Back to Journals » Drug Design, Development and Therapy » Volume 16
Ibrutinib-Associated Cardiotoxicity: From the Pharmaceutical to the Clinical
Authors Dong R, Yan Y, Zeng X, Lin N, Tan B
Received 7 June 2022
Accepted for publication 6 September 2022
Published 20 September 2022 Volume 2022:16 Pages 3225—3239
DOI https://doi.org/10.2147/DDDT.S377697
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Jianbo Sun
Rong Dong,1 Youyou Yan,2 Xiaokang Zeng,3 Nengming Lin,1,2 Biqin Tan1
1Department of Clinical Pharmacy, Key Laboratory of Clinical Cancer Pharmacology and Toxicology Research of Zhejiang Province, Affiliated Hangzhou First People’s Hospital, Zhejiang University School of Medicine, Hangzhou, 310006, People’s Republic of China; 2Translational Medicine Research Center, Affiliated Hangzhou First People’s Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang, 31006, People’s Republic of China; 3Department of Critical Care Medicine, Affiliated Hangzhou First People’s Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang, 31006, People’s Republic of China
Correspondence: Biqin Tan, Department of Clinical Pharmacy, Affiliated Hangzhou First People’s Hospital, Zhejiang University School of Medicine, Room 207, No. 5 Building, Hangzhou, People’s Republic of China, Tel +86-571-56007824, Fax +86-571-56005600, Email [email protected] Nengming Lin, Department of Clinical Pharmacy, Affiliated Hangzhou First People’s Hospital, Zhejiang University School of Medicine, Room 903, No. 7 Building, Hangzhou, People’s Republic of China, Tel/Fax +86-571-56005600, Email [email protected]
Abstract: Ibrutinib is the first-in-class Bruton tyrosine kinase (BTK) inhibitor that has revolutionized the treatment of B cell malignancies. Unfortunately, increased incidences of cardiotoxicity have limited its use. Despite over a decade of research, the biological mechanisms underlying ibrutinib cardiotoxicity remain unclear. In this review, we discuss the pharmacological properties of ibrutinib, the incidence and mechanisms of ibrutinib-induced cardiotoxicity, and practical management to prevent and treat this condition. We also synopsize and discuss the cardiovascular adverse effects related to other more selective BTK inhibitors, which may guide the selection of appropriate BTK inhibitors.
Keywords: ibrutinib, cardiotoxicity, BTK inhibitors, atrial fibrillation, cardio-oncology
Introduction
The use of oral tyrosine kinase inhibitors (TKIs) is becoming increasingly common in cancer therapies because of their convenience and patient preference.1,2 However, idiosyncratic adverse events of this class of medications are relatively unknown to physicians and pharmacists.3
Bruton tyrosine kinase (BTK) is a 659 amino acid enzyme that is mainly involved in the catalyzation of phosphate groups from ATP to tyrosine residues.4 BTK consists of five domains, each having the potential to critically regulate the interactions involved in intracellular signaling.5 The PH domain can bind to several crucial signaling proteins, such as protein kinase C (PKC) and the prolyl isomerase Pin1, both of which are negative regulators of BTK.6 As a metalloprotein enzyme requiring Zn2+ for optimal activity and stability,7,8 the TH domain in BTK contains a highly conserved zinc finger motif. Together with the kinase domain, the SH2 domain forms an allosteric interface that is critical for BTK activation.9 Although tyrosine Y223 phosphorylation in the SH3 domain is important, it is Y551 in the kinase domain initiates the catalytic activation of BTK upon the upstream kinase SYK or SRC.10 The cysteine residue at position 481 (C481), which is also located in the kinase domain, is the main binding site for small-molecule chemical inhibitors.11 BTK is predominately expressed in immune cells such as B cells, macrophages, and mast cells, but not in T lymphocytes.12,13 As such, targeted inhibition of BTK has revolutionized the treatment landscape of B cells’ malignancies.14
Ibrutinib is a once-daily oral medication that acts as a BTK inhibitor by irreversibly and covalently binding to C481 of the BTK kinase domain.11 Ibrutinib has profoundly transformed the treatment landscape of B cell malignancies. Phase 3RESONATE and RESONATE-2 trials demonstrated the superiority of ibrutinib over ofatumumab, a CD20 antibody, in relapsed/refractory chronic lymphocytic leukemia (CLL) and over chlorambucil in previously untreated older patients with CLL or small lymphocytic lymphoma (SLL).15,16 The final analysis of the RESONATE study, which had a median follow-up of 65.3 months, revealed that the median progression-free survival (PFS) of patients in the ibrutinib arm remained significantly longer than that of patients in the ofatumumab arm by approximately 36.1 months.17 Furthermore, the 5-year follow-up of RESONATE-2 study demonstrated consistent benefit in patients with high prognostic risk, mainly in those with TP53 mutation, 11q deletion, and/or unmutated IGHV. The overall response rate was 92% in the ibrutinib arm.18,19 In addition, the results of a Phase 2 study that included 111 patients revealed that ibrutinib exhibited durable single-agent efficacy in relapsed or refractory mantle cell lymphoma (MCL).20 Updated safety and efficacy results after a median 26.7-month-long follow-up showed that the overall response rate (ORR) was 67%.21 Currently, ibrutinib has been approved as the first-line treatment for CLL and SLL, MCL, marginal zone lymphoma (MZL) and Waldenström macroglobulinemia (WM). Moreover, it has emerged as an interesting therapeutic candidate for coronavirus disease 2019 (COVID-19) owing to the reported elevated levels of BTK activity in critically ill patients.22–24
Despite the significant advantages of ibrutinib in the treatment of B cell neoplasms, its long-term use has resulted in adverse effects. Mediated by both on- and off-target multiple tyrosine kinases, the most common toxicity profile of ibrutinib includes cutaneous toxicity, hypertension infection, diarrhea, arthralgias, cardiotoxicity and bleeding.25,26 These side effects are generally mild. However, bleeding and cardiotoxicity may present as severe and intractable adverse effects.27 A multicenter, retrospective analysis that included 616 ibrutinib-treated CLL patients confirmed that intolerance, rather than progressive disease, is the most common reason for treatment discontinuation with atrial fibrillation (AF) and bleeding being the most common reasons for discontinuation.28–30 AF, characterized by an irregular and often rapid heart rate, is the most common cardiac arrhythmia related to ibrutinib.31–33 In patients treated with ibrutinib, AF incidence in the first 18 months is 3–7%, increasing to 9–16% in a longer follow-up period of up to 60 months. New-onset of AF is associated with a higher risk of thromboembolism, stroke, and heart failure, leading to ibrutinib discontinuation.34–37 The optimal choice of anticoagulation in ibrutinib-treated patients with AF in turn increases the risk of bleeding. In addition, significant drug–drug interactions between ibrutinib and medications that are commonly used to manage AF have been noted.38,39 Ibrutinib treatment is also associated with an increased risk of ventricular arrhythmia and sudden death, with an estimated risk of 2 in 100 person-years in ibrutinib-treated versus 0 in non-ibrutinib-treated CLL patients. Several cases of recurrent polymorphic ventricular tachycardia and sudden death have been reported since the drug approval occasionally.40,41 Although no common features have been observed among the sporadic reports, in the literature, a preclinical study has found parallel myocardial inflammation and fibrosis injury in the animal models.42 A rare case of takotsubo cardiomyopathy (TC) was recently reported.43 This study aims to review the incidence and potential underlying mechanisms of ibrutinib-associated cardiotoxicity, as well as the recommended management options from the pertinent clinical-pharmaceutical aspect.
Pharmacokinetics and Pharmacodynamics of Ibrutinib
The recommended dose of ibrutinib for patients with CLL and WM is 420 mg once daily, and 560 mg orally once daily for patients with MCL who have received at least one prior therapy.44 Pharmacokinetic data of ibrutinib collected from clinical trials demonstrated that the Tmax ranged from 1 to 2 hours and the mean elimination half-time ranged from 4 to 6 hours.45,46 A dose-proportional increase in Cmax and area under the curve (AUC) values was noted with doses up to 840 mg. Based on noncompartmental pharmacokinetic analysis, the geometric mean ibrutinib steady-state daily AUC was 707–1159 (50–72%) ng·h/mL and 865,978 (69–82%) ng·h/mL following 420 mg and 560 mg daily, respectively.47 However, the relationship between plasma/serum ibrutinib concentration and ibrutinib efficacy remains unclear because of the high inter-individual pharmacokinetic variability caused by food–drug or drug–drug interactions. The plasma concentration of ibrutinib increases approximately twofold after consuming a high-fat meal compared with that after an overnight fast.48 Cytochrome P450 (CYP) 3A4 has been identified as the primary enzyme responsible for ibrutinib metabolism, and three major metabolites have been observed. M35 is the hydroxylation product of the phenyl form of ibrutinib, and piperidine opens to form the metabolites M25 and M34. The ethylene on the acryloyl moiety epoxidized and subsequently hydrolyzed to form dihydrodiol, which is known as the metabolite dihydrodiol ibrutinib (DHI).49,50 Current evidences do not clearly show the exact relationship between the plasma level of DHI and the adverse events. However, because DHI has a longer elimination half-life than the parent drug, monitoring DHI levels in addition to ibrutinib, may be a useful evaluation for adherence to therapy.
