Back to Journals » International Journal of Nanomedicine » Volume 19
Hydrogel Loaded with Components for Therapeutic Applications in Hypertrophic Scars and Keloids
Authors Zhong Y, Zhang Y, Lu B, Deng Z, Zhang Z, Wang Q, Zhang J
Received 16 November 2023
Accepted for publication 12 January 2024
Published 25 January 2024 Volume 2024:19 Pages 883—899
DOI https://doi.org/10.2147/IJN.S448667
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor R.D.K. Misra
Yixiu Zhong,1 Youfan Zhang,2 Beibei Lu,1 Zhenjun Deng,1 Zhiwen Zhang,2 Qi Wang,2 Jianglin Zhang1
1Department of Dermatology, Shenzhen People’s Hospital (The Second Clinical Medical College, Jinan University; The First Affiliated Hospital, Southern University of Science and Technology), Shenzhen, Guangdong, People’s Republic of China; 2Department of Dermatology, Nanfang Hospital, Southern Medical University, Guangzhou, People’s Republic of China
Correspondence: Yixiu Zhong; Jianglin Zhang, Email [email protected]; [email protected]
Abstract: Hypertrophic scars and keloids are common fibroproliferative diseases following injury. Patients with pathologic scars suffer from impaired quality of life and psychological health due to appearance disfiguration, itch, pain, and movement disorders. Recently, the advancement of hydrogels in biomedical fields has brought a variety of novel materials, methods and therapeutic targets for treating hypertrophic scars and keloids, which exhibit broad prospects. This review has summarized current research on hydrogels and loaded components used in preventing and treating hypertrophic scars and keloids. These hydrogels attenuate keloid and hypertrophic scar formation and progression by loading organic chemicals, drugs, or bioactive molecules (such as growth factors, genes, proteins/peptides, and stem cells/exosomes). Among them, smart hydrogels (a very promising method for loading many types of bioactive components) are currently favoured by researchers. In addition, combining hydrogels and current therapy (such as laser or radiation therapy, etc.) could improve the treatment of hypertrophic scars and keloids. Then, the difficulties and limitations of the current research and possible suggestions for improvement are listed. Moreover, we also propose novel strategies for facilitating the construction of target multifunctional hydrogels in the future.
Keywords: hydrogel, wound healing, hypertrophic scar, keloid
Graphical Abstract:
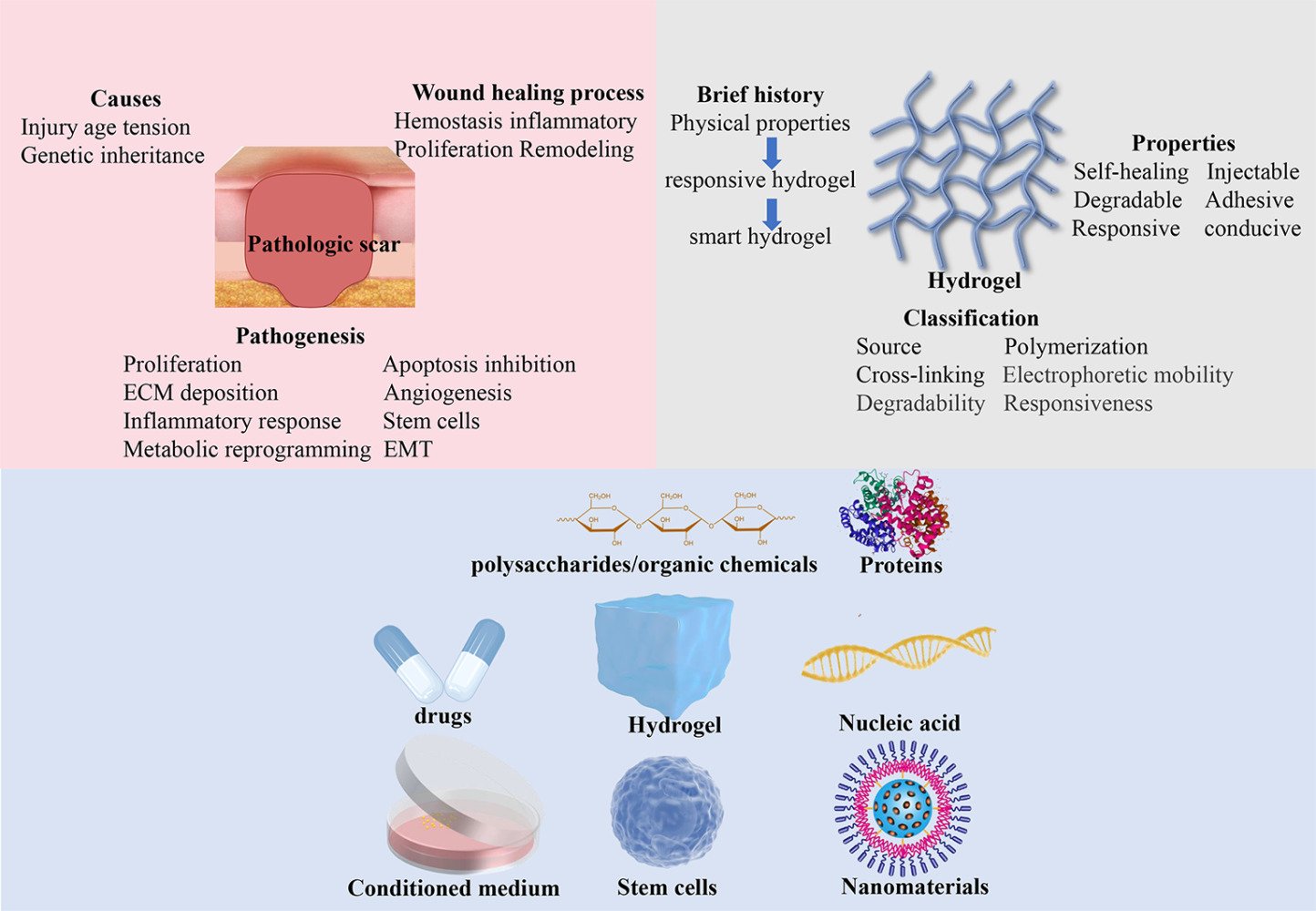
Introduction
Brief introduction of Keloid and Hypertrophic Scar
Hypertrophic scars and keloids are both benign fibrous growth diseases usually caused by skin injury. Hypertrophic scars are soft, with normal skin color, do not extend beyond the boundary of the injury, may regress over time, and histologically display well-organized type III collagen bundles. Keloids are clinically manifested as hard-raised and pigmented scars that grow beyond the original wound area, have high recurrence rates and histologically display disorganized, large thick, collagen type I and III bundles.1 Giant hypertrophic scars and keloids can cause disfiguration and movement disorders, accompanied by functional symptoms of pruritus and pain, which severely damage their quality of life and psychological health.2
In addition to injury, ethnicity, age, skin tension, and genetic inheritance were also closely associated with the occurrence and progression of keloids.3 Hispanic and African populations are more prone to keloids, with an estimated incidence of 5–16%, than white populations.4 Keloids in young individuals tend to progress faster than those in old individuals.3 The most vulnerable sites are the shoulders, backs, chests, and earlobes with high tension.5–7 Current treatments for hypertrophic scars and keloids include surgical excision, glucocorticoid injection, radiation therapy, and mesenchymal stem cells.8 Meta-analysis revealed that surgery combined with radiotherapy had a lower recurrence rate than radiotherapy (22% vs 37%) in keloids.9 However, there is no optimal treatment that can completely prevent the formation, progression, and recurrence of pathologic scars; thus, more efforts need to be dedicated to developing novel therapeutic agents for hypertrophic scars and keloids.
Pathological Scar Formation in the Wound Healing Process
Pathologic scarring is caused by abnormal wound healing (Figure 1). To improve the treatment of hypertrophic scars and keloids, we must understand the wound healing process. Wound healing is now considered a complicated process consisting of four interrelated stages: hemostasis. Inflammatory, proliferative, and remodelling, involving a variety of cells secreting different cytokines and other biomolecules.10,11
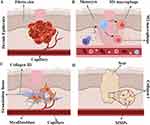 |
Figure 1 Four stages of wound healing. Note: (A) Hemostais stage, (B) inflammatory stage, (C) proliferation stage, (D) remodelling stage. |
Wound Healing Phases
Hemostasis usually lasts 2–3 hours, forming fibrin plugs and triggering the release of inflammatory mediators by platelets. Immune cells such as neutrophils and macrophages are recruited by cytokines, initiating the inflammatory phase.12 The inflammatory stage initiates immediately after skin injury and typically lasts from hours to two or three days, forming platelet plugs and the extracellular matrix to prevent blood loss, close the wound, and guide cell migration.13 The proliferation stage may last several weeks and is marked by angiogenesis, new extracellular matrix (ECM) formation, and epithelization. Endothelial cell proliferation and migration promote angiogenesis and the construction of new vessels and capillaries to deliver oxygen and nutrients essential for other cells.13 Fibroblasts activated by transforming growth factor β facilitate the construction of new ECM and immune cells, such as macrophages, and the degradation of the old matrix by secreting proteases, such as matrix metalloproteinases (MMPs).14 In addition, epithelialization is initiated by the migration of keratinocytes from the edge.15 The final phase, remodelling, can last for months to years and mainly leads to hypertrophic and keloid scar formation. A variety of proteases and their inhibitors participate in the remodelling phase. Type III collagen is converted to type I collagen to increase matrix density and stability.16 Excessive fibroblast proliferation and differentiation into myofibroblasts, as well as the imbalance between ECM disposition and degradation, is mainly responsible for pathologic scar formation.
