Back to Journals » Infection and Drug Resistance » Volume 14
Hyperlipidemia Caused by Voriconazole: A Case Report
Authors Wu J, Chen N, Yao Y, Zhou J , Zhou H
Received 9 January 2021
Accepted for publication 28 January 2021
Published 10 February 2021 Volume 2021:14 Pages 483—487
DOI https://doi.org/10.2147/IDR.S301198
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Jiasheng Wu,1,2,* Na Chen,3,* Yake Yao,1 Jianying Zhou,1 Hua Zhou1
1Department of Respiratory and Critical Care Medicine, The First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang, 310003, People’s Republic of China; 2Department of Respiratory and Critical Care Medicine, Jiaxing Second Hospital, Jiaxing, Zhejiang, 314000, People’s Republic of China; 3Department of Pharmaceutical, The First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang, 310003, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Hua Zhou; Jianying Zhou Email [email protected]; [email protected]
Abstract: Voriconazole has been widely used in clinical practice for nearly 20 years. The adverse reactions caused by voriconazole have been reported gradually, such as visual impairment, hepatotoxicity, skin rash. At present, there are few reports about triazole antifungal drugs causing the increase of triglyceride and total cholesterol. Thus, the present study reported a case of chronic pulmonary aspergillosis with significantly increased blood lipids after treatment with voriconazole. In this case, the patient’s total cholesterol was normal, and triglyceride was 2.64 times of the upper limit of the reference value at the time of admission. On the 30th day after oral administration of voriconazole 200mg q12h, triglyceride and total cholesterol were 4.55 times and 3.31 times of the baseline levels, respectively, with the trough concentration of voriconazole of 6.6 μ g/mL. After 28 days of voriconazole withdrawal and itraconazole administration, triglyceride decreased to 1.45 times of baseline level and total cholesterol decreased to the normal range. After another 24 days of treatment with voriconazole 200mg q12h, triglyceride increased again to 3.25 times of the baseline level and cholesterol was within the normal range. At the same time, the trough concentration of voriconazole was 3.2 μ g/mL. After 14 days of treatment with voriconazole 100mg q12h, the triglyceride level recovered to the baseline level, with the trough concentration of voriconazole of 1.5 μ g/mL. The Naranjo′s rating scale was used, the final score was 10 points, indicating that the causal relationship between voriconazole and dyslipidemia was positive, which was likely to be related to the trough concentration of voriconazole.
Keywords: voriconazole, chronic pulmonary aspergillosis, adverse reactions, hyperlipidemia
Introduction
Voriconazole (VRCZ) is the second generation of triazole antifungal drug that exerts antifungal effects by inhibiting 14 -sterol demethylation via cytochrome P450 in fungi to inhibit the biosynthesis of ergosterol. The common adverse reactions of VRCZ include hepatotoxicity and visual impairment, which are often related to the concentration of the drug. However, the effect of voriconazole on lipid metabolism has not been reported. Thus, the present study reported a case of chronic pulmonary aspergillosis (CPA) with significantly increased blood lipids after treatment with VRCZ and reviewed and analyzed the case of dyslipidemia caused by triazole antifungal drugs.
Case Description
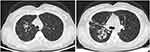
A 44-year-old female patient, 148 cm height and 43 kg weight, was admitted to the First Affiliated Hospital, Zhejiang University School of Medicine for “blood in sputum for 11 months.” Chest computed tomography (CT) of the patient showed dilatation of the bronchi in both lungs and nodular high-density shadows and air crescent sign in some cavities (Figure 1); hence, diagnosis of aspergilloma was considered. The patient was positive for specific IgG of Aspergillus fumigatus and galactomannan (GM) positive for alveolar lavage fluid in the right upper lobe. Subsequently, A. fumigatus was cultured, and the diagnosis was CPA. Voriconazole (VRCZ) tablets (200 mg every 12 hours) were taken orally from January 22, 2019. The patient had a history of rheumatoid arthritis and regularly administered leflunomide tablets 10 mg QD for a long duration and had a history of sinus tachycardia, as well as metoprolol sustained-release tablets (47.5mg QD) for a long period.
