Back to Journals » OncoTargets and Therapy » Volume 11
Hypoxia-regulated lncRNA CRPAT4 promotes cell migration via regulating AVL9 in clear cell renal cell carcinomas
Authors Zhang W, Wang J , Chai R, Zhong G, Zhang C , Cao W, Yan L, Zhang X , Xu Z
Received 24 March 2018
Accepted for publication 23 May 2018
Published 3 August 2018 Volume 2018:11 Pages 4537—4545
DOI https://doi.org/10.2147/OTT.S169155
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Geoffrey Pietersz
Wenhua Zhang,1,* Jue Wang,2,* Rong Chai,3 Guangxin Zhong,1 Cong Zhang,1 Wenjia Cao,4 Lei Yan,1 Xiang Zhang,1 Zhonghua Xu1
1Department of Urology, Qilu Hospital of Shandong University, Jinan, People’s Republic of China; 2Central Laboratory, The Second Hospital of Shandong University, Jinan, People’s Republic of China; 3Department of First Operating Room, Qilu Hospital of Shandong University, Jinan, People’s Republic of China; 4Department of Oncology, Shandong Cancer Hospital and Institute, Jinan, People’s Republic of China
*These authors contributed equally to this work
Introduction: Long noncoding RNAs (lncRNAs) are proven to be key regulators in cancer biology. Our screening effort for clear cell renal cell carcinoma (ccRCC) prognosis-associated lncRNAs identified a novel lncRNA, ccRCC prognosis-associated transcript 4 (CRPAT4), as one of the top candidates that was previously uncharacterized. The aim of this study was to verify the clinical significance of CRPAT4 in ccRCC patients and to explore its biological role as well as the underlying mechanisms, in ccRCC cell lines.
Materials and methods: Quantitative real-time polymerase chain reaction (PCR) was performed to demonstrate that CRPAT4 was differentially expressed between ccRCC and the normal controls and that high CRPAT4 expression significantly associated with advanced Fuhrman nuclear grades.
Results: Kaplan–Meier survival analysis with The Cancer Genome Atlas KIRC RNA sequencing data indicated that high CRPAT4 expression was significantly associated with poor overall survival and progression-free survival. Functional studies indicated that CRPAT4 was an HIF-1α regulated gene, and CRPAT4 knockdown significantly inhibited cell migration and proliferation in the absence of HIF-1α. In addition, a mechanistic study revealed that CRPAT4 could regulate the expression of the migration-associated protein AVL9.
Conclusion: Collectively, our study first identified CRPAT4 as a hypoxia-regulated lncRNA, acting as an oncogene in ccRCC progression via regulating AVL9 protein, thus expanding our knowledge on the hypoxia pathway in ccRCC biology from a noncoding perspective. Moreover, CRPAT4 has the potential to be a prognostic marker in ccRCC patients.
Keywords: CRPAT4, AVL9, hypoxia, clear cell renal cell carcinoma, migration
Introduction
Kidney cancer is among the 10 most common cancers in both men and women worldwide Renal cell carcinoma (RCC) is the most common pathological type of kidney cancer and represents the most lethal urologic cancer, with a 5-year survival rate of 69.4%.1 Clear cell RCC (ccRCC) is the most common subtype of RCC. For unknown reasons, the incidence of ccRCC has been rising since the 1990s.2 ccRCC rarely causes symptoms in its early stages. Unfortunately, there are currently no routine tests used to screen for kidney cancer in the absence of symptoms. Symptomatic ccRCCs are very likely to be in advanced stages, and thus are associated with extremely poor prognosis.3 In patients with metastatic diseases, the 5-year survival rate drops to <10%.4 Therefore, a better understanding of the molecular mechanisms of ccRCC initiation and progression are urgently required.