Mechanical Forces Contributes to Abnormal Scar Formation
Notably, increasing evidence suggests that mechanical forces play a key role in abnormal scar formation. As stated above, keloids are prone to occur at sites with high skin tension. High skin tension can elongate or expand the ECM and cells, increasing ECM accumulation, the cell cytoskeleton, and membrane forces, leading to an increase in whole tissue stiffness.17,18 In the inflammatory phase, mechanical forces can promote macrophage proliferation and M1 macrophage polarization, aggravating the inflammatory response.19,20 In the proliferative phase, mechanical forces can facilitate the abnormal growth of fibroblasts and their transformation to myoblasts via mechanical transduction pathways, such as FAK/ERK and YAP/TAZ, which produce excessive ECM and contribute to the formation of hypertrophic scars and keloids.21,22 In the remodelling phase, excessive ECM accumulation leads to increased ECM stiffness, inhibiting the apoptosis of myoblasts, which continuously generate new ECM.19,23,24
Keloid and Hypertrophic Scar Pathogenesis
Hypertrophic scars and keloids possess common pathological processes to varying degrees, involving proliferation, apoptosis inhibition, ECM deposition, angiogenesis, inflammatory response, metabolic reprogramming, epithelial-mesenchymal transition (EMT), and stem cells (Figure 2).
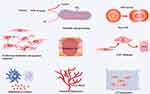 |
Figure 2 Pathogenesis of hypertrophic scars and keloids. |
Proliferation and Apoptosis Inhibition
Excessive fibroblast proliferation and apoptosis inhibition are crucial to keloid and hypertrophic scar formation and progression. Continuous activation of the TGFβ/SMAD pathway promotes fibroblast proliferation, which is necessary for collagen synthesis in hypertrophic scars and keloids. β-Catenin, Wnt5A, and Wnt10A might contribute to fibroblast growth by regulating the Wnt/β-catenin signaling pathway.25–27 High levels of cMYC and Bcl-2 (anti-apoptotic proteins), transcription factors c-Jun and c-Fos, which facilitate continuous fibroblast growth signals, and decreased levels of TP53 (anti-apoptotic protein) were observed in keloids.28–30
Deposition of ECM
Histologically, Hypertrophic scars exhibit well-organized type III collagen bundles, while Keloids manifest disorganized, large thick, collagen type I and III bundles. Excessive collagen deposition, or fibrosis, was observed in hypertrophic scars and keloids. Cytokines (TGF-β1, IL-6, and IL-8) can facilitate the production of collagen, fibronectin, and fibrotic proteins (SPARC and tenascin) in hypertrophic scar fibroblasts.31 Meanwhile, it was reported that TGF-β1 induces collagen type I, type III, and fibronectin accumulation in keloids.32 In addition, matrix metalloproteinases (MMPs) play a complex role in abnormal scar formation and progression. On the one hand, MMP was activated by IWR-1, an inhibitor of the Wnt/β-catenin pathway, to attenuate the production of collagen in keloids and normal fibroblasts.33 On the other hand, the level of MMP-2 was elevated in collagen bundle regions, which might collagen bundle remodelling and invasion of keloid fibroblasts by degrading ECM.34
Angiogenesis
As stated above, in the proliferative phase, angiogenesis could deliver oxygen and nutrients essential for other cells. It was suggested that hypertrophic scar myoblasts contribute to the construction of microvessels, resulting in excessive vascularization of hypertrophic scars. Hypertrophic scar myoblasts can release microvesicles to facilitate endothelial cell proliferation, migration, and assembly, leading to excessive vascularization of hypertrophic scars.35 Angiogenesis factors and their receptors vascular endothelial growth factor (VEGF) and VEGF/KDR complex, and platelet-derived growth factor (PDGF) and its receptor-PDGFR-α were all expressed in keloid-derived fibroblasts, phagocytes, endothelial cells, adventitial cells, epidermal cells, sustaining the vascularization for nutrient delivery and metabolism by enhancing the growth, migration and assembly of endothelial cells.36
Metabolic Reprogramming
Sparse, occluded, and flattened microvessels suggested a hypoxic microenvironment in the keloid centers. The hypoxic zone exhibited an elevated level of hypoxia-inducible factor 1 α (HIF-1α).37 Similar to tumors, keloids display metabolic reprogramming, including enhanced anaerobic glycolysis and weakened oxidative phosphorylation under hypoxia. Compared with normal skin, lactic acid levels were higher in keloids. HIF-1α may regulate glucose metabolic reprogramming via the PI3K/AKT pathway.38 Enhanced anaerobic glycolysis facilitates tumor progression by providing energy and materials for macromolecule synthesis. Likewise, under hypoxia, the proliferation of keloid fibroblasts increases, while the apoptosis of cells decreases.38,39
EMT
EMT is a biological process in which epithelial cells adjust to the mesenchymal phenotype with enhanced invasiveness.40,41 EMT plays a key role in the progression of hypertrophic scars and keloids. Mesenchymal markers such as vimentin and N-cadherin are significantly increased in hypertrophic scar tissue.42 Hypertrophic scar fibroblast-derived exosomes upregulated the EMT markers of keratinocytes.43 Moreover, increased WNT5A promotes interleukin-6-dependent EMT in keloid keratinocytes by regulating the JAK/STAT pathway.44 It was reported that pirfenidone, an antifibrotic drug, could reduce the EMT-like phenotype in keloid keratinocytes.45 Hypoxia also contributes to EMT alteration in keratinocytes. Keloid keratinocytes acquire an EMT phenotype and exhibit elevated invasiveness under hypoxia.46
Stem Cell
Keloid fibroblasts display high self-renewal capability and drug resistance and maintain themselves by asymmetric division, producing new cells to replace cells killed by medicines, laser or radiation therapy, resulting in expansion outwards from the boundary of keloids and recurrence.47,48 Haematopoietic and mesenchymal-like stem cells were identified in keloid lesions.47,48 Meanwhile, embryonic stem cell markers (OCT4, SOX2, pSTAT3, and NANOG) were positive in keloid-associated lymphoid tissue,49 while Axin2 was overexpressed in patients with pathologic scars.50 In addition, hypertrophic scar fibroblasts highly express mesenchymal stem cell markers (CD73, CD105, CD44, CD90, and OCT4), which might contribute to the multilineage differentiation potential of hypertrophic scars.51 These findings suggested the existence of stem cells in hypertrophic scars and keloids.
Hydrogel
Brief History
Hydrogels are hydrophilic polymers that chemically or physically crosslink together to form a three-dimensional (3D) network. It can absorb water without dissolving and swell while maintaining a 3D structure. The concept of proto-hydrogels was first proposed in 1894,52 and the properties of biocompatibility and high water affinity were defined in 1960.53 Near-exponential growth of hydrogel research started in the 1990s due to the versatility of hydrogels. It was suggested that hydrogels develop through three stages: the first stage, a simple polymer with physical properties; the second stage, a more complicated material responsive to stimuli such as temperature and pH; and the third stage, a smart hydrogel with a variety of variable properties and applications such as small interfering RNA (siRNA) delivery.54
Classification
Hydrogels can be classified according to their source, polymerization method, crosslinking method, electrophoretic mobility, degradability, and responsiveness (Figure 3).55 According to the source, hydrogels are classified into natural hydrogels, generally consisting of polysaccharide chains, such as hyaluronic acid, sodium alginate, and collagen; synthetic hydrogels composed of polymers, such as polyethylene glycol (PEG), poly-acrylamide (PAM) and polyvinyl pyrrolidone (PVP); and hybrid hydrogels, consisting of both natural and synthetic polymers.56–59 As for the polymerization method, hydrogels can be made up of homopolymers, copolymers, semi-interpenetrating networks (semi-IPNs), or IPNs (Figure 4).60 When it comes to the crosslinking method, hydrogels are divided into physical hydrogels and chemical hydrogels. Physical hydrogels are transient, and polymers interact with hydrogen bonds and van der Waals forces, while chemical hydrogels are permanent, and polymers crosslink with chemical bonds. Four categories of hydrogels exist based on electrophoretic mobility: nonionic hydrogels and ionic hydrogels, including cationic, anionic, and amphiphilic hydrogels. Degradable and nondegradable hydrogels were classified according to degradability: unresponsive hydrogels and responsive hydrogels, such as thermosensitive and pressure-sensitive hydrogels, in regard to responsiveness to stimuli.61 This review mainly focuses on the classification of hydrogels based on their source (Table 1).