Before administering VRCZ, the liver enzymes and the total cholesterol were normal, while the triglyceride was 2.64-fold of the upper limit of the reference value. After ingesting VRCZ tablets for 14 days, the liver enzyme levels were higher, while the blood lipids were similar to the baseline values. After administering the tablets for 30 days, the symptoms of blood in sputum disappeared. And the liver enzymes were normal while the blood lipids were significantly higher than the baseline values. Simultaneously, the trough concentration of VRCZ was 6.6 μg/mL. Considering the possibility of hyperlipidemia caused by the drug, VRCZ was withdrawn, and itraconazole (200 mg every 12 hours) was administered.
After 28 days of itraconazole treatment, the liver enzymes and blood lipids recovered to baseline levels. While, the patient’s symptoms of blood in sputum occurred.Then, the patient was given VRCZ tablets (200 mg every 12 hours) again.
After 24 days of VRCZ treatment, the liver enzyme levels were normal while triglycerides increased significantly, and the trough concentration of VRCZ was 3.2 μg/mL. The symptom of blood in sputum was improved significantly. The increase in blood lipids may be related to the trough concentration of VRCZ. The patient agreed to continue taking VRCZ tablets after communication. Therefore, the therapeutic regimen of voriconazole was adjusted to 100 mg every 12 hours. After 49 days of this therapeutic regimen, the liver enzymes and blood lipids recovered to the baseline levels again, with the trough concentration of voriconazole of 1.5 μg/mL. After 155 days of continuous use of antifungal agents, considering the full course, the voriconazole was withdrawn. During the administration and after the withdrawal of VRCZ, the liver enzymes and blood lipids were similar to the baseline levels. During the whole treatment process, the diet and living habits of the patients were consistent with those before the treatment, and no significant change was detected in height and weight compared to those before the treatment. During the treatment, the specific values of liver enzymes, total cholesterol, and triglyceride of the patient are shown in Table 1, Figure 2.
 |
Table 1 The Specific Values of Liver Enzymes, Total Cholesterol and Triglyceride of the Patient During the Treatment |
 |
Figure 2 Changes in triglyceride, cholesterol, and trough concentration of VRCZ during treatment. |
According to the treatment process of the patient, Naranjo′s rating scale was used to evaluate the adverse reactions, and the final score was 10 points, establishing a positive correlation between VRCZ and dyslipidemia, which was likely to be related to the trough concentration of VRCZ.1
Discussion
CPA is an uncommon and problematic pulmonary disease. Long-term oral antifungal therapy (voriconazole or itraconazole) is recommended for CPA to improve overall health status and respiratory symptoms, arrest haemoptysis and prevent progression.2 VRCZ is the second generation of triazole antifungal drug that has been widely used in clinical practice for about 20 years. In addition to common adverse reactions, such as visual impairment, hepatotoxicity, and skin rash,3–5 unique reactions, such as phototoxic skin diseases and malignant tumors, periostitis, hair loss, alopecia, and nail changes,6 have also been found with long-term use. However, adverse reactions of hyperlipidemia have not yet been reported. Hong and Lin7 reported a case of dyslipidemia in a 48-year-old female patient caused by itraconazole. In the second week after taking itraconazole, the patient showed elevated triglycerides and total cholesterol and normal alanine aminotransferase (ALT) and glutamic oxaloacetic transaminase (AST). The blood lipids recovered to normal 1–2 months after the withdrawal of itraconazole.
Presently, only one study7 has reported triazole antifungal drugs causing the increase in triglyceride and total cholesterol, and the mechanism is yet unclear. Studies have shown that among triazoles, VRCZ has the strongest affinity for steroid 24-hydroxylase (CYP46A1) and inhibits the conversion of cholesterol to 24-hydroxycholesterol in the brain.8,9 However, bile acids are generated from cholesterol through cholesterol 7 α hydroxylase (CYP7A1), which is a critical metabolic pathway of cholesterol in the human body. Therefore, the inhibition of cholesterol metabolism in the brain by voriconazole cannot explain the cause of elevated serum cholesterol in this patient.10,11 In addition, cytochrome P4503A (CYP3A) is the only enzyme that converts retinoic acid to cis-9-retinoic acid, and cis-9-retinoic acid is the only ligand of retinoic acid X receptor (RXR). When combined, the upregulation of apoptosis and differentiation and proliferation of adipocytes is inhibited. After the inhibition of CYP3A, the synthesis of cis-9-retinoic acid is reduced, leading to a decrease in RXR activity, thus resulting in the decreased differentiation and the increase of apoptosis of peripheral adipocytes.12 As a CYP3A inhibitor, the commonly used ritonavir has a high incidence of hypertriglyceridemia, which is related to this mechanism. However, whether the abnormal increase of triglycerides is related to VRCZ as a CYP3A drug is yet to be verified. When the patient was given VRCZ again, and when the trough concentration of the drug was half of the first dose, serum cholesterol recovered to the baseline level, and serum triglyceride level also recovered to normal with the decrease in the trough concentration of VRCZ. However, current studies still cannot explain the causes of VRCZ-induced dyslipidemia, and further research is needed.