Advances in high-throughput sequencing technologies enabled comprehensive characterization of cancer-associated transcriptomes,5 including the “dark matter,” noncoding genes. In particular, long noncoding RNAs (lncRNAs) emerge as biomarkers due to their cancer specific expression patterns.6 Our previous screening effort for ccRCC prognosis-associated lncRNAs identified RP11-225B17.2 as one of the top candidates, which was previously uncharacterized. As a result, we named it as ccRCC prognosis-associated transcript 4 (CRPAT4). CRPAT4 is a monoexonic lncRNA, located at chr7:32798494-32798965, a gene desert region within the gene locus of the migration-associated gene AVL9. Our study proved that CRPAT4 was differentially expressed between ccRCC and normal renal tissues, and was upregulated in high-grade ccRCC tissues. Endogenous expression of CRPAT4 is mainly in the cytoplasma. Survival analysis indicated that the high expression of CRPAT4 was significantly associated with bad prognosis in ccRCC patients. Functional studies revealed that CRPAT4 is regulated by HIF-1α, and its knockdown significantly inhibited cell proliferation and migration by inhibiting the migration-associated gene AVL9. In addition, CRPAT4 is regulated by the hypoxia pathway and may act as a regulatory target and functional target of HIF-1α. Thus, our study first validated the novel lncRNA CRPAT4 in ccRCC, which has the potential to be a prognostic marker as well as a novel therapeutic target.
Materials and methods
Ethics statement
The study was approved by the Ethics Boards of Qilu Hospital of Shandong University. All surgical sample acquisitions were carried out strictly according to the institutional guidelines. Written informed consents were acquired from each patient for the use of these clinical materials in this research.
Patients and specimens
Thirty fresh-frozen ccRCC tissues were acquired from pathologically diagnosed ccRCC patients at the Qilu Hospital of Shandong University between January 2016 and February 2017, and immediately transferred to liquid nitrogen until use.
Cell lines
Human ccRCC cell lines A498, 786-O, Caki-1, and 769-P and the immortalized normal renal epithelial cell line HK-2 were purchased from ATCC (Manassas, VA, USA). A498 was cultured in Dulbecco’s Modified Eagle’s Medium with 10% fetal bovine serum (FBS: Gibco, Grand Island, NY, USA). The cell lines 786-O, Caki-1, and 769-P were maintained in Roswell Park Memorial Institute 1640 medium containing 10% FBS. HK-2 cells were cultured in Keratinocyte Serum Free Medium (Gibco). All cells were incubated in a humidified incubator with 5% CO2 at 37°C.
RNA preparation, reverse-transcription polymerase chain reaction (PCR), and quantitative real-time PCR
Total RNA was extracted from fresh surgical samples and cell lines transfected for 48 h using Trizol reagent (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s instructions and reverse transcribed into cDNA using M-MLV reverse transcriptase (TaKaRa Biotechnology, Dalian, People’s Republic of China). Quantitative PCR (qPCR) was performed in a Mastercycler ep realplex real-time PCR system (Eppendorf, Hamburg, Germany) using SYBR Green kit (Applied Biosystems, Foster City, CA, USA), using the following primers: CRPAT4 (forward): 5′-CTGTATGCAAAGAGTGATGGCA-3′ and CRPAT4 (reverse): 5′-AGCCGTCTTTGTGGCAATTT-3′, GADPH (forward): 5′-GAAGGTGAAGGTCGGAGT-3′ and GADPH (reverse): 5′-GAAGATGGTGATGGGATTTC-3′. GADPH was used as an endogenous control. Relative expression level was computed using 2−ΔΔCt method.
Subcellular fractionation
Nuclear/cytoplasmic subcellular fraction of 786-O cells was assessed using an NE-PER Nuclear and Cytoplasmic Extraction Kit (Thermo Fisher Scientific, Waltham, MA, USA) per manufacturer’s instructions, followed by qPCR.
siRNA treatment
The chemically modified siRNAs targeting CRPAT4, HIF-1α or HIF-2α, and control siRNA were purchased from Thermo Fisher Scientific. The sequence of CRPAT4-specific siRNA1 was 5′-CCA CAA AGA CGG CTG GAA A-3′. The sequence of CRPAT4-specific siRNA2 was 5′-ATG CAA AGA GTG ATG GCA A-3′. Cells were transfected with 100 nM siRNAs, using Lipofectamine 2000 (Thermo Fisher Scientific) per manufacturers’ instructions.
Hypoxia treatment
Cells were seeded into 6-well plates and cultured in the incubator for 24 h (37°C, 5% CO2). Subsequently, the cells were incubated in a hypoxia chamber (Stemcell Technology, Vancouver, Canada), inflated with 95% nitrogen and 5% CO2. Western blotting or qPCR was performed immediately after the cells were taken out of the hypoxia chamber at indicated time points.