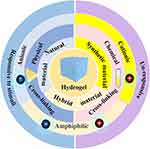 |
Figure 3 Classification of hydrogels based on different methods. |
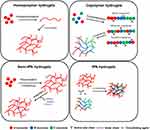 |
Figure 4 Simple diagram of homopolymer hydrogels, copolymer hydrogels, semi-IPNs, and IPNs. Note: Reproduced from Ho T-C, Chang C-C, Chan H-P, et al. Hydrogels: Properties and Applications in Biomedicine. Molecules. 2022;27(9):2902, https://creativecommons.org/licenses/by/4.0/.60 |
 |
Table 1 Classification of Hydrogels According to Source |
Natural Hydrogels
As natural components of the ECM, natural hydrogels display the best biocompatibility. Matrigel™ is a basement membrane derived from Engelbrecht–Holm–Swarm mouse sarcoma cells, which are composed of hyaluronic acid, laminin, fibrin and collagen and are rich in growth factors and MMPs.62 Alginate is a polysaccharide extracted from brown algae or bacteria that is widely used in food, medicine, and engineering fields due to its stability, safety, adhesiveness, and liquid-absorbing quality.63 Chitosan is the only canonic polysaccharide;64 hyaluronic acid is also a natural polysaccharide. Hyaluronic acid-based hydrogels are widely applied in wound healing and tumor inhibition through their ability to be adjusted by chemical modification, physical blending, and nanocomposites.65 Besides, gelatin is extracted from the hydrolysis of animal-origin collagen and gelatin-based hydrogels were also extensively used in biomedical fields.66 However, natural hydrogels have relatively poor mechanical properties and a high degree of swelling, limiting their use in different areas. Meanwhile, it is difficult to control the differences between batches when extracting these natural products. In addition, the tumour-derived natural restricts the clinical application of natural hydrogels.75 For instance, Matrigel TM could facilitate tumor progression by promoting cell migration and invasion.76
Synthetic Hydrogels
Synthetic hydrogels are composed of engineered polymers, which are polymerized by a monomer, such as PEG, polyethylene oxide (PEO), and PAM.67–69 Synthetic hydrogels are reproducible, possess tunable chemical or mechanical properties and can be standard controlled between different batches.77 For instance, PEG hydrogels are stable, highly stretchable, self-healing and injectable, exhibiting anti-infection properties.67 Besides, synthetic PVP hydrogels were widely used in drug delivery of cancer.70 Polyvinyl alcohol (PVA) hydrogels display excellent mechanical properties, dictating its applications in medical fields, and can be blended with PVP to improve their properties.71,78 Synthetic hydrogels are not as biocompatible as natural hydrogels due to their sources, but their biodegradability and biocompatibility can be improved by modifying functional group or incorporating with natural polymers.60
Hybrid Hydrogels
Hybrid hydrogels are chemically modified natural polymers or a combination of natural and synthetic materials to gain the advantages of these two types.79 A widely used hybrid hydrogel is methacryloyl-modified gelatin (GelMA), which is a chemically modified natural material,72 while PEG crosslinked fibrinogen, or collagen, is a combination of natural and synthetic matrices.73,74 Hybrid hydrogels can not only possess the multi-tuneable mechanical properties of synthetic hydrogels by adjusting chemical parameters but also preserve the biocompatibility and bioactivity of natural hydrogels.80
Properties
Hydrogel is extensively applied in biomedical files, such as tissue engineering scaffolds, bone generation, wound dressing, and drug delivery, due to its multifunctional properties. The properties of hydrogels mainly include swelling, self-healing, injectable and degradable capability, adhesiveness and responsiveness.81 In this review, we mainly focus on the injectability, self-healing, adhesiveness, and responsiveness of hydrogels (Figure 5).
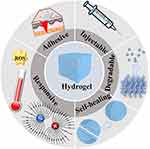 |
Figure 5 Properties of hydrogels: injectable, self-healing, adhesiveness and responsiveness. |
Injection and Self-Healing Capability
Injectable hydrogels could flow through a medical needle and aggregate into a whole bulk hydrogel at the targeted site. Injectable hydrogels are composed of precursor and self-healable hydrogels. Precursor hydrogels are referred to as hydrogels, which gelatinize under physical or chemical conditions such as light, temperature or pH.82,83 Self-healing hydrogels could automatically reconnect their network via chemical or physical bonds after being broken.84 Notably, injectable hydrogels are characterized by high flowability, given the dynamic variation of cross-linking bonds and motion of polymer chains.85 Therefore, hydrogels can be easily injected and then recover to their original structure or mechanical properties at the targeted area.
Adhesiveness to Tissue
Recently, increasing research has focused on developing different types of adhesive mechanisms of hydrogels, such as mussel-inspired hydrogels, Schiff base-bond hydrogels and bioactive proteins.86 The adhesiveness of mussel-inspired hydrogels lies in the pyrocatechol of mussel foot proteins, which form chemical interactions with and cross links with different matrices.87 The amino group of surfaces of tissue could bind with the aldehyde group of hydrogels, which is called the Schiff-base bond, contributing to the tissue adhesiveness of hydrogels.88 Bioactive proteins such as silk fibroin and elastin can form hydrogen bonds or electrostatic interactions, leading to adhesion to cells and tissues.89,90 Moreover, adhesive hydrogels applied in wound healing could reduce the boundary tension.91 These studies revealed that adhesive hydrogels show great potential in wound closure following different types of injury.
Responsiveness
Responsive hydrogels, also referred to as smart hydrogels, can respond to physical stimuli, such as light, electricity, temperature, and pressure, or chemical stimuli, such as pH and reactive oxygen species (ROS). Responsive hydrogels could undergo changes in phase, volume, or other physicochemical characteristics under different stimuli. Compared with traditional hydrogels, smart hydrogels display multifunctional variables and tunable characteristics and are thus more widely used in biomedical fields, especially in wound healing.81 It was reported that a pH/ROS dual-responsive injectable hydrogel displayed antibacterial and antioxidant effects in the wound healing process.92 Li et al designed a pressure-sensitive antibacterial hydrogel to monitor wound pressure and facilitate skin healing in bedridden patients.93 More importantly, skin tension is closely associated with the formation, progression, and recurrence of pathologic scars;17 thus, smart hydrogels, which also display promising potential in reducing skin tension in wound healing,94 might be novel therapies for preventing hypertrophic scar and keloid occurrence and recurrence.
Loaded Components of Hydrogels
Given their three-dimensional and porous structure, hydrogels can carry drugs, biomolecules such as exosomes, cytokines, and proteins, or nanomaterials. More importantly, sustained and prolonged release of drugs and biomolecules can be achieved by slow diffusion and degradation of hydrogels or by their responses to stimuli, such as temperature or pressure. This review focuses on five categories of loaded components in composite hydrogel dressings for therapeutic use in hypertrophic scars and keloids: organic chemicals, mesenchymal stem cells and their conditioned medium, drugs, nucleic acids, and proteins (Table 2). More importantly, this review also proposes a variety of components that have not been explored but possess promising therapeutic potential to be delivered by hydrogels.
