Back to Journals » Clinical and Experimental Gastroenterology » Volume 16
Hypertension and Histopathology Severity of Non-Alcoholic Fatty Liver Disease Among Adults with Obesity: A Cross-Sectional Study
Authors Chambergo-Michilot D, Rodrigo-Gallardo PK, Huaman MR, Vasquez-Chavesta AZ, Salinas-Sedo G, Toro-Huamanchumo CJ
Received 13 February 2023
Accepted for publication 13 June 2023
Published 14 August 2023 Volume 2023:16 Pages 129—136
DOI https://doi.org/10.2147/CEG.S402498
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Santosh Shenoy
Diego Chambergo-Michilot,1,* Paola K Rodrigo-Gallardo,2 Mariella R Huaman,3,* Angie Z Vasquez-Chavesta,4 Gustavo Salinas-Sedo,5 Carlos J Toro-Huamanchumo6,7,*
1Universidad Científica del Sur, Lima, Peru; 2Hospital Docente Las Mercedes, Chiclayo, Peru; 3Facultad de Medicina Humana, Universidad Nacional Mayor de San Marcos, Lima, Peru; 4Facultad de Medicina Humana, Universidad Católica Santo Toribio de Mogrovejo, Chiclayo, Peru; 5Unidad de Investigación Multidisciplinaria, Clínica Avendaño, Lima, Peru; 6Unidad de Investigación para la Generación y Síntesis de Evidencias en Salud, Universidad San Ignacio de Loyola, Lima, Peru; 7OBEMET Centro de Obesidad y Salud Metabólica, Lima, Peru
*These authors contributed equally to this work
Correspondence: Carlos J Toro-Huamanchumo, Universidad San Ignacio de Loyola, Av. la Fontana 550, La Molina, Lima, 15024, Peru, Tel + 51944942888, Email [email protected]
Background: Cardiovascular diseases are responsible for the majority of deaths resulting from non-alcoholic fatty liver disease (NAFLD). NAFLD is associated with hypertension and this is a key predictor of severe liver outcomes and an indicator of nonspecific portal fibrosis.
Aim: To assess the association between hypertension and NAFLD severity.
Methods: We conducted a secondary analysis of data from Peruvian adults with obesity and NAFLD who attended a Peruvian bariatric center. The severity of NAFLD was assessed using the Fatty Liver Inhibition of Progression algorithm / Steatosis, Activity and Fibrosis score. Hypertension was determined by either being recorded in the medical records or if the patient had a systolic pressure ≥ 140 mmHg or diastolic pressure ≥ 90 mmHg. To evaluate the association of interest, we calculated crude and adjusted prevalence ratios (aPR) using Poisson generalized linear models with logarithmic link function and robust variances. For the multivariable models, we adjusted for age, sex, physical activity and smoking.
Results: Our study included 234 participants. The prevalence of hypertension was 19.2%, while the prevalence of severe NAFLD was 46.2%. After adjusting for confounders, the prevalence of hypertension was found to be significantly higher in the severe NAFLD group compared to the non-severe group (aPR = 1.33; 95% CI: 1.03– 1.74). When stratified by the presence of metabolic syndrome (MetS), the association remained significant only in the group without MetS (aPR = 1.80; 95% CI: 1.05– 3.11).
Conclusion: We found an association between hypertension and severe NAFLD in adults with obesity, particularly in those without MetS.
Keywords: non-alcoholic fatty liver disease, hypertension, obesity
Introduction
Obesity is a multifactorial disease and is considered a public health problem worldwide. In 2014, the prevalence in male and female adults was 11% and 15%, respectively.1 In 2019, the National Institute of Statistics and Informatics (INEI, by its acronym in Spanish), reported that 22.3% and 14.1% of the Peruvian population older than 15 years had obesity and hypertension, respectively.2 Obesity coexists with other chronic diseases, including non-alcoholic fatty liver disease (NAFLD).3
NAFLD has a worldwide prevalence of 25%; it is higher in the Middle East (32%) and South America (31%).4 Its progression leads to non-alcoholic steatohepatitis (NASH) and cirrhosis.5,6 Besides liver complications, the prevalence of several non-communicable diseases (NCDs) in patients with NAFLD is high: obesity (51.3%), diabetes mellitus (22.5%), dyslipidemia (69.2%) and hypertension (39.3%). Furthermore, it was seen that the prevalence of these pathologies increased if patients with NASH.4 As a result, patients with NAFLD have a higher risk of cardiovascular disease (CVD) than patients without it.7,8 Besides, the risk of CVD mortality may be greater in patients with NASH and advanced fibrosis rather than simple steatosis.6 This suggests a relationship between liver disease progression and cardiovascular pathology.