Characteristics of Ibrutinib-Induced Cardiotoxicity
Cardiac diseases and cancer are two major public health concerns. Emerging evidence supports a strong and independent association between the risk of new-onset cardiac diseases and cancer incidence of cancer therapies. For instance, it has been suggested that there are many drugs that increase the risk of new-onset cardiac diseases.51,52 The magnitude of this risk depends on the choice of therapeutic drugs. Traditional chemotherapy, such as the anthracycline-based cancer drug doxorubicin, can cause heart failure in a dose-dependent manner and decrease the asymptomatic left ventricular ejection fraction by approximately 10–15%.53–55 Increased use of targeted therapies and indefinite inhibition of critical kinases may result in more specific cardiac toxicities. Immune checkpoint inhibitors (ICIs), such as anti-CTLA-4 antibodies or anti-PD-1 monoclonal antibodies, cause fatal toxic myocarditis, with a mortality rate up to 40%.56 Tyrosine kinase inhibitors (TKIs) targeting vascular endothelial growth factor receptor (VEGFR), sunitinib or sorafenib cause hypertension in at least 25% of all patient cases.57
Incidence of Ibrutinib-Induced Atrial Arrhythmia
CLL is the most common type of leukemia, with a significantly higher prevalence in older patients (aged >65 years), especially in the Western countries.58 Ibrutinib, which targets the B cell receptor (BCR) signaling pathway, is an ideal therapeutic candidate for CLL. However, its cardiotoxicity prevents its long-term use in patients with CLL. Atrial arrhythmia (AA) disorder is the most common adverse event in ibrutinib-induced arrhythmias. A retrospective study that quantified the incidence rates and risk of AA in 137 patients treated with ibrutinib and in 106 patients diagnosed with B cell malignancies and treated with chemotherapy confirmed that the use of ibrutinib is an independent risk factor for the incidence of AA.59 Ibrutinib use was also associated with a 5.2-fold increased risk of AA development, 14% in the ibrutinib-treated group versus 3% in the chemotherapy group.
AF is the most common atrial rhythm disorder. The incidence of AF in CLL patients is rapidly increasing with increasing age of this population. Early in 2014, the RESONATE clinical trial demonstrated the superiority of ibrutinib over ofatumumab chemotherapy regardless of age or clinical stage, with an AF incidence as high as 5% (10/195).16 Later, the extended follow-up study revealed that approximately 4% AF cases occurred within the 12-month treatment period. The RESONATE 2 trial studied the efficacy of ibrutinib in patients who were aged ≥65 years, and found that AF occurred in 6% (8/136) of the study population.60 With a median follow-up of 60 months from the RESONATE 2 study, AF with any grade occurred in 16% of patients.15 A pooled analysis from four randomized controlled trials (RESONATE, RESONATE 2, HELIOS, and RAY) reappraised the incidence of ibrutinib-induced-AF.61,62 A total of 1505 patients with CLL and MCL were analyzed, with 756 patients randomized to the ibrutinib group. The pooled rate of AF in the ibrutinib-treated group was 6.5% at the 16.6-month follow-up, and 10.4% at the 36-month follow-up, respectively. An additional updated long-term safety analysis of the RESONATE study reported that 11% (10/94) of the total population had grade 3 AF.62
AF is frequently paroxysmal and often asymptomatic. The prevalence of AF significantly increases from < 0.2% in adults aged < 55 years to 10% those aged ≥85 years, with a median age at CLL diagnosis of approximately 72 years.63–66 Though the latest recommendation statement from the United States Preventive Services Task Force cannot determine the balance of advantages of screening for AF in asymptomatic adults, the potential benefits of early AF detection in patients with cancer with concurrent hypercoagulable changes should be extended beyond stroke prevention.35,67–69 AF is usually accompanied by an increased heart rate.34 The profile of BTK-treated patients presenting with AF exhibits remarkable increases when this condition is intensively screened. Consequently, screening for asymptomatic AF or monitoring the cardiac rhythm before ibrutinib prescription should be considered an excellent recommendation. However, the precise timepoint of AF screening remains uncertain. According to safety analyses from randomized clinical trials (RCTs), ibrutinib-induced AF is more likely to occur in the first 3 months, with a median time of onset of 2.8 months and late onset of 18 months post-medication.47 Therefore, continuous monitoring during the period of long-term inhibitory effect of ibrutinib may guide for future clinical management of this condition.
Incidence of Ibrutinib-Induced Ventricular Arrhythmia
In addition to AF, ventricular arrhythmia (VA) also results in a significant burden of arrhythmia, leading to high rates of medication discontinuation and morbidity in the patient population. The use of ibrutinib has been constantly associated with recurrent polymorphic ventricular tachycardia and sudden death.41 Severe electrical storms have also been thought to be associated with ibrutinib.40 Limited information is available on ibrutinib-induced VA compared with ibrutinib-induced AF. In a single-center retrospective data analysis including 72 patients, 43% were documented with non-sustained ventricular tachycardia. Long-term follow-up of the incidence of symptomatic VAs was investigated using data from patients in the registry cohort. The results revealed that 11/582 patients developed VA symptoms, 7 of whom had a probable association with ibrutinib.70 A disproportionality analysis using the VigiBase database (the EROCA study) identified 0.52% VAs in 13,572 individual case safety reports, with a median onset time of 70 days, ranging from 28.5 to 152.5 days. Furthermore, rare unexplained sudden cardiac death may occur.26 Heart failure was also observed with a median time of 54 days. Concerns about ibrutinib-associated VAs have been emerged as a result of an increasing trend reported in the VigiBase database. Moreover, the occurrence of VAs was associated with older age in the spontaneously hypertensive rats.71
Incidence of Ibrutinib-Induced QT Interval Changes
According to current clinical trials, the effect of ibrutinib on other electrocardiographic parameters has been controversial, and this aspect has thus attracted less attention compared with other cardiac arrhythmias. In the EROCA study, QT interval prolongation was detected in 0.07% patients, and no correlation between the medication and cardiac repolarization was noted.26 However, in another study, Fradley et al retrospectively analyzed 137 patients and found that the incidence of short QT intervals tended to increase after ibrutinib exposure.59 Furthermore, a sequential administration of single doses of therapeutic and super-therapeutic concentrations of ibrutinib revealed that ibrutinib shortened the QTc interval and prolonged the PR interval, which was consistent to the results from the Phase 1b/2 study 1102.72 In conclusion, QT interval change is not strongly correlated with ibrutinib-induced cardiotoxicity.
Incidence of Other Cardiac Complications or Cardiomyopathy
Despite much focus on ibrutinib-induced arrhythmias, little information is available from individual case reports on ibrutinib-associated cardiomyopathy that is irrelevant to arrhythmias. Reversible nonischemic cardiomyopathy was reported to be associated with the use of ibrutinib. Systolic dysfunction, deterioration of the left ventricular ejection fraction, and reversible heart failure were probable symptoms.42,73,74 Takotsubo cardiomyopathy (TC), also called apical ballooning syndrome or stress cardiomyopathy, was a reported adverse effect in patients with cancer treated with TKIs.75 A case report described mid-cavitary Takotsubo cardiomyopathy post ibrutinib therapy.43
Cardiac disorders are a common condition seen in the ibrutinib-treated cancer population because of multiple shared risk factors. Although AF remains a major concern in ibrutinib-related cardiotoxicity, an increasing number of case reports documenting cardiomyopathy and sudden death at home have been published to describe long-term outcomes76 (Figure 1A). The intrinsic relationship between arrhythmia and heart failure remains difficult to define.