 |
Table 2 Loaded Components of Hydrogels in Hypertrophic Scars and Keloids |
Polysaccharides/Organic Chemicals
Hydrogels are 3D hydrophilic polymer networks. The polymer, which directly composes the hydrogel, exhibits therapeutic effects through its inherent properties. Below, we discuss polymers alone that inhibit hypertrophic scar and keloid formation, progression, and recurrence. It was reported that biocompatible polyurethane-based hydrogels could ease pain and prevent skin maceration and hypertrophic scar formation. However, this study did not establish a control group, and the selection criteria are confusing and should be improved to further confirm the efficacy of hydrogels in burn patients.95 In addition to hypertrophic scar formation, Zhang et al utilized carboxymethyl chitosan hydrogels chemically crosslinked with genipin to attenuate hypertrophic scar progression by inhibiting a-SMA and facilitating MMP-1 generation, showing the potential use of chitosan-based wound dressings in hypertrophic scar treatment.96 A polyvinyl alcohol-collagen-glycosaminoglycan-based semisynthetic hydrogel decreased the thickness, ECM deposition, and vessel-like structure of hypertrophic scars in a rabbit ear model.99 Keloids have a higher recurrence rate than hypertrophic scars. Berman et al found that injecting gelatin-dextran hydrogels could effectively reduce the symptoms and recurrence of keloid scar postsurgical excision. Dextran could modulate the immune response, prevent infection, and facilitate wound healing, which might contribute to the anti-keloid recurrence effect.97 Additionally, pregnancy might be associated with the progression of hypertrophic or keloidal scarring. A clinical trial was conducted to evaluate the erythema fading of hypertrophic scars that progressed in 45 Caucasian pregnant women. Polysaccharides and polyacrylamide hydrogel combined with laser therapy decreased the redness of the scar by cooling the skin. However, this study lacks a corresponding control and has a limited number of samples, which needs further investigation.98
Medicine/Drug
Research suggests that hydrogels are a promising and ideal vehicle to deliver drugs in hypertrophic scars and keloids due to their biocompatibility and prolonged release of drugs. Ma et al utilized caffeine embedded in hydrogel to treat hypertrophic scar tissue on rabbit ears, and the results showed that caffeine hydrogel significantly downregulated the expression of Type 1 collagen by inhibiting TGFβ.104 Chen et al developed a GelMA/polyethylene glycol diacrylate (PEGDA) hydrogel containing betamethasone as a microneedle patch, which significantly reduced the scar elevation index, collagen I/III, and TGF-β1 expression.102 Imiquimod was proven to be useful in keloids but had few effects, including erythema, pain, pruritus, and slight hyperpigmentation. To better control the release of imiquimod, Lin et al developed a hydrogel delivering imiquimod for the treatment of keloids, which significantly decreased the growth of keloid fibroblasts.103 In addition to imiquimod, triptolide was extracted from Tripterygium, displaying anti-inflammatory and antifibrotic effects. As a triptolide derivative, LA67 possesses the original activity of triptolide and improved antitumour effects, with less toxicity compared to triptolide. Wan et al used a thermosensitive hydrogel to deliver liposome-encapsulated LA67, which significantly prevents keloid progression by downregulating collagen and αSMA expression.106
In addition to a single drug, a dual-drug delivery hydrogel might be more effective in the treatment of hypertrophic scars and keloids. Bao et al loaded 5-fluorouracil and dexamethasone in a thermosensitive hydroxybutyl chitosan hydrogel, which prevents keloid fibroblast growth and VEGF expression in keloid tissues. It was revealed that the codelivery of 5-fluorouracil and dexamethasone in hydrogels exhibited promising potential in keloid therapy.100 In addition, Chen et al fabricated a dual drug-gelatin hydrogel carrying gallic acid and quercetin, which inhibited the growth and mRNA level of type I and III collagen in keloid fibroblasts and the production of reactive oxygen species and might be effective in keloid formation and progression.101 Patients with pathologic scars often suffer from itch and pain, which might be associated with the inflammatory response. A case study showed that a 2% salicylic acid-based hydrogel could significantly reduce redness, itch, and burning pain in hypertrophic scar patients, which might be due to the anti-inflammatory effects of salicylic acid, such as preventing prostaglandin production and NF-κB generation.105 Wu et al developed a guar gum-based hydrogel to deliver menthol and methyl salicylate, which was proven to effectively alleviate the pruritus of burn-induced hypertrophic scars in a multicenter, controlled trial.107 More importantly, combined therapy could increase the transdermal delivery of drugs in hypertrophic scars. CO2 fractional laser therapy combined with a silk nanofiber hydrogel could enhance the skin penetration of hydrophilic and hydrophobic substances into rabbit ear hypertrophic scar tissue.108
Stem Cells and Conditioned Medium
Hydrogels are considered ideal scaffolds for stem cells and their secretome due to their 3D dimensional properties and superior biocompatibility (Figure 6). Qu et al first used a thermosensitive Arg-Gly-Asp (RGD)-modified hydroxybutyl chitosan hydrogel to deliver bone marrow-derived mesenchymal stem cells (BMSCs) in treating keloids, and HE staining showed that ex vivo keloid tissue in the BMSC/Hydrogel group histologically displayed thin, loose nodule collagen fibres and small collagen bundles.109 Adipose-derived stem cells encapsulated in hyaluronic acid hydrogel inhibited hypertrophic scar formation by decreasing the collagen type I/collagen type I ratio and α-SMA expression in fibroblasts. It was reported that hyaluronic acid gel containing mesenchymal stem cells could effectively limit excessive fibroblasts and collagen together with chronic inflammation and correct the imbalance of ECM transformation by reducing fibronectin and tenascin-C expression.110 More importantly, clinical studies are crucial to confirm the therapeutic use of transdermal MSC hydrogels in hypertrophic scars. A random, controlled clinical trial was registered to test the effectiveness of transdermal MSC hydrogels in treating cesarean section skin scars, but the results have not been published thus far.117 Conditioned medium is referred to as the medium of MSCs and contains a variety of growth factors, cytokines, chemokines, and extracellular vesicles, such as ectosomes and exosomes.118 A polysaccharide hydrogel containing lyophilized adipose-derived stem cell conditioned medium was fabricated to inhibit hypertrophic scarring of rabbit ears by decreasing fibroblast proliferation and collagen disposition.111 It was reported that hybrid hydrogels containing xenofibroblasts reduced ECM accumulation and vessel-like structures in hypertrophic scars.99 In addition to stem cells and conditioned medium, exosomes perform therapeutic roles in hypertrophic scars and keloids.119–121 Thus, the role of exosome-based hydrogels in pathologic scars is worth exploring in the future.
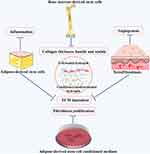 |
Figure 6 Stem cell and conditioned medium-loaded hydrogels. |
Nucleic Acids and Proteins
Given the increasing understanding of the molecular mechanisms underlying hypertrophic scars and keloids, researchers found that siRNA, which directly targets genes, and several protein products were effective in attenuating pathologic scars. It was reported that siRNA-TGFβ1-337 embedded in a pressure-sensitive adhesive hydrogel was developed for hypertrophic scar treatment, which resulted in TGFβ1 and collagen type I inhibition and regularly arranged fibroblasts in a hypertrophic scar nude mouse model.112 The poly (γ-glutamic acid)/chitosan/papain hydrogel promoted cell migration and significantly attenuated abnormal growth of fibroblasts, collagen deposition, and hyperplastic scar formation. Papain might inhibit fibroblast proliferation and degrade ECM, contributing to the prevention of the development of hypertrophic scars.113 As an elastic and firm protein, silk fibroin has attracted attention due to its wound healing-facilitating effects, but its antihypertrophic scar effects remain elusive. Thus, Li et al designed silk fibroin-based PEG hydrogels, which exhibited excellent biocompatibility and decreased the density of hypertrophic scar tissue and collagen expression.114 These results suggest that silk fibroin or silk fibre-based hydrogels are promising treatments for hypertrophic scars. Further studies are needed to compare the therapeutic efficacy and loading efficiency to keloid and hypertrophic scar tissues, fostering the application of silk-based hydrogels in pathologic scars.
Nanomaterials (Metal Ions/Carbon Materials)
Nanomaterials are referred to as materials with diameters of 1–100 nm that possess unique physical-chemical properties due to their structure. To further improve therapeutic efficacy, nanomaterials combined with hydrogels could form a hybrid biomaterial system for their controlled and prolonged release.122 Nanomaterials applied in hypertrophic scars and keloids mainly include nanoparticles, liposomes, carbon nanomaterials, and metallic nanoparticles. Liposomes and exosomes were described above;106,121 thus, in this section, we mainly discuss metal ions and carbon nanomaterials in pathologic scars. Silver ions are widely used in skin dressings and facilitate wound healing, given their antimicrobial effects. It was revealed that silver ion dressing could prevent pathologic scar formation in postburn patients.116 Jia et al found that silicone-derived silver hydrogel significantly decreased the scar elevation index.115 In addition, cuprous oxide nanoparticles in glucose solution were also proven effective in treating hypertrophic scars by inhibiting fibroblast growth and promoting cell apoptosis.123 In addition to metal ions, carbon nanomaterials were also effective in inhibiting hypertrophic scars. Weng et al demonstrated that carbon nanotubes directed hypertrophic scar fibroblast growth and decreased excessive proliferation and collagen expression in fibroblasts by suppressing TGF β.124 Carbonate apatite nanoparticle-loaded siTIMP1 significantly suppressed hypertrophic scar formation and collagen expression and density and attenuated the thickness and disorganization of collagen bundles.125
Current Limitations and Future Prospects
To date, researchers are facing some challenges that restrict the fabrication and applications of hydrogels in hypertrophic scars and keloids. We herein propose the main challenges and potential possibilities as follows to shed light on the future direction of hydrogel-based studies in keloids and hypertrophic scars.
1. The mechanism underlying these hydrogel components remains unclear, while high-throughput sequencing, such as transcriptome, spatial and single-cell transcriptome analysis, as well as proteomics, has been carried out to elucidate the molecular mechanism.126–128 Therefore, when designing hydrogels, researchers should look deeper into the mechanism and develop more specific therapies targeting abnormal cells, genes, and proteins.
2. The clinical application of hydrogels in hypertrophic scars and keloids is mainly confronted with three pitfalls: lack of animal model of keloid; lack of standard random clinical trials; and high manufacturing cost. It is believed that developing animal models of keloids, carrying out clinical trials and optimizing materials will accelerate the clinical use of hydrogels in hypertrophic scars and keloids.