The coexistence of NAFLD and hypertension quadruples the risk of atherosclerosis in adults compared to those with only hypertension or only NAFLD, which represent a significantly risk for CVD.9 This underlines the importance of investigating the relationship between these two pathologies. Previous studies have reported that hypertension may be a consequence of NAFLD,10–12 as much as a predictor of severe liver outcomes.13,14 The association between the presence of NAFLD and an increased risk for CVD is well-documented. Among these associated conditions, hypertension is particularly notable.15 It has been demonstrated that the pathological changes brought about by NAFLD can significantly impact myocardial structure and endothelial function, both of which are crucial factors in the development and progression of hypertension.16 However, the information is still limited regarding NAFLD severity and its linkage with hypertension. For example, previous research only studied Asian and European populations with small sample sizes. Likewise, it did not use biopsy (the gold standard) for the diagnosis of NAFLD,10–12 which may provide more accurate results. Therefore, this study aimed to assess the association between hypertension and the severity of biopsy-proven NAFLD in adults with obesity.
Methods
Study Design
Cross-sectional analysis of secondary data from a private bariatric center.
Population and Sample
Adults with obesity and NAFLD, who attended a private bariatric center located in Lima (Peru) between 2017 and 2020.
We included people aged between 18–59 years old with a body mass index (BMI) ≥ 30 kg/m2 and biopsy-proven NAFLD. We excluded adults with self-reported harmful alcohol consumption, those with a history of acute myocardial infarction (history or ischemia found in electrocardiogram), history of hepatitis B or C, autoimmune liver diseases, hemosiderosis, hemochromatosis, chronic consumption of drugs that can induce secondary NAFLD/NASH (eg tamoxifen, methotrexate, amiodarone, corticosteroids, valproate and nitrofurantoin), pregnant women, and patients with less than 10 portal spaces and/or depth <5mm in the biopsy.
Outcome
The severity of NAFLD according to the liver biopsies of all patients was evaluated using the Fatty Liver Inhibition of Progression algorithm / Steatosis, Activity and Fibrosis score (FLIP/SAF).17 The SAF score assessed the grade of steatosis (S): S0 (<5%), S1 (5–33%), S2 (34–66%) and S3 (>67%), the grade of activity (A): A0 to A4, according to the grade of hepatocellular ballooning with lobular inflammation, and the fibrosis (F): from F0 to F4. Severe NAFLD was defined by A≥3 and/or F≥3 at SAF score. These assessments were performed by two external pathologists. Their findings were then provided to the clinical team of the bariatric center, who incorporated this information into their database.
Exposure
Hypertension was defined if the patient had a mean systolic pressure (SBP) ≥140 mmHg or diastolic pressure (DBP) ≥90 mmHg using ambulatory blood pressure monitoring at admission, or if hypertension was stated in the clinical history regardless of whether it was controlled or not.
Other Variables
We collected the following variables: age, sex (female or male), T2DM, insulin resistance (defined as an Homeostasis Model Assessment (HOMA-IR) ≥2.5), metabolic syndrome (MetS) (defined according International Diabetes Federation criteria),18 history of tobacco use, morbid obesity (BMI ≥40 kg/m2), physical activity (defined as 150 min/week, or at least 30 min/5 days a week, of moderate-intensity activity), BMI (kg/m2), abdominal circumference (cm), hemoglobin (g/dL), glucose (mgdL), cholesterol (mg/dL), high-density-lipoprotein (mg/dL), low-density-lipoprotein (LDL, mg/dL), triglycerides (mg/dL), HOMA-IR and serum insulin (uU/mL).