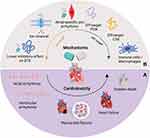 |
Figure 1 (A) Schematic diagram of the ibrutinib-induced cardiotoxicities. Ibrutinib is associated with several cardiotoxicities: atrial arrhythmias, mainly atrial fibrillation; ventricular arrhythmias. Serious ventricular tachycardia may cause sudden death. Myocardial fibrosis and injury are also reported in the clinical trials and research studies. (B) Schematic diagram of the mechanism of ibrutinib-induced cardiotoxicity. Both on- and off- target effects are involved in the ibrutinib-induced cardiotoxicities. Ibrutinib present and atrial-specific pro-arrhythmic effect. Ion transient disturbance was noticed in the chamber-specific toxicity. Low inhibitory effect of ibrutinib on target kinase is considered be another on- target effect, but clear evidence is still needed to address the issue. PI3K- and CSK- related signaling pathway inhibition are linked to ibrutinib-induced cardiotoxicity in an off-target aspect. Macrophages are also hypothesized to participate in the cardiotoxicity. Created with BioRender.com. Abbreviations: BTK, Bruton tyrosine kinase; CSK, C-terminal Src kinase; PI3K, Phosphoinositide-3 kinase. |
Mechanisms of Ibrutinib-Induced Cardiotoxicity
Ibrutinib has demonstrated impressive efficacy against at least 19 kinases, including BTK, tyrosine kinase expressed in hepatocellular carcinoma (TEC), vascular endothelial growth factor (EGFR), B-lymphoid tyrosine kinase (BLK), bone marrow kinase on the X chromosome (Bmx), C-terminal Src kinase (CSK), fetal growth restriction (FGR) and Janus kinase 3 (JAK3) (Table 1).77–79 Multiple mechanisms have been documented regarding the pathogenesis of ibrutinib-induced arrhythmia in both on- and off-target effects (Figure 1B).
 |
Table 1 The IC50 of Approved Irreversible BTK Inhibitors |
On-Target Effect of the Cardiotoxic Adverse Events
Atrial-Specific Pro-Arrhythmic Effect of Ibrutinib
Although ibrutinib presents an atrial-specific pro-arrhythmic effect in the clinical trials and real-world studies, it is hard to reproduce the drug response with a predominance of ventricular cells in the in-vitro model. Astem cell–derived cardiomyocyte model was used to investigate ibrutinib-induced AF in vitro.80 Unlike acalabrutinib and tirabrutinib, which are second-generation BTK inhibitors, ibrutinib demonstrated atrial-specific pro-arrhythmic effect not observed in ventricular cardiomyocytes. The number of differentially expressed genes was markedly decreased in the RTK pathway after treatment with ibrutinib, followed by a decrease in action potential duration (APD80) and increased calcium transient duration (CaTD80). A mouse model also suggested that the arrhythmogenic mechanisms are associated with structural remodeling and Ca2+ handling disorders in the atrium.81 These results provide another off-target possibility for ibrutinib with respect to its chamber-specific cardiotoxicity and disturbance of ion transient.
Lower Inhibitory Effect of Ibrutinib on Target Kinase
The relation between ibrutinib’s inhibitory effect on BTK and its pro-arrhythmia is controversial. No clear evidence has been shown to prove the issue. The first head-to-head comparison of two irreversible BTK inhibitors, namely ibrutinib and acalabrutinib, revealed that a 100-mg twice-daily dose of acalabrutinib resulted in noninferior PFS with fewer cardiovascular adverse events compared with ibrutinib.82 As ibrutinib can irreversibly bind to BTK and non-BTK kinases with analogous cysteine residues at lower nanomolar concentrations, it is presumed that an on-target effect may be contributing to the observed cardiotoxic adverse events. However, in another phase 3 trial of zanubrutinib vs ibrutinib, ibrutinib patients experienced a 10-fold higher incidence of atrial fibrillation than zanubrutinib patients, while zanubrutinib exhibited a higher degree of selectivity of BTK. As we conclude in Table 1, the safety and tolerability profiles of BTK inhibitors may be consistent with high selectivity vs off-target kinases.
Off-Target Effect of the Cardiotoxic Adverse Events
Involvement of the PI3K Pathway
Upon activation by antigen binding, BTK can be phosphorylated by upstream SYK or LYN, activating the downstream NF-κB and phosphatidylinositol 3-kinase (PI3K) cascades.83–86 PI3K(p110α) activation is a key regulator in sinus rhythm maintenance.87 According to the study of Jenny et al, the expression of BTK and TEC was higher in AF tissue than in the normal heart.88 When ibrutinib impaired the activity of PI3K/Akt, patients were highly susceptible to AF. PI3K was also hypothesized to increase susceptibility to VAs via disruption of calcium cycling and membrane repolarization in a spontaneously hypertensive rat model.71 These observations highlight that the cardiotoxicity of ibrutinib is an off-target adverse effect.
Involvement of C-Terminal Src Kinase (CSK)
In a study by Xiao et al, an off-target effect for AF induction was also identified as the mechanism leading to AF.89 Chemoproteomic kinase profiling of ibrutinib and acalabrutinib, which has a low cardiotoxicity profile, was performed to screen the potential mechanism associated with AF. The results revealed CSK as the culprit. In a previous study using a transgenic mouse model, it was suggested that CSK loss in the myocardium led to an increase in AF susceptibility. Pharmaceutical profiling of the CSK inhibitor also confirmed that kinase inhibitors with higher Cmax/IC50 values had the tendency to induce AF.80
Role of Macrophages in the Ibrutinib-Induced Cardiotoxicity
It is worth mentioning that infiltration of macrophage lineage cells was detected in an acute toxicity mouse model of ibrutinib, reminding us that macrophages are involved in other immunotherapies associated with cardiotoxicity, such as PD-1 antibodies and CTLA-4 inhibitors.90,91 Latest studies have unmasked the role of macrophages in the normal functioning of the heart. For example, tissue-resident macrophages in the normal myocardium were found to couple electrically to cardiomyocytes via the connexin-43-containing gap junctions and facilitate their electrical activity.92 They also have also been proved to be involved in mitochondrial elimination from the myocardial tissue and in the preservation of metabolic homeostasis.93 The anthracycline-based cancer drug doxorubicin, which was notorious for its dose-dependent cardiotoxicity and heart failure side effects, was found to intoxicate splenic marginal zone macrophages, thereby causing splenic contraction and impairing the immediate deployment of monocytes.94 Interestingly, ibrutinib treatment was reported to sustain the M2 macrophage population and immunosuppressive profile of nurse-like cells in CLL patients, whereas it inhibited M2 macrophage activation in a BTK-PI3Kγ-dependent manner in patients with pancreatic ductal adenocarcinoma.95,96 In a zymosan-induced peritonitis model of sterile inflammation, BTK inhibition was correlated with reduced C5a and CCL2 levels, both of which regulate myeloid cell recruitment.97 As BTK is predominantly expressed in immune cells including macrophages, it is logical to hypothesize that macrophages may actively be involved in ibrutinib-induced arrhythmias. Further studies may be necessary to clarify this potential involvement.