Western blot
Proteins were extracted from cells transfected for 72 h, or proteins were immediately extracted from the hypoxia-treated cells, and these were immunoblotted with different primary antibodies as described previously.7 The primary antibodies used were anti-HIF1α (1:500; Cell Signaling Technology, Danvers, MA, USA), anti-HIF2α (1:500; R&D Systems, Minneapolis, MN, USA), anti-AVL9 (1:100; abcam, Cambridge, MA, USA), and anti-GAPDH (1:1,000; Cell Signaling Technology).
Wound healing assay
The wound healing assay was performed as described earlier.7 In brief, 24 h after transfection, uniform wounds were created across the confluent cell monolayer using sterile 200 μL pipette tips. The plates were washed twice to remove the detached cells and then incubated in serum-free medium for 24 h. Digital pictures were taken at 0 and 24 h after the scratch. ImageJ (National Institutes of Health, Bethesda, MD, USA) was used to calculate the width of the wound at four locations within each well. The percentage of wound closure was quantified by dividing the wound width that had healed at 24 h by the initial width at 0 h. Each experiment was performed three times using triplicate wells.
Transwell invasion assay
Transwell migration assay was used to determine the migratory capacity of ccRCC cell lines. Briefly, cells with or without CRPAT4 siRNAs treatment were seeded onto the Transwell chamber (pore size, 8 mm; Corning Inc., Corning, NY, USA) with FBS-free medium. Five hundred microliters of medium with 10% FBS was added into the lower chamber as chemoattractant. After incubation for 24 h, the nonmigrated cells on the upper surface of the membrane were removed. The membranes were fixed and stained with 0.1% crystal violet. Stained cells were photographed and counted in four randomly selected high-microscopic fields (400×).
Cell proliferation assay
About 5.0×103 cells were seeded into each well of 24-well plates and then cultured in the incubator for 24 h (37°C, 5% CO2). The cells were than transfected with either CRPAT4-specific siRNA or control siRNA with Lipofectamine 2000 according to the protocol. The medium was changed 12 h after the transfection, and then the cells were incubated for 6 days without changing the medium. Subsequently, cells were counted using the cell Countstar (IC1000) at various points (from day 1 to 5). The cell growth curve was drawn according to the data, and all points were measured in triplicate.
RNA sequencing data processing
Fastq files of the KIRC project (n=541) from The Cancer Genome Atlas Research Network (TCGA, http://cancergenome.nih.gov) were acquired from the CGhub. Reads were aligned using the STAR 2.4.2,8 and the read abundance was calculated using FeatureCounts 1.4.6.9
Statistical analysis
The CRPAT4 expression in surgical samples and the matched normal renal tissues was calculated using paired t-test. The survival analysis was calculated using Kaplan–Meier curves compared by the log rank test. The data of independent cell functional experiments were expressed as mean ± standard error of the mean and analyzed by Student’s t-test. Multiple group statistic comparisons were analyzed by one-way analysis of variance followed with post hoc tests. All statistical analyses were performed with SPSS 16.0 statistical software (SPSS Inc., Chicago, IL, USA). P-value <0.05 was considered statistically significant.
Results
CRPAT4 is upregulated in ccRCC tissues and cell lines
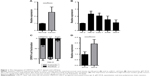
We applied qPCR to quantitatively analyze the CRPAT4 expression levels in ccRCC tissues and cell lines. Results showed that CRPAT4 was significantly increased in ccRCC tissues compared to in the matched normal renal tissues (Figure 1A, P=0.0357). Additionally, as shown in Figure 1B, CRPAT4 was remarkably highly expressed in all four ccRCC cell lines, as compared with the normal renal epithelial cell line HK-2. And, the expression of CRPAT4 was highest in 786-O and Caki-1, among the four common ccRCC cell lines. Cell fractionation followed by qPCR revealed that CRPAT4 was mainly localized in the cytoplasm of 786-O cells (Figure 1C).