3. Current research in hypertrophic scars and keloids only explores a limited number of drugs, stem cells, nucleic acids, and proteins. Nanomaterials such as metal ions and carbon nanomaterials exhibit multifunctional roles in medical fields;129,130 thus, these nanomaterials are worth investigating in keloid treatment.
4. Furthermore, traditional therapy, such as surgery, X-ray, laser and cryotherapy, could be combined with hydrogels to enhance therapeutic efficacy and even exert synergistic effects for the treatment of keloids and hypertrophic scars.
5. Although various types of hydrogels have been designed, their function is mainly restricted to one effect, such as anti-inflammatory, antiangiogenic or antifibrotic effects. Therefore, developing multifunctional hydrogels by loading different materials or tuning the physical and chemical properties of hydrogels will definitely promote their therapeutic efficacy in the future.
Conclusion
Hydrogels have been gaining increasing attention in hypertrophic scars and keloids due to its biocompatibility and effectiveness. In this review, the pathogenesis of pathologic scars and the classification and properties of hydrogels, as well as their loaded components, are discussed and highlighted, followed by the advances in nanomaterials in the area of hydrogels for biomedical applications. And smart hydrogel, which could respond to different stimuli is a future trend of hydrogels in biomedical fields. However, challenges still exist, such as tumor-related risks of tumor-derived natural hydrogels and elusive mechanism of pathologic scars. Associating hypertrophic scar and keloid pathogenesis with hydrogels as well as loaded components will shed light on further exploration and clinical applications. Thus, it is crucial to elucidate pathologic scar pathogenesis and improve efficacy and biocompatibility of hydrogels, in order to guide the therapeutic use of hydrogels in hypertrophic scars and keloids.
Data Sharing Statement
Data sharing is not applicable to this article, as no datasets were generated or analysed during the current study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the National Natural Science Foundation of China (82073019, 82073018 and 82202851), Shenzhen Science and Technology Innovation Commission, China (Natural Science Foundation of Shenzhen), (JCYJ20210324113001005, JCYJ20210324114212035, JCYJ20220530151817038). This work was supported by Grant No. from the National Natural Science Foundation and Grant No. from Shenzhen Science and Technology Planning Project.
Disclosure
The authors declare they have no conflicts of interest.
References
1. Gauglitz GG, Korting HC, Pavicic T, Ruzicka T, Jeschke MG. Hypertrophic scarring and keloids: pathomechanisms and current and emerging treatment strategies. Mol Med. 2011;17(1–2):113–125. doi:10.2119/molmed.2009.00153
2. Limandjaja GC, Niessen FB, Scheper RJ, Gibbs S. The keloid disorder: heterogeneity, histopathology, mechanisms and models. Front Cell Dev Biol. 2020;8:360. doi:10.3389/fcell.2020.00360
3. Knowles A, Glass DA. Keloids and hypertrophic scars. Dermatol Clin. 2023;41:509–517. doi:10.1016/j.det.2023.02.010
4. Morelli Coppola M, Salzillo R, Segreto F, Persichetti P. Triamcinolone acetonide intralesional injection for the treatment of keloid scars: patient selection and perspectives. Clin Cosmet Invest Dermatol. 2018;11:387–396. doi:10.2147/CCID.S133672
5. Hunasgi S, Koneru A, Vanishree M, Shamala R. Keloid: a case report and review of pathophysiology and differences between keloid and hypertrophic scars. J Oral Maxillofac Pathol. 2013;17:116–120. doi:10.4103/0973-029X.110701
6. Alfenas ER, Moreno A, Tanner PB, Netto HD, Fonseca MFL, Rios FG. Management of peri-implant hypertrophic scarring for an ear prosthesis. J Craniofac Surg. 2017;28:e777–e778. doi:10.1097/SCS.0000000000003976
7. Martin MS, Collawn SS. Combination treatment of CO2 fractional laser, pulsed dye laser, and triamcinolone acetonide injection for refractory keloid scars on the upper back. J Cosmet Laser Ther. 2013;15:166–170. doi:10.3109/14764172.2013.780448
8. Frech FS, Hernandez L, Urbonas R, Zaken GA, Dreyfuss I, Nouri K. Hypertrophic scars and keloids: advances in treatment and review of established therapies. Am J Clin Dermatol. 2023;24:225–245. doi:10.1007/s40257-022-00744-6
9. Mankowski P, Kanevsky J, Tomlinson J, Dyachenko A, Luc M. Optimizing radiotherapy for keloids: a meta-analysis systematic review comparing recurrence rates between different radiation modalities. Ann Plast Surg. 2017;78:403–411. doi:10.1097/SAP.0000000000000989
10. Broughton G, Janis JE, Attinger CE. Wound healing: an overview. Plast Reconstr Surg. 2006;117:1e-S-32e–S. doi:10.1097/01.prs.0000222562.60260.f9
11. Sorg H, Tilkorn DJ, Hager S, Hauser J, Mirastschijski U. Skin wound healing: an update on the current knowledge and concepts. Eur Surg Res. 2016;58:81–94. doi:10.1159/000454919
12. Falanga V. Wound healing and its impairment in the diabetic foot. Lancet. 2005;366:1736–1743. doi:10.1016/S0140-6736(05)67700-8
13. Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453:314–321. doi:10.1038/nature07039
14. Schreml S, Szeimies RM, Prantl L, Karrer S, Landthaler M, Babilas P. Oxygen in acute and chronic wound healing. Br J Dermatol. 2010;163:257–268. doi:10.1111/j.1365-2133.2010.09804.x
15. O’Toole EA. Extracellular matrix and keratinocyte migration. Clin Exp Dermatol. 2001;26:525–530. doi:10.1046/j.1365-2230.2001.00891.x
16. Hinz B. Formation and function of the myofibroblast during tissue repair. J Invest Dermatol. 2007;127:526–537. doi:10.1038/sj.jid.5700613
17. Harn HI-C, Ogawa R, Hsu C-K, Hughes MW, Tang M-J, Chuong C-M. The tension biology of wound healing. Exp Dermatol. 2019;28:464–471. doi:10.1111/exd.13460
18. Rosińczuk J, Taradaj J, Dymarek R, Sopel M. Mechanoregulation of wound healing and skin homeostasis. Biomed Res Int. 2016;2016:3943481. doi:10.1155/2016/3943481
19. Pakshir P, Hinz B. The big five in fibrosis: macrophages, myofibroblasts, matrix, mechanics, and miscommunication. Matrix Biol. 2018;68–69:81–93. doi:10.1016/j.matbio.2018.01.019
20. Ballotta V, Driessen-Mol A, Bouten CVC, Baaijens FPT. Strain-dependent modulation of macrophage polarization within scaffolds. Biomaterials. 2014;35:4919–4928. doi:10.1016/j.biomaterials.2014.03.002
21. Deng Z, Subilia M, Chin IL, et al. Keloid fibroblasts have elevated and dysfunctional mechanotransduction signaling that is independent of TGF-β. J Dermatol Sci. 2021;104:11–20. doi:10.1016/j.jdermsci.2021.09.002
22. Lu H, Wang H, Huang G, Wang X, Bu X. Therapeutic targeting of mechanical stretch-induced FAK/ERK signaling by fisetin in hypertrophic scars. Eur J Pharmacol. 2022;932:175228. doi:10.1016/j.ejphar.2022.175228
23. El Ayadi A, Jay JW, Prasai A. Current approaches targeting the wound healing phases to attenuate fibrosis and scarring. Int J Mol Sci. 2020;21:1105. doi:10.3390/ijms21031105
24. Van De Water L, Varney S, Tomasek JJ. Mechanoregulation of the myofibroblast in wound contraction, scarring, and fibrosis: opportunities for new therapeutic intervention. Adv Wound Care. 2013;2:122–141. doi:10.1089/wound.2012.0393
25. Hamburg E, DiNuoscio GJ, Mullin NK, Lafayatis R, Atit RP. Sustained β-catenin activity in dermal fibroblasts promotes fibrosis by up-regulating expression of extracellular matrix protein-coding genes. J Pathol. 2015;235:686–697. doi:10.1002/path.4481
26. Igota S, Tosa M, Murakami M, et al. Identification and characterization of wnt signaling pathway in keloid pathogenesis. Int J Med Sci. 2013;10:344–354. doi:10.7150/ijms.5349
27. Yu D, Shang Y, Yuan J, Ding S, Luo S, Hao L. Wnt/β-catenin signaling exacerbates keloid cell proliferation by regulating telomerase. Cell Physiol Biochem. 2016;39:2001–2013. doi:10.1159/000447896
28. Teofoli P, Barduagni S, Ribuffo M, Campanella A, De Pita’ O, Puddu P. Expression of Bcl-2, p53, c-jun and c-fos protooncogenes in keloids and hypertrophic scars. J Dermatol Sci. 1999;22:31–37. doi:10.1016/s0923-1811(99)00040-7
29. Hu Z, Lou L, Luo S. 病理性瘢痕中c-myc、c-fos和ras原癌基因表达的实验研究 [Experimental study of the expression of c-myc, c-fos and proto-oncogenes on hypertrophic and scars]. Zhonghua Zheng Xing Wai Ke Za Zhi. 2002;18:165–167. Chinese.