Statistical Analysis
Data analysis was performed in Stata v16 (StataCorp, TX, USA). Qualitative variables were presented as absolute and relative frequencies. Quantitative variables were expressed as mean (with standard deviation) or median (with p25-p75) according to their distribution, which was evaluated with the histogram, skewness and kurtosis.
Bivariate analyses were presented according to the presence of hypertension and severe NAFLD. For assessing the relationship between hypertension or severe NAFLD with numerical independent variables, the Student’s t-test or Mann Whitney U-test was used, according to the distribution. For assessing the relationship with categorical variables, the Chi2 test or Fisher’s exact test was used.
To assess the association between hypertension and severe NAFLD, crude (cPR) and adjusted prevalence ratios (aPR) were calculated using Poisson generalized linear models with logarithmic link function and robust variances. For the multivariable models, we followed an epidemiological approach, adjusting for age, sex, physical activity and smoking status. Additionally, we evaluated whether the variables of morbid obesity and MetS were effect modifiers, using the Wald test and the comparison of models with the log-likelihood ratio test. Since we found that MetS was an effect modifier, we presented our models stratified. We also assessed the presence of multicollinearity using the variance inflation factor (VIF), considering values less than 10 as acceptable. All models were presented with their 95% confidence intervals (95% CI), and a p-value <0.05 was considered significant for all tests.
Ethics
The present study was approved by the Institutional Review Board of the Clínica Avendaño. In addition, this was an analysis of a secondary database and the data were not personally identifiable. The project adhered to the principles of confidentiality and anonymity, according to the Declaration of Helsinki and its subsequent revisions.
Results
Characteristics of the Study Population
We included 234 participants in the study. A total of 63.2% (n=148) were women. The prevalence of hypertension was 19.2% (n=45), while the prevalence of severe NAFLD was 53.8% (n=126).
Characteristics of the Study Population According to the Presence of Severe NAFLD
We observed a higher frequency of severe NAFLD in participants with hypertension (68.9% vs 3.1%, p = 0.024), MetS (62.1% vs 43.1%; p = 0.004), and insulin resistance (56.3% vs 28.6%, p = 0.015) compare to participants without these conditions. Furthermore, we found significantly higher medians for glucose (94 vs 89; p<0.001) and HOMA-IR (5.6 vs 5.1; p = 048) in participants with severe disease (Table 1).
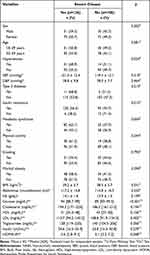 |
Table 1 Sociodemographic, Clinical, Anthropometric and Laboratory Characteristics According to NAFLD Severity (n=234) |
Characteristics of the Study Population According to the Presence of Hypertension
We observed a significantly higher frequency of hypertension in participants with severe NAFLD compared to non-severe NAFLD (24.6% vs 13.0%, p =0.024). In patients with hypertension, the means were significantly higher for SBP (131.7 vs 117.6, p = <0.001), DBP (85.3 vs 76.8, p = <0.001), BMI (41.0 vs 38.3, p = 0.005), abdominal circumference (123.1 vs 114.4, p<0.001), and Hb (14.5 vs 13.9, p = 0.030). Furthermore, we found significantly higher medians for glucose (94 vs 92; p =0.002) and HOMA-IR (7.5 vs 5.3; p = 0.003) in participants with hypertension (Table 2).