CRPAT4 expression predicts poor prognosis in ccRCC patients
Analysis of the CRPAT4 expression in 30 ccRCC surgical specimens revealed that CRPAT4 was significantly highly expressed in the advanced Fuhrman nuclear grade tumors (G3+ G4, n=11) compared to in the low-grade tumors (G1+ G2, n=19) (Figure 1D, P=0.0357). We further queried whether CRPAT4 expression correlated with the patients’ outcomes, and TCGA KIRC RNA sequencing data was extracted for the survival analysis. All 541 subjects of the TCGA KIRC project were included. To do extremity analysis, the top quarter of patients with the highest CRPAT4 expression were defined as the CRPAT4 high-expression group (n=135), and the bottom quarter of patients with the lowest CRPAT4 expression were defined as the CRPAT4 low-expression group (n=136). Kaplan–Meier curve analysis showed that high expression of CRPAT4 is significantly associated with bad prognosis in terms of overall survival (Figure 2A, P<0.0001), as well as progression-free survival (Figure 2B, P<0.0001).
CRPAT4 is regulated by hypoxia pathway
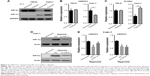
As is known, the hypoxia pathway plays a crucial role in kidney cancer pathophysiology. To examine the possible regulatory mechanism of the differential expression of CRPAT4 in ccRCC and normal renal tissues, we first checked the effect of hypoxia treatment on the CRPAT4 expression in ccRCC cell lines. As is shown in Figure 3A, both HIF-1α and HIF-2α were successfully induced in Caki-1 cells (vhl wild-type) under hypoxia treatment, and HIF-2α was constitutively expressed in 786-O cells (vhl mutant) no matter under hypoxia or normoxia conditions. CRPAT4 was significantly downregulated in Caki-1 cells treated with hypoxia, than in normoxia, but not in 786-O cells (Figure 3B, P=0.0025). On the contrary, the well-known hypoxia-regulated gene PLOD2 was significantly upregulated in Caki-1 cells treated with hypoxia, than in normoxia (Figure 3C, P=0.0015), and this dysregulation was not observed in 786-O cells. To further investigate whether HIF-1α or HIF-2α induced this regulatory effect, siRNA targeting either HIF-1α or HIF-2α was adopted. The knockdown efficacy of HIF-specific siRNAs was verified in Caki-1 cells by Western blotting (Figure 3D). The inhibitory effect of hypoxia on CRPAT4 expression was significantly counteracted by HIF-1α siRNA treatment in Caki-1 cells (Figure 3E), but not by the HIF-2α siRNA treatment.
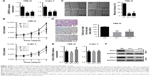
CRPAT4 knockdown significantly inhibits cell migration and proliferation in vitro
Loss-of-function experiments were adopted to investigate the biological function of CRPAT4 in ccRCC cell lines. CRPAT4 expression was significantly inhibited in 786-O and Caki-1 cells treated with CRPAT4-specific siRNA1 and siRNA2 compared with the negative control siRNA (Figure 4A, all P<0.05). Cell counting on seven consecutive days showed that the cell proliferation was significantly inhibited in 786-O cells treated with CRPAT4 siRNAs compared with the control siRNA group, while only modest inhibitory effect on cell proliferation was observed in Caki-1 cells treated with CRPAT4 siRNAs compared with the control siRNA group (Figure 4B). Wound healing assay showed that significantly less percentages of the initial wound width had been healed in the CRPAT4 siRNA-treated 786-O cells as compared with the control siRNA group (Figure 4C, all P<0.0001). Transwell migration assay showed that significantly less cells penetrated through the membrane in the CRPAT4 siRNA-treated 786-O cells than in the control siRNA group (Figure 4D, all P<0.0001). No marked changes were observed in Caki-1 cells in the wound healing assay and Transwell migration assay (data not shown). As CRPAT4 gene resides within the gene locus of migration-associated gene AVL9, we further assessed the effect of CRPAT4 knockdown on the expression of AVL9. Marked downregulation of AVL9 protein was observed in the CRPAT4 siRNA-treated 786-O cells compared with the control siRNA group; however, only a modest effect was observed in Caki-1 cells following the CRPAT4 knockdown (Figure 4F). Furthermore, no significant changes of the AVL9 mRNA expression were observed following CRPAT4 knockdown in both 786-O and Caki-1 cells (Figure 4E).