Clinical Management
Risk Factors of Ibrutinib-Induced Cardiotoxicity
Several risk factors have been implicated in ibrutinib-related cardiotoxicity in clinical trials and clinical practice. A retrospective study of 242 patients receiving ibrutinib or another chemotherapy drug was performed to quantify the real-world incidence rates and risk of AF. The results revealed that prior hypertension, ACEI/ARB use, beta-blocker use, and aspirin use were associated with increased AA incidence.98 Prior history of AF, enlarged left atrial diameter and age over 65 years were also variables associated with the development of ibrutinib induced adverse effects.34
Evaluation of Ibrutinib-Induced Cardiotoxicity
Cardiovascular magnetic resonance imaging (CMR) or echocardiography has also been recommended for assessment of the myocardial injury.99 A single-center study examined the relationship between myocardial damage and myocardial strain using cardiac biomarkers. The findings revealed that evaluating myocardial strain in conjunction with measuring high-sensitive troponin T is useful for detecting early cardiac drug toxicity secondary to ibrutinib.100,101
For patients with AF, clinical assessment should be performed to estimate the risk of bleeding and stroke. The CHA2DS2-VASc or HAS-BLED scoring systems could provide a guide to control this issue. For patients with a low score, mainly CHA2DS2-VASc 0 or 1, it is recommended to continue the use of ibrutinib.102–104 However, for patients with more than two risk factors, or a score of ≥2, discontinuation of ibrutinib is an optional choice. Although the bleeding risk can also be accessed via the HAS-BLED scoring systems, it is not always appropriate for patients with cancer, according to the European Society of Cardiology position paper. This is because, neither the CHA2DS2-VASc nor the HAS-BLED scoring systems consider cancer-specific high-risk bleeding characteristics.105 Treatment should be patient-customized to manage the bleeding and stroke associated with ibrutinib use.100
Considerations with Anticoagulants
Patients with ibrutinib-induced AF have a substantially increased risk of stroke, heart failure, and all-cause death, the optimal strategies to prevent or treat this condition remain uncertain.101 In cases where stroke and thromboembolism are major concerns, the selection of systemic anticoagulation agent should be based upon the risk and benefit for a given patient. The intravenous administration of low-molecular-weight heparin (LMWH) is frequently used but required close monitoring. Traditional vitamin K antagonists, mainly warfarin, should be avoided, as an increased rate of subdural hematomas has been reported in MCL patients.21 Direct oral anticoagulants (DOACs), such as apixaban, rivaroxaban, or dabigatran, are introduced in the management of AF. In some cases, low dose of aspirin has also been used. Nevertheless, ibrutinib is also associated with bleeding, and it is possible that significant bleeding complications may emerge with concomitant use of anticoagulants or antiplatelets.106 Over-the counter supplements, such as vitamin E or fish oil, are not acceptable, either.104
Rate Control vs Rhythm Control
Paroxysmal and asymptomatic AF patients without continuous electrocardiogram monitoring are less likely to be and yet need to be handle by oncologists; however, it is hard to make the decision for cases of symptomatic AF, such as those with palpitations and fluttering. In the case report by Essa, the Atrial Fibrillation Better Care (ABC) pathway was recommended to provide a holistic management for AF patients. Factors favoring rhythm control were as follows: 1) hemodynamic instability; 2) younger cancer patients without structural heart disease; 3) AF secondary to correctable causes; and 4) persistent symptoms despite medical management.107 A retrospective descriptive study reviewed patients with rhythm-controlled strategies. In that study, amiodarone, flecainide, digoxin and dofetilide were used as rhythm-controlled medication. The Cleveland experience suggests temporarily hold ibrutinib and reintroducing it at either a full or reduced dose after the AF is controlled.108 Patients with advanced disease and palliative care, proarrhythmogenic chemotherapeutics, drug interactions or frailty were better provided with a rate control policy. Pertaining to rate control, the majority of studies selected a beta-blocker over nondihydropyridine calcium channel blockers (verapamil and diltiazem) or P-glycoprotein substrates (amiodarone).
Drug–Drug Interactions
Ibrutinib is metabolized by the CYP 3A4.109 For this reason, co-administration of ibrutinib with cytochrome inhibitors should be avoided. Considering the drug–drug interactions of CYP 3A4/5, cardiologist often chose beta-blockers as the first-line therapy. Calcium channel blockers such as diltiazem and verapamil, which inhibit CYP 3A4, increased the concentration of ibrutinib. Amiodarone also inhibits cytochrome 3A4 and can increase the serum concentration of ibrutinib. Ketoconazole is a strong CYP 3A4 inhibitor. In a dedicated drug–drug interaction trial, ketoconazole was found to increased ibrutinib Cmax by 29-fold and AUC by 24-fold.39,109 Ibrutinib also inhibits P-glycoprotein, as well as increases the intracellular concentration of P-glycoprotein substrates, such as digoxin and dabigatran.
Adjustment of Drug Intensity
It remains controversial whether it is appropriate to modify or temporarily interrupt the drug intensity. A clinical trial to examine the impact of prolonged dose delays and reductions in the RESONATE study was conducted, and the results suggested that adherence to the recommended ibrutinib dosage correlated with a higher PFS and improved OS.47 When encountered with adverse event management or perioperative holds, dose reduction or interruption are inevitable In Xiao’s work, the in vitro chemoproteic profiling experiments demonstrated that the Cmax/IC50 values were strongly associated with the manifestation of AF adverse effects.89 Pertaining to the pharmacology aspect, ibrutinib achieved full occupancy at a dose of 2.5 mg/kg, while the proposed dose of ibrutinib in CLL patients equates to a mean dose of 7.22/kg/day. A pilot study identified a step-down dose cycle, from 420 mg to 280 mg and 140 mg, which did not impact BTK occupancy, and biological and molecular consequences in CLL cells, which is in agreement with the real-world experience from a retrospective analysis where reduced did not affect PFS or OS.110–113 Further evidence showed that BTK expression decreased in a time-dependent manner with the prescription of ibrutinib compared with baseline, which supports the lower doses of ibrutinib administration in future clinical management.114
Alternative Treatment Options and Their Cardiotoxicity
Given the crucial role of BTK in B cell maturation and success of ibrutinib, four additional BTK inhibitors have been approved globally and more than 70 inhibitors are currently undergoing clinical or preclinical trials. Second-generation irreversible BTK inhibitors include acalabrutinib, tirabrutinib, zanubrutinib, and orelabrutinib, all of which demonstrated well tolerability regarding cardiovascular diseases (Table 2).115–118 Acalabrutinib was approved by the US Food and Drug Administration in 2017 for the treatment of patients with CLL, SLL, or R/R MCL. The IC50 of acalabrutinib of BTK in vitro is 3 nM. In a previous study, among the acalabrutinib monotherapy-treated CLL patients, AF was reported in 2 out of 25 patients.115 Long-term data revealed its durable efficacy and safety with a 2% rate of AF-related adverse events.119 A phase 2 study of acalabrutinib in ibrutinib-intolerant patients with R/R CLL demonstrated an ORR of 73% with a median PFS that did not reach the demonstrated durable disease control.120 Neither AF nor arrythmias were included among the most frequent adverse events associated with acalabrutinib use in this population, although two patients experienced a lower grade AF. Zanubrutinib is a highly selective second-generation irreversible BTK inhibitor developed by the Chinese company Beigene. Structural differences in the pyrimidine ring make zanubrutinib more selective than ibrutinib, with a 10-fold lower IC50 concentration. Pooled analysis to explore the efficacy of zanubrutinib monotherapy demonstrated an equally efficacious and favorable safety profile in R/R MCL patients.117 A trial comparison of acalabrutinib and ibrutinib revealed that acalabrutinib had noninferior PFS with fewer cardiovascular adverse events.82 The ASPEN study assessed the effect of zanubrutinib versus ibrutinib on symptomatic WM patients and found that zanubrutinib had a higher rate of complete response and a lower cardiotoxicity profile compared with ibrutinib (Table 3).121 As data continue to accumulate, more direct head-to-head comparisons will provide a better insight into the selection of BTK inhibitors.
 |
Table 2 Clinical Trials of Approved BTK Inhibitors Monotherapy |
 |
Table 3 Clinical Trials of Head-to-Head Comparison of Approved BTK Inhibitors |
Prospects and Conclusions
Cardiovascular diseases are the second leading cause of morbidity and mortality in cancer patients, next to the development of a second malignancy.122 Arrhythmias are a pivotal complication associated with unique management challenges in patients with cancer. Not only atrial and VAs but also other electrolyte abnormalities, such as QT interval changes, are the most common drug related side effects. Among them, AF is a leading cause of significant thrombotic morbidity and overall cardiovascular mortality. AF has become an increasingly common concern, particularly in older patients diagnosed with cancer. In a multi-ethnic representative cohort of patients with early- and late-stage breast cancer, patients with AF onset after 1 year from the onset of breast cancer had a higher mortality rate than patients with new-onset AF.123 Cardiotoxicity from chemotherapies has recently attracted considerable attention from clinicians. The growing awareness of cancer therapy-related cardiac dysfunction has led to the emergence of cardio-oncology.124,125 Its goal is to provide optimal prevention and treatment of cancer therapy-related cardiotoxicity and present a better understanding of the relationship between cancer therapeutics and cardiac function.
As data continue to emerge regarding the use of next-generation BTK inhibitors, idiosyncratic drug-specific toxicities may arise, including ibrutinib-related cardiotoxicity, acalabrutinib-related headache or zanubrutinib-related neutrophilia. The unique cardiotoxicity of ibrutinib does not appear to be class-related specific adverse events as lower rates are reported with the use of second-generation BTK inhibitors. Studies have reported that direct comparison data and longer follow-up periods are required to elucidate the safety profile of BTK inhibitors. Furthermore, the continuous and increasingly more common use of selective inhibitors may provide a greater insight into the specific mechanism pertaining to the on-/off-target cardiotoxicity of ibrutinib. To date, ibrutinib remains the most widely used BTK inhibitor which takes up to approximately 97% of market share. Patients or their caregivers should be appropriately informed about cardiotoxicity when initiating therapy. However, it is still too early to make any decision and choose new inhibitors.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant number 82104288) and the Medical and Health Technology Project of Hangzhou (Grant number 0020190228).
Disclosure
The authors declare that they have no competing interests.