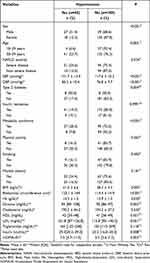 |
Table 2 Sociodemographic, Clinical, Anthropometric and Laboratory Characteristics According to Hypertension (n=234) |
Association Between Hypertension and Severe NAFLD
In the crude model, the prevalence of hypertension was significantly higher in the severe NAFLD group compared to the non-severe group (cPR = 1.37; 95% CI: 1.08–1.75). Similarly, when we adjusted for confounders, the association remained statistically significant (aPR = 1.33; 95% CI: 1.03–1.74). When we stratified by metabolic syndrome and adjusted for sex, age, physical activity and smoking, the association remained significant only in the non-metabolic syndrome group (aPR = 1.80; 95% CI: 1.05–3.11) (Table 3).
 |
Table 3 Association Between Hypertension and Severe NAFLD |
Discussion
Main Findings
Our study in adults with obesity and NAFLD showed an association between hypertension and severe disease. However, this association was not evidenced in adults with metabolic syndrome. Additionally, we found that the prevalence of severe NAFLD in adults with hypertension and obesity was 68.9%.
Comparison with Other Studies
Although a bidirectional relationship is considered to exist between hypertension and NAFLD,19 the conditional probability of NAFLD given the presence of hypertension is 23.6%.20 Our study has shown that, in patients with NAFLD and obesity, hypertension increases the prevalence rates of severe NAFLD compared to patients with non-hypertension. Similar to our results, in patients with obesity undergoing laparoscopic obesity surgery, systemic hypertension was an independent predictor of an advanced form of NAFLD.21 Besides, a national study of adults from the United States22 reported that the prevalence of advanced fibrosis, using different noninvasive scores, was higher in those with hypertension compared to those with optimal blood pressure (3% to 9.6% vs < 1%).
Interpretation of Findings
Animal studies have shown how hypertension promotes the inflammatory response and fibrosis of the liver by inducing increased aggregation of CD68-positive Kupffer cells and cytokines release, and by reduction of liver antioxidant capacity.23,24 This could explain why hypertension also worsens liver fibrosis status, leading to the progression of NAFLD. Moreover, a trial evidenced that an antihypertensive drug was associated with improvement of liver fibrosis25, which could be a clue to the association between hypertension and NAFLD severity that should be studied further.
We found that hypertension was associated with severe NAFLD in absence of metabolic syndrome. Since MetS could be a mediator of the relationship between hypertension and steatosis,26 it is plausible to expect that the association between hypertension and NAFLD severity is not significant in the metabolic syndrome group, considering also that one of the criteria for MetS includes hypertension. Conversely, we found a significant association in the non-metabolic syndrome group. Indeed, hypertension has a role in the development of NAFLD independently from metabolic factors,27 which could explain our results. Regarding pathophysiology, hypertension-related changes in the liver could be explaining its role in NAFLD severity.28 Nevertheless, despite we found an important role of hypertension in NAFLD severity, that does not imply that other factors should be ignored. Metabolic syndrome is a combination of cardiovascular parameters that could influence NAFLD severity even more than a sole factor like hypertension.
Relevance in Clinical Practice
An association between hypertension and NAFLD severity means that clinicians should boost the healthcare of hypertensive patients with NAFLD. Better care includes a better monitoring of hypertension and NAFLD and a more aggressive change of harmful habits that influence NAFLD progression.
Prognostic factors must be included in clinical practice in the best possible way. For example, hypertension influence could be included in risk scores for NAFLD. Also, further studies should assess severity-prediction models that include hypertension. It may help the clinician to better classify patient’s disease progression in order to make evidence-based decisions. Currently, some guidelines29 recommend that patients with NASH with fibrosis associated with hypertension should receive closer monitoring because of a higher risk of disease progression.
Limitations
Interestingly, antihypertensive drugs may reduce the fibrosis degree in the liver. We did not collect the information about drug use, frequency and dose. Since antihypertensive treatment reduces hypertension severity and probably liver fibrosis, the absence of this variable may introduce a limitation when interpreting the results. For further studies we highly recommend not only collecting antihypertensive use, but adherence to therapy.
Some other limitations should be highlighted. First, we carried out the study in a single-center in a Latin American country, which may reduce the external validity of our results. Second, since the stratified analysis was exploratory, it might have been not statistically powered enough, being subject to a type 2 error. Third, our cross-sectional analysis does not allow us to assume causality; however, the results give readers insights for further studies that might improve our limitations: multicentric, greater sample size, longitudinal analysis.