Discussion
Recently, a growing number of studies have implicated the important roles of lncRNAs in cancer biology,6,10 including in ccRCC.11 One of the first characterized lncRNAs, HOTAIR, was demonstrated to be differentially expressed in ccRCC and to promote ccRCC cell proliferation and migration through repressing target gene transcription by upregulating histone H3K27 demethylase JMJD3.12,13 MALAT-1 is generally expressed at a higher level in cancerous tissue compared with normal tissue with enhancement of cell proliferation and distant metastasis.14–16 And, in accordance, MALAT-1 is also upregulated in ccRCC, and promotes cell proliferation and invasion by inducing the epithelial-to-mesenchymal transition.17,18 RCCRT1 was proven to be a reliable prognostic biomarker in ccRCC due to its cancer- and lineage-specific expression patterns.19 Also, lncRNAs GAS5,20 MEG3,21 and NBAT122 represent important tumor suppressors in ccRCC. Studies on ccRCC-associated lncRNAs improved our knowledge on kidney cancer biology and provided promising candidates offering potentials as biomarkers or therapeutic targets. However, on the whole, RCC lncRNA studies still remain in their infancy.23
In our screening study for ccRCC prognosis-associated lncRNAs, we identified CRPAT4 as one of the top candidates, which remained previously uncharacterized. In our present study, we confirmed the differential expression of CRPAT4 in clinical ccRCC samples and matched normal renal tissues. In addition, the expression of CRPAT4 was found to be significantly increased in the more advanced tumors (Fuhrman nuclear grade G3–G4) compared with the less-advanced tumors (Fuhrman nuclear grade G1–G2). Our findings supported its potential as a diagnostic marker, and more importantly, a risk stratification factor in the Chinese ccRCC population, thus warranting further confirmation in a larger ccRCC cohort. As CRPAT4 expression is associated with the Fuhrman nuclear grades, we further explored its prognostic value in a large ccRCC RNA sequencing cohort from TCGA KIRC project. Survival analysis by Kaplan–Meier curves demonstrated that high CRPAT4 expression was associated with poor outcomes, in terms of overall survival as well as progression-free survival. Whether CRPAT4 could help evaluate patient outcome prospectively is not known, and this thus warrants further studies in the future.
As is well know, the vhl tumor suppressor gene acts as a critical gatekeeper with respect to the development of RCC.24 It is reported that the inactivating mutation of vhl was found in >80% of sporadic ccRCC cases.25 Inactive mutation of the vhl gene, either genetic or epigenetic, leads to vhl protein deficiency, and further leads to the accumulation of HIF as well as the abnormal activation of the hypoxia pathway. To investigate the potential regulatory effect of the hypoxia pathway on the CRPAT4 expression, we examined the CRPAT4 expression in the vhl mutant cell line 786-O and vhl wild-type cell line Caki-1 under hypoxic treatment. The 786-O cells constitutively and exclusively expressed HIF-2α but not HIF-1α due to the HIF-1α gene mutation, while Caki-1 cells expressed inducible HIF-1α and HIF-2α under hypoxic treatment. The results finally showed that CRPAT4 expression was downregulated in the hypoxia-treated Caki-1 cells (vhl wild-type), with the known hypoxia-regulated gene PLOD2 successfully induced, but remained unchanged in 786-O cells (vhl mut). And, further knockdown studies demonstrated that HIF-1α, but not HIF-2α, carries out the inhibitory effect upon CRPAT4 expression. Interestingly, a functional study revealed that CRPAT4 knockdown could significantly inhibit cell proliferation and migration only in vhl mut 786-O cells, but the effect remained modest in vhl wild-type Caki-1 cells. There might be two reasons for this. First, during the 6-day cell proliferation assay, hypoxia condition will inevitably be induced. HIF-1α protein is induced, too, which leads to downregulation of CRPAT4. Thus, further knockdown of CRPAT4 might be less effective. Second, CRPAT4 represents a potential oncogene in ccRCC and is a potential functional target of the tumor suppressor HIF-1α.26 Also, the oncogenic effect of CRPAT4 will be compromised in the HIF-1α positive cell line, but not the oncoprotein HIF-2α positive cell line.27 These findings indicate that CRPAT4 acts as an oncogene in ccRCC and is a regulatory target as well as a functional target of the tumor suppressor HIF-1α (Figure 5).