References
1. Mato AR, Nabhan C, Barr PM, et al. Outcomes of CLL patients treated with sequential kinase inhibitor therapy: a real world experience. Blood. 2016;18128:2199–2205. doi:10.1182/blood-2016-05-716977
2. Burger JA, O’Brien S. Evolution of CLL treatment - from chemoimmunotherapy to targeted and individualized therapy. Nat Rev Clin Oncol. 2018;815:510–527. doi:10.1038/s41571-018-0037-8
3. Fleming MR, Xiao L, Jackson KD, et al. Vascular impact of cancer therapies: the case of BTK (Bruton Tyrosine Kinase) inhibitors. Circ Res. 2021;12128:1973–1987. doi:10.1161/CIRCRESAHA.121.318259
4. Smith CI, Islam TC, Mattsson PT, et al. The Tec family of cytoplasmic tyrosine kinases: mammalian Btk, Bmx, Itk, Tec, Txk and homologs in other species. Bioessays. 2001;523:436–446. doi:10.1002/bies.1062
5. Bradshaw JM. The Src, Syk, and Tec family kinases: distinct types of molecular switches. Cell Signal. 2010;822:1175–1184. doi:10.1016/j.cellsig.2010.03.001
6. Yu L, Mohamed AJ, Vargas L, et al. Regulation of Bruton tyrosine kinase by the peptidylprolyl isomerase Pin1. J Biol Chem. 2006;26281:18201–18207. doi:10.1074/jbc.M603090200
7. Vihinen M, Nore BF, Mattsson PT, et al. Missense mutations affecting a conserved cysteine pair in the TH domain of Btk. FEBS Lett. 1997;2413:205–210. doi:10.1016/S0014-5793(97)00912-5
8. Yao L, Janmey P, Frigeri LG, et al. Pleckstrin homology domains interact with filamentous actin. J Biol Chem. 1999;28274:19752–19761. doi:10.1074/jbc.274.28.19752
9. Hyvonen M, Saraste M. Structure of the PH domain and Btk motif from Bruton’s tyrosine kinase: molecular explanations for X-linked agammaglobulinaemia. EMBO J. 1997;1216:3396–3404. doi:10.1093/emboj/16.12.3396
10. Rawlings DJ, Scharenberg AM, Park H, et al. Activation of BTK by a phosphorylation mechanism initiated by SRC family kinases. Science. 1996;5250271:822–825. doi:10.1126/science.271.5250.822
11. Pan Z, Scheerens H, Li SJ, et al. Discovery of selective irreversible inhibitors for Bruton’s tyrosine kinase. ChemMedChem. 2007;12:58–61. doi:10.1002/cmdc.200600221
12. Genevier HC, Hinshelwood S, Gaspar HB, et al. Expression of Bruton’s tyrosine kinase protein within the B cell lineage. Eur J Immunol. 1994;1224:3100–3105. doi:10.1002/eji.1830241228
13. Smith CI, Baskin B, Humire-Greiff P, et al. Expression of Bruton’s agammaglobulinemia tyrosine kinase gene, BTK, is selectively down-regulated in T lymphocytes and plasma cells. J Immunol. 1994;2152:557–565.
14. Pal Singh S, Dammeijer F, Hendriks RW. Role of Bruton’s tyrosine kinase in B cells and malignancies. Mol Cancer. 2018;117:57.
15. Byrd JC, Hillmen P, O’Brien S, et al. Long-term follow-up of the RESONATE phase 3 trial of ibrutinib vs ofatumumab. Blood. 2019;19133:2031–2042. doi:10.1182/blood-2018-08-870238
16. Byrd JC, Brown JR, O’Brien S, et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med. 2014;3371:213–223. doi:10.1056/NEJMoa1400376
17. Munir T, Brown JR, O’Brien S, et al. Final analysis from RESONATE: up to six years of follow-up on ibrutinib in patients with previously treated chronic lymphocytic leukemia or small lymphocytic lymphoma. Am J Hematol. 2019;1294:1353–1363. doi:10.1002/ajh.25638
18. Burger JA, Barr PM, Robak T, et al. Long-term efficacy and safety of first-line ibrutinib treatment for patients with CLL/SLL: 5 years of follow-up from the phase 3 RESONATE-2 study. Leukemia. 2020;334:787–798. doi:10.1038/s41375-019-0602-x
19. O’Brien S, Furman RR, Coutre S, et al. Single-agent ibrutinib in treatment-naive and relapsed/refractory chronic lymphocytic leukemia: a 5-year experience. Blood. 2018;17131:1910–1919. doi:10.1182/blood-2017-10-810044
20. Wang ML, Rule S, Martin P, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2013;6369:507–516. doi:10.1056/NEJMoa1306220
21. Wang ML, Blum KA, Martin P, et al. Long-term follow-up of MCL patients treated with single-agent ibrutinib: updated safety and efficacy results. Blood. 2015;6126:739–745. doi:10.1182/blood-2015-03-635326
22. Treon SP, Castillo JJ, Skarbnik AP, et al. The BTK inhibitor ibrutinib may protect against pulmonary injury in COVID-19-infected patients. Blood. 2020;21135:1912–1915. doi:10.1182/blood.2020006288
23. Thibaud S, Tremblay D, Bhalla S, et al. Protective role of Bruton tyrosine kinase inhibitors in patients with chronic lymphocytic leukaemia and COVID-19. Br J Haematol. 2020;2190:e73–e76.
24. Roschewski M, Lionakis MS, Sharman JP, et al. Inhibition of Bruton tyrosine kinase in patients with severe COVID-19. Sci Immunol. 2020;485:eabd0110.
25. Dickerson T, Wiczer T, Waller A, et al. Hypertension and incident cardiovascular events following ibrutinib initiation. Blood. 2019;22134:1919–1928. doi:10.1182/blood.2019000840
26. Salem JE, Manouchehri A, Bretagne M, et al. Cardiovascular toxicities associated with ibrutinib. J Am Coll Cardiol. 2019;1374:1667–1678. doi:10.1016/j.jacc.2019.07.056
27. Christensen BW, Zaha VG, Awan FT. Cardiotoxicity of BTK inhibitors: ibrutinib and beyond. Expert Rev Hematol. 2022;415:321–331. doi:10.1080/17474086.2022.2067526
28. Thorp BC, Badoux X. Atrial fibrillation as a complication of ibrutinib therapy: clinical features and challenges of management. Leuk Lymphoma. 2018;259:311–320. doi:10.1080/10428194.2017.1339874
29. Lasica M, Tam CS. Management of ibrutinib toxicities: a practical guide. Curr Hematol Malig Rep. 2020;315:177–186. doi:10.1007/s11899-020-00576-3
30. Mato AR, Nabhan C, Thompson MC, et al. Toxicities and outcomes of 616 ibrutinib-treated patients in the United States: a real-world analysis. Haematologica. 2018;5103:874–879. doi:10.3324/haematol.2017.182907
31. Kapelios CJ, Bonou MS, Diamantopoulos P, et al. Ibrutinib-related atrial fibrillation: therapeutic challenges. J Oncol Pharm Pract. 2019;525:1258–1260. doi:10.1177/1078155218785983
32. Leong DP, Caron F, Hillis C, et al. The risk of atrial fibrillation with ibrutinib use: a systematic review and meta-analysis. Blood. 2016;1128:138–140. doi:10.1182/blood-2016-05-712828
33. Zimetbaum P. Atrial fibrillation. Ann Intern Med. 2017;5166:ITC33–ITC48. doi:10.7326/AITC201703070
34. Brown JR, Moslehi J, O’Brien S, et al. Characterization of atrial fibrillation adverse events reported in ibrutinib randomized controlled registration trials. Haematologica. 2017;10102:1796–1805. doi:10.3324/haematol.2017.171041
35. Jonas DE, Kahwati LC, Yun JDY, et al. Screening for atrial fibrillation with electrocardiography: evidence report and systematic review for the US preventive services task force. JAMA. 2018;5320:485–498. doi:10.1001/jama.2018.4190
36. Friberg L, Rosenqvist M, Lindgren A, et al. High prevalence of atrial fibrillation among patients with ischemic stroke. Stroke. 2014;945:2599–2605. doi:10.1161/STROKEAHA.114.006070
37. Tang CPS, McMullen J, Tam C. Cardiac side effects of Bruton tyrosine kinase (BTK) inhibitors. Leuk Lymphoma. 2018;759:1554–1564. doi:10.1080/10428194.2017.1375110
38. Cheng M, Yang F, Liu J, et al. Tyrosine kinase inhibitors-induced arrhythmias: from molecular mechanisms, pharmacokinetics to therapeutic strategies. Front Cardiovasc Med. 2021;8:758010. doi:10.3389/fcvm.2021.758010
39. de Zwart L, Snoeys J, De Jong J, et al. Ibrutinib dosing strategies based on interaction potential of CYP3A4 perpetrators using physiologically based pharmacokinetic modeling. Clin Pharmacol Ther. 2016;5100:548–557. doi:10.1002/cpt.419
40. Beyer A, Ganti B, Majkrzak A, Theyyunni N. A perfect storm: tyrosine kinase inhibitor-associated polymorphic ventricular tachycardia. J Emerg Med. 2017;452:e123–e127. doi:10.1016/j.jemermed.2016.10.019
41. Tomcsanyi J, Nenyei Z, Matrai Z, Bozsik B. Ibrutinib, an approved tyrosine kinase inhibitor as a potential cause of recurrent polymorphic ventricular tachycardia. JACC Clin Electrophysiol. 2016;72:847–849. doi:10.1016/j.jacep.2016.07.004
42. Buck B, Chum A, Patel M, et al. Myocardial injury after ibrutinib initiation for hematologic malignancies. J Am Coll Cardiol. 2022;979:1938. doi:10.1016/S0735-1097(22)02929-1
43. Giza DE, Moudgil R, Lopez-Mattei J, Kim P, Iliescu C. Association between ibrutinib and mid-cavitary Takotsubo cardiomyopathy: a case report and a review of chemotherapy-induced Takotsubo's cardiomyopathy. Eur Heart J Case Rep. 2017;21:ytx006.