Although drug-induced hyperlipidemia is clinically common, VRCZ has not been reported previously to cause hyperlipidemia. Based on the data of this case, it is inferred that VRCZ-induced hyperlipidemia is a reversible concentration-dependent adverse reaction. In addition, the side effects of drug-induced hyperlipidemia require large scale population studies to observe whether it has implications for patients with cardiovascular and cerebrovascular disease especially in terms of incidence to stroke and myocardial infraction.
Conclusion
We clearly observed drug-induced hyperlipidemia caused by voriconazole in a patient with chronic pulmonary aspergillosis.The level of triglycerides and cholesterol was positively correlated with the trough concentration of voriconazole. Therapeutic drug monitoring and blood lipid level monitoring were both important during the use of voriconazole.
Ethics Approval and Consent for Publication
This study has been reviewed and approved by the Research Ethics Committee of the First Affiliated Hospital of Zhejiang University (ref#2020-1502). The patient provided informed consent for publication of the clinical details including lung CT images, and written informed consent was obtained.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
No funding was granted.
Disclosure
The authors declare that they have no conflicts of interest.
References
1. Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):
2. Denning DW, Cadranel J, Beigelman-Aubry C, et al. Chronic pulmonary aspergillosis: rationale and clinical guidelines for diagnosis and management. Eur Respir J. 2016;47(1):45–68. doi:10.1183/13993003.00583-2015
3. Dolton MJ, McLachlan AJ. Voriconazole pharmacokinetics and exposure–response relationships: assessing the links between exposure, efficacy and toxicity. Int J Antimicrob Agents. 2014;44(3):183–193. doi:10.1016/j.ijantimicag.2014.05.019
4. Levine MT, Chandrasekar PH. Adverse effects of voriconazole: over a decade of use. Clin Transplant. 2016;30(11):1377–1386. doi:10.1111/ctr.12834
5. Purkins L, Wood N, Greenhalgh K, et al. Voriconazole, a novel wide‐spectrum triazole: oral pharmacokinetics and safety. Br J Clin Pharmacol. 2016;56(s1):2–9. doi:10.1046/j.1365-2125.2003.01993.x
6. Benitez LL, Carver PL. Adverse effects associated with long-term administration of azole antifungal agents. Drugs. 2019;1–21. doi:10.1007/s40265-019-01127-8
7. Hong L, Lin W. A case report of elevated serum cholesterol and triglyceride induced by itraconazole. Chin J Clin Pharm Ther. 2010;08:886–887.
8. Björkhem I, Lütjohann D, Breuer O, Sakinis A, Wennmalm A. Importance of a novel oxidative mechanism for elimination of brain cholesterol. Turnover of cholesterol and 24(S)-hydroxycholesterol in rat brain as measured with 18O2 techniques in vivo and in vitro. J Biol Chem. 1997;272(48):30178–30184. doi:10.1074/jbc.272.48.30178
9. Lund EG, Xie C, Kotti T, Turley SD, Dietschy JM, Russell DW. Knockout of the cholesterol 24-hydroxylase gene in mice reveals a brain-specific mechanism of cholesterol turnover. J Biol Chem. 2003;278:22980–22988. doi:10.1074/jbc.M303415200
10. Liu H, Pathak P, Boehme S, et al. Cholesterol 7α-hydroxylase protects the liver from inflammation and fibrosis by maintaining cholesterol homeostasis. J Lipid Res. 2016;57(10):1831–1844. doi:10.1194/jlr.M069807
11. Li T, Chiang JYL. Bile acids as metabolic regulators. Curr Opin Gastroenterol. 2015;31(2):159–165. doi:10.1097/MOG.0000000000000156
12. Zanetti HR, Roever L, Goncalves A, et al. Human immunodeficiency virus infection, antiretroviral therapy, and statin: a clinical update. Curr Atheroscler Rep. 2018;20(2):9. doi:10.1007/s11883-018-0708-z
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.