  | Figure 5 Schematic diagram of CRPAT4 as an oncogene in ccRCC progression. |
CRPAT4 is a monoexon lncRNA which resides in a gene desert region within the intron of the largest isoform of AVL9. AVL9 was first described in budding yeast as an exocytosis gene,28 and recent studies demonstrated that AVL9 has an important role in cell polarity, cell migration, and cell cycle progression.29,30 Notably, AVL9 knockdown significantly inhibited the human adenocarcinomic alveolar basal epithelial cell A549 migration.30 Interestingly, remarked downregulation of AVL9 protein, but not the AVL9 mRNA, was observed following CRPAT4 knockdown, indicating that CRPAT4 promotes ccRCC cell migration via facilitating AVL9 translation. Considering the cytoplasmic localization of CRPAT4, it is possible that CRPAT4 acts as a scaffold, facilitating the binding of translation initiation complex and the AVL9 mRNA, promoting the translation of AVL9 proteins. However, due to the limited laboratory core facilities, this hypothesis was not verified. Further studies focusing on the detailed mechanism of the regulation of CRPAT4 on AVL9 are encouraged.
The limitation of this study is the lack of detailed descriptions of the molecular mechanisms underlying the interaction between the HIF proteins and CRPAT4, and between CRPAT4 and its target gene AVL9, which warrant further studies to unveil the HIF-1α tumor-suppressing effect from a noncoding perspective.
In conclusion, our study first demonstrated a novel lncRNA CRPAT4 as a potential prognostic marker as well as a risk stratification factor in ccRCC patients. The functional study revealed that CRPAT4 acts as an oncogene in ccRCC progression, promoting cell migration via regulating the translation process of the migration-associated gene AVL9. Moreover, CRPAT4 is regulated by HIF-1α, representing a regulatory target as well as a functional target of the tumor suppressor HIF-1α in ccRCC, which thus expands our knowledge on the hypoxia pathway in ccRCC biology from a noncoding perspective. Our study also encourages further efforts to study the detailed molecular mechanisms underlying the CRPAT4–HIF-1α and the CRPAT4–AVL9 interactions in ccRCC progression.
Acknowledgment
This work was supported by the National Natural Science Foundation of China (no. 81702521) and Provincial Natural Science Foundation of Shandong (no. ZR2018BH018 and no. ZR2017PH019).
Disclosure
The authors report no conflicts of interest in this work.
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. | ||
Gandaglia G, Ravi P, Abdollah F, et al. Contemporary incidence and mortality rates of kidney cancer in the United States. Can Urol Assoc J. 2014;8(7–8):247–252. | ||
Kroeger N, Seligson DB, Signoretti S, et al. Poor prognosis and advanced clinicopathological features of clear cell renal cell carcinoma (ccRCC) are associated with cytoplasmic subcellular localisation of hypoxia inducible factor-2α. Eur J Cancer. 2014;50(8):1531–1540. | ||
Minardi D, Quaresima L, Santoni M, et al. Recent aspects of sunitinib therapy in patients with metastatic clear-cell renal cell carcinoma: a systematic review of the literature. Curr Urol Rep. 2015;16(2):3. | ||
Wajnberg G, Passetti F. Using high-throughput sequencing transcriptome data for INDEL detection: challenges for cancer drug discovery. Expert Opin Drug Discov. 2016;11(3):257–268. | ||
Iyer MK, Niknafs YS, Malik R, et al. The landscape of long noncoding RNAs in the human transcriptome. Nat Genet. 2015;47(3):199–208. | ||
Zhang X, Ren J, Yan L, et al. Cytoplasmic expression of pontin in renal cell carcinoma correlates with tumor invasion, metastasis and patients’ survival. PLoS One. 2015;10:e0118659. | ||
Dobin A, Davis CA, Schlesinger F, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29(1):15–21. | ||
Liao Y, Smyth GK, Shi W. featurecounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics. 2014;30(7):923–930. | ||