Conclusion
There is an association between hypertension and severity of NAFLD in our sample of adults with obesity and NAFLD. After stratification, this association remains in the absence of metabolic syndrome.
Funding
This study was self-funded.
Disclosure
The authors declare no competing interests for this work.
References
1. Arroyo-Johnson C, Mincey KD. Obesity epidemiology worldwide. Gastroenterol Clin North Am. 2016;45(4):571–579. doi:10.1016/j.gtc.2016.07.012
2. Instituto Nacional de Estadisticas e Informatica (INEI). Peru: Enfermedades No Transmisibles y Transmisibles, 2019 [Internet]; 2020. Available from: https://proyectos.inei.gob.pe/endes/2019/SALUD/ENFERMEDADES_ENDES_2019.pdf.
3. Godoy-Matos AF, Silva Júnior WS, Valerio CM. NAFLD as a continuum: from obesity to metabolic syndrome and diabetes. Diabetol Metab Syndr. 2020;12(1):60. doi:10.1186/s13098-020-00570-y
4. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease—Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84. doi:10.1002/hep.28431
5. Peng C, Stewart AG, Woodman OL, Ritchie RH, Qin CX. Non-alcoholic steatohepatitis: a review of its mechanism, models and medical treatments. Front Pharmacol. 2020;11:603926.
6. Armstrong MJ, Adams LA, Canbay A, Syn WK. Extrahepatic complications of nonalcoholic fatty liver disease. Hepatol Baltim Md. 2014;59(3):1174–1197.
7. Velarde-Ruiz Velasco JA, García-Jiménez ES, García-Zermeño KR, et al. Extrahepatic complications of non-alcoholic fatty liver disease: its impact beyond the liver. Rev Gastroenterol Mex. 2019;84(4):472–481.
8. Veracruz N, Hameed B, Saab S, Wong RJ. The association between nonalcoholic fatty liver disease and risk of cardiovascular disease, stroke, and extrahepatic cancers. J Clin Exp Hepatol. 2021;11(1):45–81.
9. Cattazzo F, Lombardi R, Mantovani A, et al. Subclinical and clinical atherosclerosis in non-alcoholic fatty liver disease is associated with the presence of hypertension. Nutr Metab Cardiovasc Dis. 2022;32(12):2839–2847.
10. Ryoo JH, Ham WT, Choi JM, et al. Clinical significance of non-alcoholic fatty liver disease as a risk factor for prehypertension. J Korean Med Sci. 2014;29(7):973–979. doi:10.3346/jkms.2014.29.7.973
11. Ryoo JH, Suh YJ, Shin HC, Cho YK, Choi JM, Park SK. Clinical association between non-alcoholic fatty liver disease and the development of hypertension. J Gastroenterol Hepatol. 2014;29(11):1926–1931. doi:10.1111/jgh.12643
12. Roh JH, Park JH, Lee H, et al. A close relationship between non-alcoholic fatty liver disease marker and new-onset hypertension in healthy Korean adults. Korean Circ J. 2020;50(8):695–705. doi:10.4070/kcj.2019.0379
13. Kanwal F, Kramer JR, Li L, et al. Effect of metabolic traits on the risk of cirrhosis and hepatocellular cancer in nonalcoholic fatty liver disease. Hepatology. 2020;71(3):808–819. doi:10.1002/hep.31014
14. Alexander M, Loomis AK, van der Lei J, et al. Risks and clinical predictors of cirrhosis and hepatocellular carcinoma diagnoses in adults with diagnosed NAFLD: real-world study of 18 million patients in four European cohorts. BMC Med. 2019;17(1):95. doi:10.1186/s12916-019-1321-x
15. Gutiérrez-Cuevas J, Santos A, Armendariz-Borunda J. Pathophysiological molecular mechanisms of obesity: a link between MAFLD and NASH with cardiovascular diseases. Int J Mol Sci. 2021;22(21):11629. doi:10.3390/ijms222111629