44. Akbulut M, Urun Y. Onco-cardiology: drug-drug interactions of antineoplastic and cardiovascular drugs. Crit Rev Oncol Hematol. 2020;145:102822. doi:10.1016/j.critrevonc.2019.102822
45. Mukai Y, Yoshida T, Kondo T, Inotsume N, Toda T. Novel high-performance liquid chromatography-tandem mass spectrometry method for simultaneous quantification of BCR-ABL and Bruton’s tyrosine kinase inhibitors and their three active metabolites in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2020;1137:121928. doi:10.1016/j.jchromb.2019.121928
46. Yasu T, Momo K, Yasui H, Kuroda S. Simple determination of plasma ibrutinib concentration using high-performance liquid chromatography. Biomed Chromatogr. 2019;333:e4435. doi:10.1002/bmc.4435
47. Barr PM, Brown JR, Hillmen P, et al. Impact of ibrutinib dose adherence on therapeutic efficacy in patients with previously treated CLL/SLL. Blood. 2017;19129:2612–2615. doi:10.1182/blood-2016-12-737346
48. Scheers E, Leclercq L, de Jong J, et al. Absorption, metabolism, and excretion of oral (1)(4)C radiolabeled ibrutinib: an open-label, Phase I, single-dose study in healthy men. Drug Metab Dispos. 2015;243:289–297. doi:10.1124/dmd.114.060061
49. de Vries R, Huang M, Bode N, et al. Bioanalysis of ibrutinib and its active metabolite in human plasma: selectivity issue, impact assessment and resolution. Bioanalysis. 2015;207:2713–2724. doi:10.4155/bio.15.159
50. Tobinai K, Ogura M, Ishizawa K, et al. Safety and tolerability of ibrutinib monotherapy in Japanese patients with relapsed/refractory B cell malignancies. Int J Hematol. 2016;1103:86–94. doi:10.1007/s12185-015-1900-3
51. Jarkowski A, Glode AE, Spangenthal EJ, Wong MK. Heart failure caused by molecularly targeted therapies for cancer. Pharmacotherapy. 2011;131:62–75. doi:10.1592/phco.31.1.62
52. Hahn VS, Zhang KW, Sun L, et al. Heart failure with targeted cancer therapies: mechanisms and cardioprotection. Circ Res. 2021;10128:1576–1593. doi:10.1161/CIRCRESAHA.121.318223
53. Cardinale D, Colombo A, Bacchiani Get al, Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation. 2015;22131:1981–1988. doi:10.1161/CIRCULATIONAHA.114.013777
54. Rawat PS, Jaiswal A, Khurana A, Bhatti JS, Navik U. Doxorubicin-induced cardiotoxicity: an update on the molecular mechanism and novel therapeutic strategies for effective management. Biomed Pharmacother. 2021;139:111708. doi:10.1016/j.biopha.2021.111708
55. Moutabian H, Ghahramani-Asl R, Mortezazadeh T, et al. The cardioprotective effects of nano-curcumin against doxorubicin-induced cardiotoxicity: a systematic review. Biofactors. 2022;348:597–610. doi:10.1002/biof.1823
56. Lyon AR, Yousaf N, Battisti NML, Moslehi J, Larkin J. Immune checkpoint inhibitors and cardiovascular toxicity. Lancet Oncol. 2018;919:e447–e458. doi:10.1016/S1470-2045(18)30457-1
57. Alexandre J, Moslehi JJ, Bersell KR, et al. Anticancer drug-induced cardiac rhythm disorders: current knowledge and basic underlying mechanisms. Pharmacol Ther. 2018;189:89–103. doi:10.1016/j.pharmthera.2018.04.009
58. Yao Y, Lin X, Li F, Jin J, Wang H. The global burden and attributable risk factors of chronic lymphocytic leukemia in 204 countries and territories from 1990 to 2019: analysis based on the global burden of disease study 2019. Biomed Eng Online. 2022;121:4. doi:10.1186/s12938-021-00973-6
59. Fradley MG, Welter-Frost A, Gliksman M, et al. Electrocardiographic changes associated with ibrutinib exposure. Cancer Control. 2020;127:1073274820931808.
60. Burger JA, Tedeschi A, Barr PM, et al. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med. 2015;25373:2425–2437. doi:10.1056/NEJMoa1509388
61. Coutre SE, Byrd JC, Hillmen P, et al. Long-term safety of single-agent ibrutinib in patients with chronic lymphocytic leukemia in 3 pivotal studies. Blood Adv. 2019;123:1799–1807. doi:10.1182/bloodadvances.2018028761
62. Yun S, Vincelette ND, Acharya U, Abraham I. Risk of atrial fibrillation and bleeding diathesis associated with ibrutinib treatment: a systematic review and pooled analysis of four randomized controlled trials. Clin Lymphoma Myeloma Leuk. 2017;117(31–37):e13.
63. Archibald WJ, Rabe KG, Kabat BF, et al. Atrial fibrillation in patients with chronic lymphocytic leukemia (CLL) treated with ibrutinib: risk prediction, management, and clinical outcomes. Ann Hematol. 2021;1100:143–155. doi:10.1007/s00277-020-04094-3
64. Shanafelt TD, Parikh SA, Noseworthy PA, et al. Atrial fibrillation in patients with chronic lymphocytic leukemia (CLL). Leuk Lymphoma. 2017;758:1630–1639. doi:10.1080/10428194.2016.1257795
65. Lin HJ, Wolf PA, Benjamin EJ, Belanger AJ, D’Agostino RB. Newly diagnosed atrial fibrillation and acute stroke. The Framingham Study. Stroke. 1995;926:1527–1530. doi:10.1161/01.STR.26.9.1527
66. Sposato LA, Klein FR, Jauregui A, et al. Newly diagnosed atrial fibrillation after acute ischemic stroke and transient ischemic attack: importance of immediate and prolonged continuous cardiac monitoring. J Stroke Cerebrovasc Dis. 2012;321:210–216. doi:10.1016/j.jstrokecerebrovasdis.2010.06.010
67. Force USPST, Curry SJ, Krist AH, et al. Screening for atrial fibrillation with electrocardiography: US preventive services task force recommendation statement. JAMA. 2018;5320:478–484.
68. Force USPST, Davidson KW, Barry MJ, et al. Screening for atrial fibrillation: US preventive services task force recommendation statement. JAMA. 2022;4327:360–367.