Kondo Y, Shinjo K, Katsushima K. Long non-coding RNAs as an epigenetic regulator in human cancers. Cancer Sci. 2017;108(10):1927–1933. | ||
Blondeau JJ, Deng M, Syring I, et al. Identification of novel long non-coding RNAs in clear cell renal cell carcinoma. Clin Epigenetics. 2015;7:10. | ||
Xia M, Yao L, Zhang Q, et al. Long noncoding RNA HOTAIR promotes metastasis of renal cell carcinoma by up-regulating histone H3K27 demethylase JMJD3. Oncotarget. 2017;8(12):19795–19802. | ||
Wu Y, Liu J, Zheng Y, You L, Kuang D, Liu T. Suppressed expression of long non-coding RNA HOTAIR inhibits proliferation and tumourigenicity of renal carcinoma cells. Tumour Biol. 2014;35(12):11887–11894. | ||
Kan JY, Wu DC, Yu FJ, et al. Chemokine (C-C Motif) ligand 5 is involved in tumor-associated dendritic cell-mediated colon cancer progression through non-coding RNA MALAT-1. J Cell Physiol. 2015;230(8):1883–1894. | ||
Han T, Jiao F, Hu H, et al. EZH2 promotes cell migration and invasion but not alters cell proliferation by suppressing E-cadherin, partly through association with MALAT-1 in pancreatic cancer. Oncotarget. 2016;7(10):11194–11207. | ||
Chen D, Liu L, Wang K, et al. The role of MALAT-1 in the invasion and metastasis of gastric cancer. Scand J Gastroenterol. 2017;52(6–7):790–796. | ||
Chen S, Ma P, Zhao Y, et al. Biological function and mechanism of MALAT-1 in renal cell carcinoma proliferation and apoptosis: role of the MALAT-1-Livin protein interaction. J Physiol Sci. 2017;67(5):577–585. | ||
An J, Liu H, Magyar CE, et al. Hyperactivated JNK is a therapeutic target in pVHL-deficient renal cell carcinoma. Cancer Res. 2013;73(4):1374–1385. | ||
Song S, Wu Z, Wang C, et al. RCCRT1 is correlated with prognosis and promotes cell migration and invasion in renal cell carcinoma. Urology. 2014;84(3):730.e1–e7. | ||
Qiao HP, Gao WS, Huo JX, Yang ZS. Long non-coding RNA GAS5 functions as a tumor suppressor in renal cell carcinoma. Asian Pac J Cancer Prev. 2013;14(2):1077–1082. | ||
Wang M, Huang T, Luo G, et al. Long non-coding RNA MEG3 induces renal cell carcinoma cells apoptosis by activating the mitochondrial pathway. J Huazhong Univ Sci Technolog Med Sci. 2015;35(4):541–545. | ||
Xue S, Li QW, Che JP, Guo Y, Yang FQ, Zheng JH. Decreased expression of long non-coding RNA NBAT-1 is associated with poor prognosis in patients with clear cell renal cell carcinoma. Int J Clin Exp Pathol. 2015;8(4):3765–3774. | ||
Seles M, Hutterer GC, Kiesslich T, et al. Current insights into long non-coding RNAs in renal cell carcinoma. Int J Mol Sci. 2016;17(4):573. | ||
Moch H. [Von-Hippel-Lindau (VHL) protein function by initiation and progression of renal cancer]. Pathologe. 2008;29(Suppl 2):149–152. | ||
Moore LE, Nickerson ML, Brennan P, et al. Von Hippel-Lindau (VHL) inactivation in sporadic clear cell renal cancer: associations with germline VHL polymorphisms and etiologic risk factors. PLoS Genet. 2011;7(10):e1002312. | ||
Gudas LJ, Fu L, Minton DR, Mongan NP, Nanus DM. The role of HIF1α in renal cell carcinoma tumorigenesis. J Mol Med (Berl). 2014;92(8):825–836. | ||
Morris MR, Hughes DJ, Tian YM, et al. Mutation analysis of hypoxia-inducible factors HIF1A and HIF2A in renal cell carcinoma. Anticancer Res. 2009;29(11):4337–4343. | ||
Harsay E, Schekman R. Avl9p, a member of a novel protein superfamily, functions in the late secretory pathway. Mol Biol Cell. 2007;18(4):1203–1219. | ||
Li Y, Xu J, Xiong H, et al. Cancer driver candidate genes AVL9, DENND5A and NUPL1 contribute to MDCK cystogenesis. Oncoscience. 2014;1(12):854–865. | ||
Linford A, Yoshimura S, Nunes Bastos R, et al. Rab14 and its exchange factor FAM116 link endocytic recycling and adherens junction stability in migrating cells. Dev Cell. 2012;22(5):952–966. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.