30. De Felice B, Ciarmiello LF, Mondola P, et al. Differential p63 and p53 expression in human keloid fibroblasts and hypertrophic scar fibroblasts. DNA Cell Biol. 2007;26:541–547. doi:10.1089/dna.2007.0591
31. Chawla S, Ghosh S. Regulation of fibrotic changes by the synergistic effects of cytokines, dimensionality and matrix: towards the development of an in vitro human dermal hypertrophic scar model. Acta Biomater. 2018;69:131–145. doi:10.1016/j.actbio.2018.01.002
32. Jiao H, Dong P, Yan L, et al. TGF-β1 induces polypyrimidine tract-binding protein to alter fibroblasts proliferation and fibronectin deposition in keloid. Sci Rep. 2016;6:38033. doi:10.1038/srep38033
33. Zhou M-W, Yin W-T, Jiang R-H, et al. Inhibition of collagen synthesis by IWR-1 in normal and keloid-derived skin fibroblasts. Life Sci. 2017;173:86–93. doi:10.1016/j.lfs.2016.12.003
34. Imaizumi R, Akasaka Y, Inomata N, et al. Promoted activation of matrix metalloproteinase (MMP)-2 in keloid fibroblasts and increased expression of MMP-2 in collagen bundle regions: implications for mechanisms of keloid progression. Histopathology. 2009;54:722–730. doi:10.1111/j.1365-2559.2009.03287.x
35. Laberge A, Merjaneh M, Arif S, Larochelle S, Moulin VJ. Shedding of proangiogenic microvesicles from hypertrophic scar myofibroblasts. Exp Dermatol. 2021;30:112–120. doi:10.1111/exd.14178
36. Jiang D, Fu X, Chen W, Sun T. 血管生成因子及其受体过表达与瘢痕疙瘩侵袭性生长 [Relationship of overexpression of angiogenesis factors and their receptors with invasive growth of keloid]. Zhonghua Zheng Xing Wai Ke Za Zhi. 2004;20:128–131. Chinese.
37. Okuno R, Ito Y, Eid N, Otsuki Y, Kondo Y, Ueda K. Upregulation of autophagy and glycolysis markers in keloid hypoxic-zone fibroblasts: morphological characteristics and implications. Histol Histopathol. 2018;33:1075–1087. doi:10.14670/HH-18-005
38. Wang Q, Wang P, Qin Z, et al. Altered glucose metabolism and cell function in keloid fibroblasts under hypoxia. Redox Biol. 2021;38:101815. doi:10.1016/j.redox.2020.101815
39. Li Q, Qin Z, Nie F, et al. Metabolic reprogramming in keloid fibroblasts: aerobic glycolysis and a novel therapeutic strategy. Biochem Biophys Res Commun. 2018;496:641–647. doi:10.1016/j.bbrc.2018.01.068
40. Fedele M, Sgarra R, Battista S, Cerchia L, Manfioletti G. The epithelial-mesenchymal transition at the crossroads between metabolism and tumor progression. Int J Mol Sci. 2022;23:800. doi:10.3390/ijms23020800
41. Zhang N, Ng AS, Cai S, Li Q, Yang L, Kerr D. Novel therapeutic strategies: targeting epithelial-mesenchymal transition in colorectal cancer. Lancet Oncol. 2021;22:e358–e368. doi:10.1016/S1470-2045(21)00343-0
42. Yan C, Grimm WA, Garner WL, et al. Epithelial to mesenchymal transition in human skin wound healing is induced by tumor necrosis factor-alpha through bone morphogenic protein-2. Am J Pathol. 2010;176:2247–2258. doi:10.2353/ajpath.2010.090048
43. Cui HS, Joo SY, Lee SY, Cho YS, Kim DH, Seo CH. Effect of hypertrophic scar fibroblast-derived exosomes on keratinocytes of normal human skin. Int J Mol Sci. 2023;24:6132. doi:10.3390/ijms24076132
44. Lee YI, Shim JE, Kim J, et al. WNT5A drives interleukin-6-dependent epithelial-mesenchymal transition via the JAK/STAT pathway in keloid pathogenesis. Burns Trauma. 2022;10:tkac023. doi:10.1093/burnst/tkac023
45. Satish L, Evdokiou A, Geletu E, Hahn JM, Supp DM. Pirfenidone inhibits epithelial-mesenchymal transition in keloid keratinocytes. Burns Trauma. 2020;8:tkz007. doi:10.1093/burnst/tkz007
46. Lei R, Zhang S, Wang Y, Dai S, Sun J, Zhu C. Metformin inhibits epithelial-to-mesenchymal transition of keloid fibroblasts via the HIF-1α/PKM2 signaling pathway. Int J Med Sci. 2019;16:960–966. doi:10.7150/ijms.32157
47. Qu M, Song N, Chai G, Wu X, Liu W. Pathological niche environment transforms dermal stem cells to keloid stem cells: a hypothesis of keloid formation and development. Med Hypotheses. 2013;81:807–812. doi:10.1016/j.mehy.2013.08.033
48. Macarak EJ, Wermuth PJ, Rosenbloom J, Uitto J. Keloid disorder: fibroblast differentiation and gene expression profile in fibrotic skin diseases. Exp Dermatol. 2021;30:132–145. doi:10.1111/exd.14243
49. Grant C, Chudakova DA, Itinteang T, et al. Expression of embryonic stem cell markers in keloid-associated lymphoid tissue. J Clin Pathol. 2016;69:643–646. doi:10.1136/jclinpath-2015-203483
50. Bazid HAS, Samaka RM, Mousa MEA, Seleit I. Immunohistochemical expression of Axin-2, as an implication of the role of stem cell in scar pathogenesis and prognosis. J Cosmet Dermatol. 2022;21:6010–6020. doi:10.1111/jocd.15075
51. Zhao X-F, Wang D-L, Wei Z-R, Xue Q-Y, Yu L-M. 人增生性瘢痕成纤维细胞的间充质干细胞表型及多向分化潜能 [The research of fibroblasts from human hypertrophic scar showing a mesenchymal stem cell phenotype and multilineage differentiation potentialities]. Zhonghua Zheng Xing Wai Ke Za Zhi. 2013;29:273–279. Chinese.