16. Di Sessa A, Umano GR, Miraglia Del Giudice E, Santoro N. From the liver to the heart: cardiac dysfunction in obese children with non-alcoholic fatty liver disease. World J Hepatol. 2017;9(2):69. doi:10.4254/wjh.v9.i2.69
17. Nascimbeni F, Bedossa P, Fedchuk L, et al. Clinical validation of the FLIP algorithm and the SAF score in patients with non-alcoholic fatty liver disease. J Hepatol. 2020;72(5):828–838. doi:10.1016/j.jhep.2019.12.008
18. Alberti KGMM, Zimmet P, Shaw J. Metabolic syndrome--A new world-wide definition. A consensus statement from the International Diabetes Federation. Diabet Med J Br Diabet Assoc. 2006;23(5):469–480. doi:10.1111/j.1464-5491.2006.01858.x
19. Lonardo A, Nascimbeni F, Mantovani A, Targher G. Hypertension, diabetes, atherosclerosis and NASH: cause or consequence? J Hepatol. 2018;68(2):335–352. doi:10.1016/j.jhep.2017.09.021
20. Zhang Y, Zhang T, Zhang C, et al. Identification of reciprocal causality between non-alcoholic fatty liver disease and metabolic syndrome by a simplified Bayesian network in a Chinese population. BMJ Open. 2015;5(9):e008204. doi:10.1136/bmjopen-2015-008204
21. Dixon JB, Bhathal PS, O’Brien PE. Nonalcoholic fatty liver disease: predictors of nonalcoholic steatohepatitis and liver fibrosis in the severely obese. Gastroenterology. 2001;121(1):91–100. doi:10.1053/gast.2001.25540
22. Ciardullo S, Monti T, Sala I, Grassi G, Mancia G, Perseghin G. Nonalcoholic fatty liver disease and advanced fibrosis in US adults across blood pressure categories. Hypertension. 2020;76(2):562–568. doi:10.1161/HYPERTENSIONAHA.120.15220
23. Yuan Y, Naito H, Kitamori K, Hashimoto S, Asano T, Nakajima T. The antihypertensive agent hydralazine reduced extracellular matrix synthesis and liver fibrosis in nonalcoholic steatohepatitis exacerbated by hypertension. PLoS One. 2020;15(12):e0243846. doi:10.1371/journal.pone.0243846
24. Ikuta T, Kanno K, Arihiro K, et al. Spontaneously hypertensive rats develop pronounced hepatic steatosis induced by choline-deficient diet: evidence for hypertension as a potential enhancer in non-alcoholic steatohepatitis: hypertension enhances steatohepatitis. Hepatol Res. 2012;42(3):310–320. doi:10.1111/j.1872-034X.2011.00920.x
25. Alam S, Kabir J, Mustafa G, Gupta U, Hasan SKMN, Alam AKMK. Effect of telmisartan on histological activity and fibrosis of non-alcoholic steatohepatitis: a 1-year randomized control trial. Saudi J Gastroenterol. 2016;22(1):69. doi:10.4103/1319-3767.173762
26. Brookes MJ, Iqbal TH, Cooper BT. Hypertension and hepatic steatosis. Curr Hypertens Rep. 2008;10(3):182–187. doi:10.1007/s11906-008-0035-9
27. Oikonomou D, Georgiopoulos G, Katsi V, et al. Non-alcoholic fatty liver disease and hypertension: coprevalent or correlated? Eur J Gastroenterol Hepatol. 2018;30(9):979–985. doi:10.1097/MEG.0000000000001191
28. Sciacqua A, Perticone M, Miceli S, et al. Endothelial dysfunction and non-alcoholic liver steatosis in hypertensive patients. Nutr Metab Cardiovasc Dis. 2011;21(7):485–491. doi:10.1016/j.numecd.2009.11.015
29. European Association for the Study of the Liver (EASL), European Association for the Study of Diabetes (EASD), European Association for the Study of Obesity (EASO). EASL–EASD–EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64(6):1388–1402. doi:10.1016/j.jhep.2015.11.004
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