69. Kahwati LC, Asher GN, Kadro ZO, et al. Screening for atrial fibrillation: updated evidence report and systematic review for the US preventive services task force. JAMA. 2022;4327:368–383. doi:10.1001/jama.2021.21811
70. Fazal M, Kapoor R, Cheng P, et al. Arrhythmia patterns in patients on ibrutinib. Front Cardiovasc Med. 2021;8:792310. doi:10.3389/fcvm.2021.792310
71. Du B, Chakraborty P, Azam MA, et al. Acute effects of ibrutinib on ventricular arrhythmia in spontaneously hypertensive rats. JACC CardioOncol. 2020;42:614–629. doi:10.1016/j.jaccao.2020.08.012
72. de Jong J, Hellemans P, Jiao JJ, et al. Ibrutinib does not prolong the corrected QT interval in healthy subjects: results from a thorough QT study. Cancer Chemother Pharmacol. 2017;680:1227–1237. doi:10.1007/s00280-017-3471-x
73. Kyi HH, Zayed Y, Al Hadidi S. Ibrutinib-induced cardiomyopathy. J Community Hosp Intern Med Perspect. 2019;19:50–52. doi:10.1080/20009666.2018.1555432
74. Gulsaran SK, Baysal M, Demirci U, et al. Late onset left ventricular dysfunction and cardiomyopathy induced with ibrutinib. J Oncol Pharm Pract. 2020;226:478–480. doi:10.1177/1078155219852146
75. Madhavan M, Prasad A. Proposed Mayo Clinic criteria for the diagnosis of Tako-Tsubo cardiomyopathy and long-term prognosis. Herz. 2010;435:240–243. doi:10.1007/s00059-010-3339-x
76. Lampson BL, Yu L, Glynn RJ, et al. Ventricular arrhythmias and sudden death in patients taking ibrutinib. Blood. 2017;18129:2581–2584. doi:10.1182/blood-2016-10-742437
77. Burger JA, Buggy JJ. Bruton tyrosine kinase inhibitor ibrutinib (PCI-32765). Leuk Lymphoma. 2013;1154:2385–2391. doi:10.3109/10428194.2013.777837
78. Chang BY, Huang MM, Francesco M, et al. The Bruton tyrosine kinase inhibitor PCI-32765 ameliorates autoimmune arthritis by inhibition of multiple effector cells. Arthritis Res Ther. 2011;413:R115. doi:10.1186/ar3400
79. Honigberg LA, Smith AM, Sirisawad M, et al. The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proc Natl Acad Sci U S A. 2010;29107:13075–13080. doi:10.1073/pnas.1004594107
80. Shafaattalab S, Lin E, Christidi E, et al. Ibrutinib displays atrial-specific toxicity in human stem cell-derived cardiomyocytes. Stem Cell Reports. 2019;512:996–1006. doi:10.1016/j.stemcr.2019.03.011
81. Jiang L, Li L, Ruan Y, et al. Ibrutinib promotes atrial fibrillation by inducing structural remodeling and calcium dysregulation in the atrium. Heart Rhythm. 2019;916:1374–1382. doi:10.1016/j.hrthm.2019.04.008
82. Byrd JC, Hillmen P, Ghia P, et al. Acalabrutinib versus ibrutinib in previously treated chronic lymphocytic leukemia: results of the first randomized phase III trial. J Clin Oncol. 2021;3139:3441–3452. doi:10.1200/JCO.21.01210
83. Bajpai UD, Zhang K, Teutsch M, Sen R, Wortis HH. Bruton’s tyrosine kinase links the B cell receptor to nuclear factor kappaB activation. J Exp Med. 2000;10191:1735–1744. doi:10.1084/jem.191.10.1735
84. Isakoff SJ, Cardozo T, Andreev J, et al. Identification and analysis of PH domain-containing targets of phosphatidylinositol 3-kinase using a novel in vivo assay in yeast. EMBO J. 1998;1817:5374–5387. doi:10.1093/emboj/17.18.5374
85. Satterthwaite AB, Lowell CA, Khan WN, et al. Independent and opposing roles for Btk and lyn in B and myeloid signaling pathways. J Exp Med. 1998;5188:833–844. doi:10.1084/jem.188.5.833
86. Kurosaki T, Kurosaki M. Transphosphorylation of Bruton’s tyrosine kinase on tyrosine 551 is critical for B cell antigen receptor function. J Biol Chem. 1997;25272:15595–15598. doi:10.1074/jbc.272.25.15595
87. Pretorius L, Du XJ, Woodcock EA, et al. Reduced phosphoinositide 3-kinase (p110alpha) activation increases the susceptibility to atrial fibrillation. Am J Pathol. 2009;3175:998–1009. doi:10.2353/ajpath.2009.090126
88. McMullen JR, Boey EJ, Ooi JY, et al. Ibrutinib increases the risk of atrial fibrillation, potentially through inhibition of cardiac PI3K-Akt signaling. Blood. 2014;25124:3829–3830. doi:10.1182/blood-2014-10-604272
89. Xiao L, Salem JE, Clauss S, et al. Ibrutinib-mediated atrial fibrillation attributable to inhibition of C-terminal src kinase. Circulation. 2020;25142:2443–2455. doi:10.1161/CIRCULATIONAHA.120.049210
90. Xia W, Zou C, Chen H, Xie C, Hou M. Immune checkpoint inhibitor induces cardiac injury through polarizing macrophages via modulating microRNA-34a/Kruppel-like factor 4 signaling. Cell Death Dis. 2020;711:575. doi:10.1038/s41419-020-02778-2
91. Vermeulen L, Depuydt CE, Weckx P, et al. Myositis as a neuromuscular complication of immune checkpoint inhibitors. Acta Neurol Belg. 2020;2120:355–364. doi:10.1007/s13760-020-01282-w
92. Hulsmans M, Clauss S, Xiao L, et al. Macrophages facilitate electrical conduction in the heart. Cell. 2017;3169(510–522):e520.
93. Nicolas-Avila JA, Lechuga-Vieco AV, Esteban-Martinez L, et al. A network of macrophages supports mitochondrial homeostasis in the heart. Cell. 2020;1183(94–109):e123.
94. Jadapalli JK, Wright GW, Kain V, et al. Doxorubicin triggers splenic contraction and irreversible dysregulation of COX and LOX that alters the inflammation-resolution program in the myocardium. Am J Physiol Heart Circ Physiol. 2018;5315:H1091–H1100. doi:10.1152/ajpheart.00290.2018
95. Gunderson AJ, Kaneda MM, Tsujikawa T, et al. Bruton tyrosine kinase-dependent immune cell cross-talk drives pancreas cancer. Cancer Discov. 2016;36:270–285. doi:10.1158/2159-8290.CD-15-0827
96. Galletti G, Caligaris-Cappio F, Bertilaccio MT. B cells and macrophages pursue a common path toward the development and progression of chronic lymphocytic leukemia. Leukemia. 2016;1230:2293–2301. doi:10.1038/leu.2016.261
97. Purvis GSD, Aranda-Tavio H, Channon KM, Greaves DR. Bruton’s TK Regulates myeloid cell recruitment during acute inflammation. Br J Pharmacol. 2021;179. doi:10.1111/bph.15778
98. Fradley MG, Gliksman M, Emole J, et al. Rates and risk of atrial arrhythmias in patients treated with ibrutinib compared with cytotoxic chemotherapy. Am J Cardiol. 2019;4124:539–544. doi:10.1016/j.amjcard.2019.05.029
99. Agrawal A, Kumar A, Berglund F, Klein AL. Ibrutinib induced atrial fibrillation and its associated management. J Am Coll Cardiol. 2022;979:3361. doi:10.1016/S0735-1097(22)04352-2
100. Ciuculete DC, Popescu RA, Georgescu GD, Dan GA. Evaluation of ibrutinib cardiotoxicity by comparative use of speckle-tracking technique and biomarkers. Am J Ther. 2022;129:e50–e55. doi:10.1097/MJT.0000000000001463
101. Chugh SS, Havmoeller R, Narayanan K, et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014;8129:837–847. doi:10.1161/CIRCULATIONAHA.113.005119
102. Brown JR. How I treat CLL patients with ibrutinib. Blood. 2018;4131:379–386. doi:10.1182/blood-2017-08-764712
103. Stephens DM, Byrd JC. How I manage ibrutinib intolerance and complications in patients with chronic lymphocytic leukemia. Blood. 2019;12133:1298–1307. doi:10.1182/blood-2018-11-846808
104. Lipsky AH, Farooqui MZ, Tian X, et al. Incidence and risk factors of bleeding-related adverse events in patients with chronic lymphocytic leukemia treated with ibrutinib. Haematologica. 2015;12100:1571–1578. doi:10.3324/haematol.2015.126672
105. Zamorano JL, Lancellotti P, Rodriguez Muñoz D, et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: the Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J. 2016;37:2768–2801. doi:10.1093/eurheartj/ehw211
106. O’Brien S, Hillmen P, Coutre S, et al. Safety analysis of four randomized controlled studies of ibrutinib in patients with chronic lymphocytic leukemia/small lymphocytic lymphoma or mantle cell lymphoma. Clin Lymphoma Myeloma Leuk. 2018;1018(648–657):e615.
