthesis, which is significant to the treatment of OA.
Therefore, after elucidating the relationship between ROS generation and MMP activation (Figure 2), we exposed IL-1β-treated chondrocytes to ICA. The results demonstrated that ICA could significantly alleviate the matrix degradation caused by IL-β (Figure 3), in accord with previous reports. To further investigate the mechanism by ICA rescued the matrix degradation, we employed a series of assays targeting intracellular ROS generation. The results revealed that the excess ROS generation could be reduced by ICA (Figure 4). Additionally, ICA activated the Nrf2/ARE pathway, consequently upregulating the generation of GPX and SOD, which strongly suggested that Nrf2/ARE signaling was involved in the mechanism of ICA-mediated chondroprotection (Figure 4). Taken together, these results indicate that ICA may alleviate matrix degradation by activating Nrf2/ARE signaling and eliminating excrescent ROS.
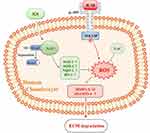
Nevertheless, the actual mechanism of chondroprotection mediated by ICA can be fairly complex, and it may include other factors except the activation Nrf2/ARE signaling. Hence, whether activation of the Nrf2/ARE pathway is necessary for the inhibition of matrix-degrading is a crucial issue. Therefore, to expound the role of Nrf2/ARE signaling in ICA-mediated chondroprotection, we eventually resorted to transfection experiments. The results demonstrated that ablation of Nrf2 completely abrogated the chondroprotective and antioxidative effects of ICA in IL-1β-treated chondrocytes (Figure 5), substantiating our hypothesis (Figure 6).
In conclusion, our research shows that ICA can activate Nrf2 to decrease oxidative stress, consequently reducing the degradation of cartilage matrix and revealing the cartilage-protective and antioxidation functions of ICA, based on activation of the Nrf2 signaling pathways. These findings offer an important theoretical basis for the admission of ICA to the clinical treatment of joint diseases. However, its clinical application requires further research, and further studies are also needed to determine whether and how ICA activates Nrf2 upstream signaling molecules.
Acknowledgment
This work was supported by National Natural Science Foundation of China (81560104 and 81860115 to Xue-Ke Zhao).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Felson DT, Anderson JJ, Naimark A, et al. The prevalence of knee osteoarthritis in the elderly. The Framingham Osteoarthritis Study. Arthritis Rheum. 1987;30:914–918. doi:10.1002/art.1780300811
2. Fernandes JC, Martel-Pelletier J, Pelletier JP. The role of cytokines in osteoarthritis pathophysiology. Biorheology. 2002;39:237–246.
3. Kapoor M, Martel-Pelletier J, Lajeunesse D, et al. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2011;7:33–42. doi:10.1038/nrrheum.2010.196
4. Hayami T, Funaki H, Yaoeda K, et al. Expression of the cartilage derived anti-angiogenic factor chondromodulin-I decreases in the early stage of experimental osteoarthritis. J Rheumatol. 2003;30:2207–2217.
5. Martel-Pelletier J, Alaaeddine N, Pelletier JP. Cytokines and their role in the pathophysiology of osteoarthritis. Front Biosci. 1999;4:694–703. doi:10.2741/A387
6. Dinarello CA, Arend W, Sims J, et al. IL-1 family nomenclature. Nat Immunol. 2010;11:973. doi:10.1038/ni1110-973
7. Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–550. doi:10.1146/annurev.immunol.021908.132612
8. Dinarello CA, Simon A, Van der Meer JW. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nature Rev Drug Discov. 2012;11:633–652. doi:10.1038/nrd3800
9. Gabay C, Lamacchia C, Palmer G. IL-1 pathways in inflammation and human diseases. Nature Rev Rheumatol. 2010;6:232–241. doi:10.1038/nrrheum.2010.4
10. Sims JE, Smith DE. The IL-1 family: regulators of immunity. Nat Rev Immunol. 2010;10:89–102. doi:10.1038/nri2691
11. Chen CJ, Kono H, Golenbock D, et al. Identification of a key pathway required for the sterile inflammatory response triggered by dying cells. Nat Med. 2007;13:851–856. doi:10.1038/nm1603
12. Rider P, Carmi Y, Guttman O, et al. IL-1α and IL-1β recruit different myeloid cells and promote different stages of sterile inflammation. J Immunol. 2011;187:4835–4843. doi:10.4049/jimmunol.1102048
13. Berda-Haddad Y, Robert S, Salers P, et al. Sterile inflammation of endothelial cell-derived apoptotic bodies is mediated by interleukin-1alpha. Proc Natl Acad Sci USA. 2011;108:20684–20689. doi:10.1073/pnas.1116848108