52. Lee SC, Kwon IK, Park K. Hydrogels for delivery of bioactive agents: a historical perspective. Adv Drug Deliv Rev. 2013;65:17–20. doi:10.1016/j.addr.2012.07.015
53. Wichterle O, Lím D. Hydrophilic gels for biological use. Nature. 1960;185:117–118. doi:10.1038/185117a0
54. Buwalda SJ, Boere KWM, Dijkstra PJ, Feijen J, Vermonden T, Hennink WE. Hydrogels in a historical perspective: from simple networks to smart materials. J Control Release. 2014;190:254–273. doi:10.1016/j.jconrel.2014.03.052
55. Sharpe LA, Daily AM, Horava SD, Peppas NA. Therapeutic applications of hydrogels in oral drug delivery. Expert Opin Drug Deliv. 2014;11:901–915. doi:10.1517/17425247.2014.902047
56. Mahapatra C, Jin G-Z, Kim H-W. Alginate-hyaluronic acid-collagen composite hydrogel favorable for the culture of chondrocytes and their phenotype maintenance. Tissue Eng Regen Med. 2016;13:538–546. doi:10.1007/s13770-016-0059-1
57. Wang J, Youngblood R, Cassinotti L, Skoumal M, Corfas G, Shea L. An injectable PEG hydrogel controlling neurotrophin-3 release by affinity peptides. J Control Release. 2021;330:575–586. doi:10.1016/j.jconrel.2020.12.045
58. Murthy PSK, Murali Mohan Y, Varaprasad K, Sreedhar B, Mohana Raju K. First successful design of semi-IPN hydrogel-silver nanocomposites: a facile approach for antibacterial application. J Colloid Interface Sci. 2008;318:217–224. doi:10.1016/j.jcis.2007.10.014
59. Masood N, Ahmed R, Tariq M, et al. Silver nanoparticle impregnated chitosan-PEG hydrogel enhances wound healing in diabetes induced rabbits. Int J Pharm. 2019;559:23–36. doi:10.1016/j.ijpharm.2019.01.019
60. Ho T-C, Chang -C-C, Chan H-P, et al. Hydrogels: properties and applications in biomedicine. Molecules. 2022;27:2902. doi:10.3390/molecules27092902
61. Ahmed EM. Hydrogel: preparation, characterization, and applications: a review. J Adv Res. 2015;6:105–121. doi:10.1016/j.jare.2013.07.006
62. Hughes CS, Postovit LM, Lajoie GA. Matrigel: a complex protein mixture required for optimal growth of cell culture. Proteomics. 2010;10:1886–1890. doi:10.1002/pmic.200900758
63. Rastogi P, Kandasubramanian B. Review of alginate-based hydrogel bioprinting for application in tissue engineering. Biofabrication. 2019;11:042001. doi:10.1088/1758-5090/ab331e
64. Patrulea V, Ostafe V, Borchard G, Jordan O. Chitosan as a starting material for wound healing applications. Eur J Pharm Biopharm. 2015;97:417–426. doi:10.1016/j.ejpb.2015.08.004
65. Lam J, Truong NF, Segura T. Design of cell-matrix interactions in hyaluronic acid hydrogel scaffolds. Acta Biomater. 2014;10:1571–1580. doi:10.1016/j.actbio.2013.07.025
66. Mohanto S, Narayana S, Merai KP, et al. Advancements in gelatin-based hydrogel systems for biomedical applications: a state-of-The-art review. Int J Biol Macromol. 2023;253:127143. doi:10.1016/j.ijbiomac.2023.127143
67. Krsko P, Kaplan JB, Libera M. Spatially controlled bacterial adhesion using surface-patterned poly(ethylene glycol) hydrogels. Acta Biomater. 2009;5:589–596. doi:10.1016/j.actbio.2008.08.025
68. Madry H, Gao L, Rey-Rico A, et al. Thermosensitive hydrogel based on PEO-PPO-PEO poloxamers for a controlled in situ release of recombinant adeno-associated viral vectors for effective gene therapy of cartilage defects. Adv Mater. 2020;32:e1906508. doi:10.1002/adma.201906508
69. Pang Q, Wu K, Jiang Z, et al. A polyaniline nanoparticles crosslinked hydrogel with excellent photothermal antibacterial and mechanical properties for wound dressing. Macromol Biosci. 2022;22:e2100386. doi:10.1002/mabi.202100386
70. Huang B, Wu C, Hu Y, et al. Osmanthus-loaded PVP/PVA hydrogel inhibits the proliferation and migration of oral squamous cell carcinoma cells CAL-27. Polymers. 2022;14:5399. doi:10.3390/polym14245399
71. Wan WK, Campbell G, Zhang ZF, Hui AJ, Boughner DR. Optimizing the tensile properties of polyvinyl alcohol hydrogel for the construction of a bioprosthetic heart valve stent. J Biomed Mater Res. 2002;63:854–861. doi:10.1002/jbm.10333
72. Yue K, Trujillo-de santiago G, Alvarez MM, Tamayol A, Annabi N, Khademhosseini A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials. 2015;73:254–271. doi:10.1016/j.biomaterials.2015.08.045
73. Bakulina AA, Musina GR, Gavdush AA, et al. PEG-fibrin conjugates: the PEG impact on the polymerization dynamics. Soft Matter. 2023;19:2430–2437. doi:10.1039/d2sm01504h
74. Stahl PJ, Romano NH, Wirtz D, Yu SM. PEG-based hydrogels with collagen mimetic peptide-mediated and tunable physical cross-links. Biomacromolecules. 2010;11:2336–2344. doi:10.1021/bm100465q
75. Cruz-Acuña R, García AJ. Synthetic hydrogels mimicking basement membrane matrices to promote cell-matrix interactions. Matrix Biol. 2017;57–58:324–333. doi:10.1016/j.matbio.2016.06.002
76. Egeblad M, Rasch MG, Weaver VM. Dynamic interplay between the collagen scaffold and tumor evolution. Curr Opin Cell Biol. 2010;22:697–706. doi:10.1016/j.ceb.2010.08.015
77. Koide H, Okishima A, Hoshino Y, et al. Synthetic hydrogel nanoparticles for sepsis therapy. Nat Commun. 2021;12:5552. doi:10.1038/s41467-021-25847-2
78. Teodorescu M, Bercea M, Morariu S. Biomaterials of PVA and PVP in medical and pharmaceutical applications: perspectives and challenges. Biotechnol Adv. 2019;37:109–131. doi:10.1016/j.biotechadv.2018.11.008
79. Chu T-W, Feng J, Yang J, Kopeček J. Hybrid polymeric hydrogels via peptide nucleic acid (PNA)/DNA complexation. J Control Release. 2015;220:608–616. doi:10.1016/j.jconrel.2015.09.035
80. Xu Z, Liu G, Huang J, Wu J. Novel glucose-responsive antioxidant hybrid hydrogel for enhanced diabetic wound repair. ACS Appl Mater Interfaces. 2022;14:7680–7689. doi:10.1021/acsami.1c23461
81. Cao H, Duan L, Zhang Y, Cao J, Zhang K. Current hydrogel advances in physicochemical and biological response-driven biomedical application diversity. Signal Transduct Target Ther. 2021;6:426. doi:10.1038/s41392-021-00830-x
82. Meng Z, Zhou X, Xu J, et al. Light-triggered in situ gelation to enable robust photodynamic-immunotherapy by repeated stimulations. Adv Mater. 2019;31:e1900927. doi:10.1002/adma.201900927
83. Malik R, Lelkes PI, Cukierman E. Biomechanical and biochemical remodeling of stromal extracellular matrix in cancer. Trends Biotechnol. 2015;33:230–236. doi:10.1016/j.tibtech.2015.01.004
84. Yang Q, Su S, Liu S, et al. Exosomes-loaded electroconductive nerve dressing for nerve regeneration and pain relief against diabetic peripheral nerve injury. Bioact Mater. 2023;26:194–215. doi:10.1016/j.bioactmat.2023.02.024
85. Guo S, Ren Y, Chang R, et al. Injectable self-healing adhesive chitosan hydrogel with antioxidative, antibacterial, and hemostatic activities for rapid hemostasis and skin wound healing. ACS Appl Mater Interfaces. 2022;14:34455–34469. doi:10.1021/acsami.2c08870
86. Li S, Cong Y, Fu J. Tissue adhesive hydrogel bioelectronics. J Mater Chem B. 2021;9:4423–4443. doi:10.1039/d1tb00523e
87. Zhang F-X, Liu P, Ding W, et al. Injectable Mussel-Inspired highly adhesive hydrogel with exosomes for endogenous cell recruitment and cartilage defect regeneration. Biomaterials. 2021;278:121169. doi:10.1016/j.biomaterials.2021.121169
88. Xu J, Liu Y, Hsu S-H. Hydrogels based on Schiff base linkages for biomedical applications. Molecules. 2019;24:3005. doi:10.3390/molecules24163005
89. Liu Y, Zhang Z, Zhang Y, et al. Construction of adhesive and bioactive silk fibroin hydrogel for treatment of spinal cord injury. Acta Biomater. 2023;158:178–189. doi:10.1016/j.actbio.2022.12.048
90. Shirzaei Sani E, Portillo-Lara R, Spencer A, et al. Engineering adhesive and antimicrobial hyaluronic acid/elastin-like polypeptide hybrid hydrogels for tissue engineering applications. ACS Biomater Sci Eng. 2018;4:2528–2540. doi:10.1021/acsbiomaterials.8b00408
91. Theocharidis G, Yuk H, Roh H, et al. A strain-programmed patch for the healing of diabetic wounds. Nat Biomed Eng. 2022;6:1118–1133. doi:10.1038/s41551-022-00905-2
92. Wu Y, Wang Y, Long L, Hu C, Kong Q, Wang Y. A spatiotemporal release platform based on pH/ROS stimuli-responsive hydrogel in wound repairing. J Control Release. 2022;341:147–165. doi:10.1016/j.jconrel.2021.11.027
93. Li D, Fei X, Xu L, Wang Y, Tian J, Li Y. Pressure-sensitive antibacterial hydrogel dressing for wound monitoring in bed ridden patients. J Colloid Interface Sci. 2022;627:942–955. doi:10.1016/j.jcis.2022.07.030
94. Cai C, Zhu H, Chen Y, et al. Mechanoactive nanocomposite hydrogel to accelerate wound repair in movable parts. ACS Nano. 2022;16:20044–20056. doi:10.1021/acsnano.2c07483
95. Osti E. Cutaneous burns treated with hydrogel (Burnshield) and a semipermeable adhesive film. Arch Surg. 2006;141:39–42. doi:10.1001/archsurg.141.1.39
96. Zhang N, Gao T, Wang Y, et al. Modulating cationicity of chitosan hydrogel to prevent hypertrophic scar formation during wound healing. Int J Biol Macromol. 2020;154:835–843. doi:10.1016/j.ijbiomac.2020.03.161
97. Berman B, Garikaparthi S, Smith E, Newburger J. A novel hydrogel scaffold for the prevention or reduction of the recurrence of keloid scars postsurgical excision. J Am Acad Dermatol. 2013;69:828–830. doi:10.1016/j.jaad.2013.06.025
98. Nizet J-L, Piérard GE, Quatresooz P. Revisiting biothermal effects on erythematous hypertrophic scars during pregnancy. J Cosmet Dermatol. 2009;8:27–31. doi:10.1111/j.1473-2165.2009.00420.x
99. Hartwell R, Poormasjedi-Meibod M-S, Chavez-Munoz C, Jalili RB, Hossenini-Tabatabaei A, Ghahary A. An in-situ forming skin substitute improves healing outcome in a hypertrophic scar model. Tissue Eng Part A. 2015;21:1085–1094. doi:10.1089/ten.TEA.2014.0271
100. Bao Z, Gao P, Xia G, et al. A thermosensitive hydroxybutyl chitosan hydrogel as a potential co-delivery matrix for drugs on keloid inhibition. J Mater Chem B. 2016;4:3936–3944. doi:10.1039/c6tb00378h
101. Chen Y-J, Cheng H-W, Yen W-Y, et al. The treatment of keloid scars via modulating heterogeneous gelatin-structured composite microneedles to control transdermal dual-drug release. Polymers. 2022;14:4436. doi:10.3390/polym14204436
102. Chen Z, Hu X, Lin Z, et al. Layered GelMA/PEGDA hydrogel microneedle patch as an intradermal delivery system for hypertrophic scar treatment. ACS Appl Mater Interfaces. 2023;15:43309–43320. doi:10.1021/acsami.3c06800
103. Lin W-C, Liou S-H, Kotsuchibashi Y. Development and characterisation of the Imiquimod Poly(2-(2-methoxyethoxy)ethyl Methacrylate) hydrogel dressing for keloid therapy. Polymers. 2017;9:579. doi:10.3390/polym9110579
104. Ma J-C, Wang Z-N, Xi M-F, Yin D, Jiang LI-F, Qi J. Experimental study on the effect of caffeine hydrogel on the expression of TGF -β1, α-SMA and collagen in hypertrophic scar of rabbit ears. J Burn Care Res. 2023;irad115. doi:10.1093/jbcr/irad115
105. Danielson JR, Walter RJ. Case studies: use of salicylic acid (Avosil) and hydrogel (Avogel) in limiting scar formation. J Burns Wounds. 2005;4:e6.