107. Essa H, Lodhi T, Dobson R, Wright D, Lip GYH. How to manage atrial fibrillation secondary to ibrutinib. Jacc-Cardiooncol. 2021;13:140–144. doi:10.1016/j.jaccao.2020.11.016
108. Khalid S, Yasar S, Khalid A, et al. Management of atrial fibrillation in patients on ibrutinib: a Cleveland Clinic experience. Cureus. 2018;510:e2701.
109. de Jong J, Skee D, Murphy J, et al. Effect of CYP3A perpetrators on ibrutinib exposure in healthy participants. Pharmacol Res Perspect. 2015;43:e00156.
110. Mato AR, Timlin C, Ujjani C, et al. Comparable outcomes in chronic lymphocytic leukaemia (CLL) patients treated with reduced-dose ibrutinib: results from a multi-centre study. Br J Haematol. 2018;2181:259–261. doi:10.1111/bjh.14540
111. Forum UC. Ibrutinib for relapsed/refractory chronic lymphocytic leukemia: a UK and Ireland analysis of outcomes in 315 patients. Haematologica. 2016;12101:1563–1572.
112. Winqvist M, Asklid A, Andersson PO, et al. Real-world results of ibrutinib in patients with relapsed or refractory chronic lymphocytic leukemia: data from 95 consecutive patients treated in a compassionate use program. A study from the Swedish Chronic Lymphocytic Leukemia Group. Haematologica. 2016;12101:1573–1580. doi:10.3324/haematol.2016.144576
113. Chen LS, Bose P, Cruz ND, et al. A pilot study of lower doses of ibrutinib in patients with chronic lymphocytic leukemia. Blood. 2018;21132:2249–2259. doi:10.1182/blood-2018-06-860593
114. Cervantes-Gomez F, Kumar Patel V, Bose P, Keating MJ, Gandhi V. Decrease in total protein level of Bruton’s tyrosine kinase during ibrutinib therapy in chronic lymphocytic leukemia lymphocytes. Leukemia. 2016;830:1803–1804. doi:10.1038/leu.2016.129
115. Eyre TA, Schuh A, Wierda WG, et al. Acalabrutinib monotherapy for treatment of chronic lymphocytic leukaemia (ACE-CL-001): analysis of the Richter transformation cohort of an open-label, single-arm, phase 1–2 study. Lancet Haematol. 2021;128:e912–e921. doi:10.1016/S2352-3026(21)00305-7
116. Narita Y, Nagane M, Mishima K, et al. Phase I/II study of tirabrutinib, a second-generation Bruton’s tyrosine kinase inhibitor, in relapsed/refractory primary central nervous system lymphoma. Neuro Oncol. 2021;123:122–133. doi:10.1093/neuonc/noaa145
117. Zhou K, Zou D, Zhou J, et al. Zanubrutinib monotherapy in relapsed/refractory mantle cell lymphoma: a pooled analysis of two clinical trials. J Hematol Oncol. 2021;114:167. doi:10.1186/s13045-021-01174-3
118. Wu JJ, Wang WH, Dong M, et al. Orelabrutinib-Bruton tyrosine kinase inhibitor-based regimens in the treatment of central nervous system lymphoma: a retrospective study. Invest New Drugs. 2022;40(3):650–659. doi:10.1007/s10637-022-01219-5
119. Byrd JC, Woyach JA, Furman RR, et al. Acalabrutinib in treatment-naive chronic lymphocytic leukemia. Blood. 2021;24137:3327–3338. doi:10.1182/blood.2020009617
120. Rogers KA, Thompson PA, Allan JN, et al. Phase II study of acalabrutinib in ibrutinib-intolerant patients with relapsed/refractory chronic lymphocytic leukemia. Haematologica. 2021;9106:2364–2373. doi:10.3324/haematol.2020.272500
121. Tam CS, Opat S, D’Sa S, et al. A randomized phase 3 trial of zanubrutinib vs ibrutinib in symptomatic Waldenstrom macroglobulinemia: the ASPEN study. Blood. 2020;18136:2038–2050. doi:10.1182/blood.2020006844
122. Okwuosa TM, Prabhu N, Patel H, et al. The cardiologist and the cancer patient: challenges to cardio-oncology (or onco-cardiology) and call to action. J Am Coll Cardiol. 2018;272:228–232. doi:10.1016/j.jacc.2018.04.043
123. Guha A, Fradley MG, Dent SF, et al. Incidence, risk factors, and mortality of atrial fibrillation in breast cancer: a SEER-Medicare analysis. Eur Heart J. 2022;443:300–312. doi:10.1093/eurheartj/ehab745
124. Tajiri K, Aonuma K, Sekine I. Cardio-oncology: a multidisciplinary approach for detection, prevention and management of cardiac dysfunction in cancer patients. Jpn J Clin Oncol. 2017;847:678–682. doi:10.1093/jjco/hyx068
125. Fradley MG, Beckie TM, Brown SA, et al. Recognition, prevention, and management of arrhythmias and autonomic disorders in cardio-oncology: a scientific statement from the American Heart Association. Circulation. 2021;3144:e41–e55.
126. Xu W, Yang S, Zhou K. Treatment of relapsed/refractory chronic lymphocytic leukemia/small lymphocytic lymphoma with the BTK inhibitor zanubrutinib: Phase 2, single-arm, multicenter study. J Hematol Oncol. 2020;113:48. doi:10.1186/s13045-020-00884-4
127. Cull G, Burger JA, Opat S. Zanubrutinib for treatment-naive and relapsed/refractory chronic lymphocytic leukaemia: long-term follow-up of the Phase I/II AU-003 study. Br J Haematol. 2022;5196:1209–1218. doi:10.1111/bjh.17994
128. An G, Zhou D, Cheng S. A phase II trial of the bruton tyrosine-kinase inhibitor zanubrutinib (BGB-3111) in patients with relapsed/refractory waldenstrom macroglobulinemia. Clin Cancer Res. 2021;2027:5492–5501. doi:10.1158/1078-0432.CCR-21-0539
129. Byrd JC, Wierda WG, Schuh A, et al. Acalabrutinib monotherapy in patients with relapsed/refractory chronic lymphocytic leukemia: updated phase 2 results. Blood. 2020;15135:1204–1213. doi:10.1182/blood.2018884940
130. Owen RG, McCarthy H, Rule S, et al. Acalabrutinib monotherapy in patients with Waldenstrom macroglobulinemia: a single-arm, multicentre, phase 2 study. Lancet Haematol. 2020;27:e112–e121. doi:10.1016/S2352-3026(19)30210-8
131. Wang M, Rule S, Zinzani PL. Durable response with single-agent acalabrutinib in patients with relapsed or refractory mantle cell lymphoma. Leukemia. 2019;1133:2762–2766. doi:10.1038/s41375-019-0575-9
132. Wang M. Acalabrutinib in relapsed or refractory mantle cell lymphoma (ACE-LY-004): a single-arm, multicentre, phase 2 trial. Lancet. 2018;10121391:659–667. doi:10.1016/S0140-6736(17)33108-2
133. Byrd JC, Harrington B, O’Brien S, et al. Acalabrutinib (ACP-196) in relapsed chronic lymphocytic leukemia. N Engl J Med. 2016;4374:323–332. doi:10.1056/NEJMoa1509981
134. Yamagami J, Ujiie H, Aoyama Y, et al. A multicenter, open-label, uncontrolled, single-arm phase 2 study of tirabrutinib, an oral Bruton’s tyrosine kinase inhibitor, in pemphigus. J Dermatol Sci. 2021;3103:135–142. doi:10.1016/j.jdermsci.2021.07.002
135. Sekiguchi N, Rai S, Munakata W. A multicenter, open-label, phase II study of tirabrutinib (ONO/GS-4059) in patients with Waldenstrom’s macroglobulinemia. Cancer Sci. 2020;9111:3327–3337. doi:10.1111/cas.14561
136. Munakata W. Phase I study of tirabrutinib (ONO-4059/GS-4059) in patients with relapsed or refractory B-cell malignancies in Japan. Cancer Sci. 2019;5110:1686–1694. doi:10.1111/cas.13983
137. Walter HS, Jayne S, Rule SA, et al. Long-term follow-up of patients with CLL treated with the selective Bruton’s tyrosine kinase inhibitor ONO/GS-4059. Blood. 2017;20129:2808–2810. doi:10.1182/blood-2017-02-765115
138. Shirley M. Bruton tyrosine kinase inhibitors in B-cell malignancies: their use and differential features. Target Oncol. 2022;117:69–84. doi:10.1007/s11523-021-00857-8
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