14. Dinarello CA. The IL-1 family and inflammatory diseases. Clin Exp Rheumatol. 2002;20:1–13.
15. Palomo J, Dietrich D, Martin P, et al. The interleukin (IL)-1 cytokine family–balance between agonists and antagonists in inflammatory diseases. Cytokine. 2015;76:25–37. doi:10.1016/j.cyto.2015.06.017
16. Haddad JJ, Safieh-Garabedian B, Saadé NE, et al. Chemioxyexcitation (delta pO2/ROS)-dependent release of IL-1 beta, IL-6 and TNF-alpha: evidence of cytokines as oxygen-sensitive mediators in the alveolar epithelium. Cytokine. 2001;13:138–147. doi:10.1006/cyto.2000.0789
17. Shin HC, Hwang HJ, Kang KJ, et al. An antioxidative and antiinflammatory agent for potential treatment of osteoarthritis from Ecklonia cava. Arch Pharm Res. 2006;29:165–171. doi:10.1007/BF02974279
18. Motohashi H, Yamamoto M. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol Med. 2004;10:549–557. doi:10.1016/j.molmed.2004.09.003
19. Sykiotis GP, Bohmann D. Keap1/Nrf2 signaling regulates oxidative stress tolerance and lifespan in Drosophila. Dev Cell. 2008;14:76–85. doi:10.1016/j.devcel.2007.12.002
20. Zhang Z, Zhou S, Jiang X, et al. The role of the Nrf2/Keap1 pathway in obesity and metabolic syndrome. Rev Endocr Metab Disord. 2015;16:35–45. doi:10.1007/s11154-014-9305-9
21. Li J, Ichikawa T, Villacorta L, et al. Nrf2 protects against maladaptive cardiac responses to hemodynamic stress. Arterioscler Thromb Vasc Biol. 2009;29:1843–1850. doi:10.1161/ATVBAHA.109.189480
22. Hintsala HR, Jokinen E, Haapasaari KM, et al. Nrf2/Keap1 pathway and expression of oxidative stress lesions 8-hydroxy-2ʹ-deoxyguanosine and nitrotyrosine in melanoma. Anticancer Res. 2016;36:1497–1506.
23. Ma H, He X, Yang Y, et al. The genus Epimedium: an ethnopharmacological and phytochemical review. J Ethnopharmacol. 2011;134:519–541. doi:10.1016/j.jep.2011.01.001
24. Zhao J, Ohba S, Shinkai M, et al. Icariin induces osteogenic differentiation in vitro in a BMP- and Runx2-dependent manner. Biochem Biophys Res Commun. 2008;369:444–448. doi:10.1016/j.bbrc.2008.02.054
25. Cui J, Zhu M, Zhu S, et al. Inhibitory effect of icariin on Ti-induced inflammatory osteoclastogenesis. J Surg Res. 2014;192:447–453. doi:10.1016/j.jss.2014.05.038
26. Li L, Sun J, Xu C, et al. Icariin ameliorates cigarette smoke induced inflammatory responses via suppression of NF-kappaB and modulation of GR in vivo and in vitro. PLoS One. 2014;9:e102345. doi:10.1371/journal.pone.0102345
27. Zhang Y, Wei Y, Zhu Z, et al. Icariin enhances radiosensitivity of colorectal cancer cells by suppressing NF-kappaB activity. Cell Biochem Biophys. 2014;69:303–310. doi:10.1007/s12013-013-9799-x
28. Zeng L, Wang W, Rong XF, et al. Chondroprotective effects and multi-target mechanisms of Icariin in IL-1 beta-induced human SW 1353 chondrosarcoma cells and a rat osteoarthritis model. Int Immunopharmacol. 2014;18:175–181. doi:10.1016/j.intimp.2013.11.021
29. Zeng L, Rong XF, Li RH, et al. Icariin inhibits MMP1, MMP3 and MMP13 expression through MAPK pathways in IL1betastimulated SW1353 chondrosarcoma cells. Mol Med Rep. 2017;15:2853–2858. doi:10.3892/mmr.2017.6312
30. Sze SC, Tong Y, Ng TB, et al. Herba Epimedii: anti-oxidative properties and its medical implications. Molecules. 2010;15:7861–7870. doi:10.3390/molecules15117861
31. Li M, Zhang Y, Cao Y, et al. Icariin ameliorates palmitate-induced insulin resistance through reducing thioredoxin-interacting protein (TXNIP) and suppressing ER stress in C2C12 myotubes. Front Pharmacol. 2018;9. doi:10.3389/fphar.2018.01180
32. Macrae VE, Davey MG, McTeir L, et al. Inhibition of PHOSPHO1 activity results in impaired skeletal mineralization during limb development of the chick. Bone. 2010;46:1146–1155. doi:10.1016/j.bone.2009.12.018
33. Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986;883:173–177. doi:10.1016/0304-4165(86)90306-5
34. Li C, Li Q, Mei Q, et al. Pharmacological effects and pharmacokinetic properties of icariin, the major bioactive component in Herba Epimedii. Life Sci. 2015;126:57–68. doi:10.1016/j.lfs.2015.01.006
35. Han Y, Li X, Yan M, et al. Oxidative damage induces apoptosis and promotes calcification in disc cartilage endplate cell through ROS/MAPK/NF-kappaB pathway: implications for disc degeneration. Biochem Biophys Res Commun. 2017;0435:S0006291X17305788.