106. Wan H, Wang S, Li C, et al. LA67 liposome-loaded thermo-sensitive hydrogel with active targeting for efficient treatment of keloid via peritumoral injection. Pharmaceutics. 2023;15:2157. doi:10.3390/pharmaceutics15082157
107. Wu J, Xu R, Zhan R, et al. Effective symptomatic treatment for severe and intractable pruritus associated with severe burn-induced hypertrophic scars: a prospective, multicenter, controlled trial. Burns. 2016;42:1059–1066. doi:10.1016/j.burns.2015.09.021
108. Yang Y, Liu L, Wu X, Wang X, Lu Q, Zhang Z. CO2 fractional laser-assisted transdermal delivery of silk nanofiber carriers in a rabbit ear hypertrophic scar model. Burns Trauma. 2022;10:tkac040. doi:10.1093/burnst/tkac040
109. Qu C, Bao Z, Zhang X, et al. A thermosensitive RGD-modified hydroxybutyl chitosan hydrogel as a 3D scaffold for BMSCs culture on keloid treatment. Int J Biol Macromol. 2019;125:78–86. doi:10.1016/j.ijbiomac.2018.12.058
110. Dong Y, Cui M, Qu J, et al. Conformable hyaluronic acid hydrogel delivers adipose-derived stem cells and promotes regeneration of burn injury. Acta Biomater. 2020;108:56–66. doi:10.1016/j.actbio.2020.03.040
111. Zhang C, Wang T, Zhang L, et al. Combination of lyophilized adipose-derived stem cell concentrated conditioned medium and polysaccharide hydrogel in the inhibition of hypertrophic scarring. Stem Cell Res Ther. 2021;12:23. doi:10.1186/s13287-020-02061-3
112. Zhao R, Yan Q, Huang H, Lv J, Ma W. Transdermal siRNA-TGFβ1-337 patch for hypertrophic scar treatment. Matrix Biol. 2013;32:265–276. doi:10.1016/j.matbio.2013.02.004
113. Xue Y, Qi C, Dong Y, et al. Poly (γ-glutamic acid)/chitooligo-saccharide/papain hydrogel prevents hypertrophic scar during skin wound healing. J Biomed Mater Res B Appl Biomater. 2021;109:1724–1734. doi:10.1002/jbm.b.34830
114. Li Z, Song J, Zhang J, et al. Topical application of silk fibroin-based hydrogel in preventing hypertrophic scars. Colloids Surf B Biointerfaces. 2020;186:110735. doi:10.1016/j.colsurfb.2019.110735
115. Jia S, Zhao Y, Mustoe TA. The effects of topically applied silicone gel and its silver derivative on the prevention of hypertrophic scarring in two rabbit ear-scarring models. J Plast Reconstr Aesthet Surg. 2011;64:e332–e334. doi:10.1016/j.bjps.2011.05.008
116. Munteanu A, Florescu I, Nitescu C. A modern method of treatment: the role of silver dressings in promoting healing and preventing pathological scarring in patients with burn wounds. J Med Life. 2016;9:306–315.
117. Fan D, Xia Q, Wu S, et al. Mesenchymal stem cells in the treatment of Cesarean section skin scars: study protocol for a randomized, controlled trial. Trials. 2018;19:155. doi:10.1186/s13063-018-2478-x
118. L PK, Kandoi S, Misra R, S V, K R, Verma RS. The mesenchymal stem cell secretome: a new paradigm towards cell-free therapeutic mode in regenerative medicine. Cytokine Growth Factor Rev. 2019;46:1–9. doi:10.1016/j.cytogfr.2019.04.002
119. Wu Z-Y, Zhang H-J, Zhou Z-H, et al. The effect of inhibiting exosomes derived from adipose-derived stem cells via the TGF-β1/Smad pathway on the fibrosis of keloid fibroblasts. Gland Surg. 2021;10:1046–1056. doi:10.21037/gs-21-4
120. Li Y, Zhang J, Shi J, et al. Exosomes derived from human adipose mesenchymal stem cells attenuate hypertrophic scar fibrosis by miR-192-5p/IL-17RA/Smad axis. Stem Cell Res Ther. 2021;12:221. doi:10.1186/s13287-021-02290-0
121. Zhong Y, Zhang Y, Yu A, et al. Therapeutic role of exosomes and conditioned medium in keloid and hypertrophic scar and possible mechanisms. Front Physiol. 2023;14:1247734. doi:10.3389/fphys.2023.1247734
122. Gao W, Zhang Y, Zhang Q, Zhang L. Nanoparticle-hydrogel: a hybrid biomaterial system for localized drug delivery. Ann Biomed Eng. 2016;44:2049–2061. doi:10.1007/s10439-016-1583-9
123. Xiao Y, Xu D, Song H, et al. Cuprous oxide nanoparticles reduces hypertrophic scarring by inducing fibroblast apoptosis. Int J Nanomed. 2019;14:5989–6000. doi:10.2147/IJN.S196794
124. Weng W, He S, Song H, et al. Aligned carbon nanotubes reduce hypertrophic scar via regulating cell behavior. ACS Nano. 2018;12:7601–7612. doi:10.1021/acsnano.7b07439
125. Aoki M, Matsumoto NM, Dohi T, et al. Direct delivery of apatite nanoparticle-encapsulated siRNA targeting TIMP-1 for intractable abnormal scars. Mol Ther Nucleic Acids. 2020;22:50–61. doi:10.1016/j.omtn.2020.08.005
126. Wang Q, Zhong Y, Li Z, et al. Multitranscriptome analyses of keloid fibroblasts reveal the role of the HIF-1α/HOXC6/ERK axis in keloid development. Burns Trauma. 2022;10:tkac013. doi:10.1093/burnst/tkac013
127. Shim J, Oh SJ, Yeo E, et al. Integrated analysis of single-cell and spatial transcriptomics in keloids: highlights on fibrovascular interactions in keloid pathogenesis. J Invest Dermatol. 2022;142:2128–2139.e11. doi:10.1016/j.jid.2022.01.017
128. Liu J, Yang C, Zhang H, et al. Quantitative proteomics approach reveals novel biomarkers and pathological mechanism of keloid. Proteomics Clin Appl. 2022;16:e2100127. doi:10.1002/prca.202100127
129. Cheng Z, Li M, Dey R, Chen Y. Nanomaterials for cancer therapy: current progress and perspectives. J Hematol Oncol. 2021;14:85. doi:10.1186/s13045-021-01096-0
130. Wang M, Huang X, Zheng H, et al. Nanomaterials applied in wound healing: mechanisms, limitations and perspectives. J Control Release. 2021;337:236–247. doi:10.1016/j.jconrel.2021.07.017
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