36. Jallali N, Ridha H, Thrasivoulou C, et al. Vulnerability to ROS-induced cell death in ageing articular cartilage: the role of antioxidant enzyme activity. Osteoarthritis Cartilage. 2005;13:614–622. doi:10.1016/j.joca.2005.02.011
37. Downs I, Liu J, Aw TY, et al. The ROS scavenger, NAC, regulates hepatic Valpha14iNKT cells signaling during Fas mAb-dependent fulminant liver failure. PLoS One. 2012;7:e38051. doi:10.1371/journal.pone.0038051
38. Shimamoto K, Hayashi H, Taniai E, et al. Antioxidant N-acetyl-L-cysteine (NAC) supplementation reduces reactive oxygen species (ROS)-mediated hepatocellular tumor promotion of indole-3-carbinol (I3C) in rats. J Toxicol Sci. 2011;36:775–786. doi:10.2131/jts.36.775
39. Delise AM, Tuan RS. Analysis of N-cadherin function in limb mesenchymal chondrogenesis in vitro. Dev Dyn. 2002;225:195–204. doi:10.1002/(ISSN)1097-0177
40. Mello MA, Tuan RS. High density micromass cultures of embryonic limb bud mesenchymal cells: an in vitro model of endochondral skeletal development. In Vitro Cell Dev Biol Anim. 1999;35:262–269. doi:10.1007/s11626-999-0070-0
41. Marchev AS, Dimitrova PA, Burns AJ, et al. Oxidative stress and chronic inflammation in osteoarthritis: can NRF2 counteract these partners in crime? Ann N Y Acad Sci. 2017;1401:114–135. doi:10.1111/nyas.2017.1401.issue-1
42. Qiu L, Song Z, Setaluri V. Oxidative stress and vitiligo: the Nrf2-ARE signaling connection. J Invest Dermatol. 2014;134:2074–2076. doi:10.1038/jid.2014.241
43. Abdul-Aziz A, MacEwan DJ, Bowles KM, et al. Oxidative stress responses and NRF2 in human leukaemia. Oxid Med Cell Longev. 2015;2015:1–7. doi:10.1155/2015/454659
44. Akino N, Wada-Hiraike O, Terao H, et al. Activation of Nrf2 might reduce oxidative stress in human granulosa cells. Mol Cell Endocrinol. 2017;470:S0303720717305221.
45. Huber M, Trattnig S, Lintner F. Anatomy, biochemistry, and physiology of articular cartilage. Invest Radiol. 2000;35:573–580. doi:10.1097/00004424-200010000-00003
46. Lai WM, Hou JS, Mow VC. A triphasic theory for the swelling and deformation behaviors of articular cartilage. J Biomech Eng. 1991;113:245–258. doi:10.1115/1.2894880
47. Blake DR, Merry P, Unsworth J, et al. Hypoxic-reperfusion injury in the inflamed human joint. Lancet. 1989;1:289–293. doi:10.1016/S0140-6736(89)91305-6
48. Merry P, Grootveld M, Lunec J, et al. Oxidative damage to lipids within the inflamed human joint provides evidence of radical-mediated hypoxic-reperfusion injury. Am J Clin Nutr. 1991;53:362–369. doi:10.1093/ajcn/53.1.362S
49. Sauer H, Wartenberg M, Hescheler J. Reactive oxygen species as intracellular messengers during cell growth and differentiation. Cell Physiol Biochem. 2001;11:173–186. doi:10.1159/000047804
50. Li T, Xiao J, Wu Z, et al. Transcriptional activation of human MMP-13 gene expression by c-Maf in osteoarthritic chondrocyte. Connect Tissue Res. 2010;51:48–54. doi:10.3109/03008200902989104
51. Tetsunaga T, Nishida K, Furumatsu T, et al. Regulation of mechanical stress-induced MMP-13 and ADAMTS-5 expression by RUNX-2 transcriptional factor in SW1353 chondrocyte-like cells. Osteoarthritis Cartilage. 2011;19:222–232. doi:10.1016/j.joca.2010.11.004
52. Bao H, Chen L. Icariin reduces mitochondrial oxidative stress injury in diabetic rat hearts. Chin Med Mag China. 2011;36:1503–1507.
53. Tang Y, Jacobi A, Vater C, et al. Icariin promotes angiogenic differentiation and prevents oxidative stress-induced autophagy in endothelial progenitor cells. Stem Cells. 2015 ;33:1863–1877. doi:10.1002/stem.2005
54. Ye R, Xu S, Liu Y, et al. Protective effect of Icariin on the development of preimplantation mouse embryos against hydrogen peroxide-induced oxidative injury. Oxid Med Cell Longev. 2017;2017:2704532
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.