Back to Journals » OncoTargets and Therapy » Volume 9
Hürthle cell carcinoma: current perspectives
Authors Ahmadi S, Stang M, Jiang XS, Sosa JA
Received 17 August 2016
Accepted for publication 9 September 2016
Published 7 November 2016 Volume 2016:9 Pages 6873—6884
DOI https://doi.org/10.2147/OTT.S119980
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Chiung-Kuei Huang
Sara Ahmadi,1 Michael Stang,2 Xiaoyin “Sara” Jiang,3 Julie Ann Sosa2,4,5
1Division of Endocrinology, Department of Medicine, 2Section of Endocrine Surgery, Department of Surgery, 3Department of Pathology, Duke University Medical Center, 4Duke Cancer Institute, 5Duke Clinical Research Institute, Duke University Medical Center, Durham, NC, USA
Abstract: Hürthle cell carcinoma (HCC) can present either as a minimally invasive or as a widely invasive tumor. HCC generally has a more aggressive clinical behavior compared with the other differentiated thyroid cancers, and it is associated with a higher rate of distant metastases. Minimally invasive HCC demonstrates much less aggressive behavior; lesions <4 cm can be treated with thyroid lobectomy alone, and without radioactive iodine (RAI). HCC has been observed to be less iodine-avid compared with other differentiated thyroid cancers; however, recent data have demonstrated improved survival with RAI use in patients with HCC >2 cm and those with nodal and distant metastases. Patients with localized iodine-resistant disease who are not candidates for a wait-and-watch approach can be treated with localized therapies. Systemic therapy is reserved for patients with progressive, widely metastatic HCC.
Keywords: thyroid cancer, thyroid nodule, follicular cell carcinoma, Hurthle cell lesion, minimally invasive HCC
Hürthle cell cytology, genotype, and clinical behavior
The oncocytic follicular cells of the thyroid continue to carry the name “Hürthle cells”, even though the cells that Karl Hürthle initially identified in 1894 ultimately proved to be the parafollicular C cells (Hürthle 1894).1 They are large, polygonal cells with marked eosinophilic, granular cytoplasm reflective of overly abundant mitochondria (Figure 1). Hürthle cells can be observed in both benign and malignant conditions of the thyroid gland. The mechanism behind mitochondrial abundance in these conditions is not completely understood; however, it is likely a reflection of excessive mitochondrial proliferation and/or decreased mitochondrial degradation (mitophagy). Oncocytic metaplasia occurs in many organs of the body secondary to various cellular stress responses. In the thyroid, oncocytic changes are associated with such benign conditions as cell-mediated autoimmune thyroiditis (Figure 2), humoral-mediated hyperthyroidism (Graves disease), and hyperplastic nodules seen in multinodular goiters.2 Focal oncocytic trans-differentiation results in neoplasia consisting predominantly of Hürthle cells that can represent benign Hürthle cell adenomas or malignant Hürthle cell carcinomas (HCCs). The histologic distinction between adenoma and carcinoma can generally only be definitively made on histologic examination of a resection specimen; like other follicular lesions, it is determined by the presence or absence of capsular and/or vascular invasion, which are the hallmarks of HCC (Figure 3). Oncocytic follicular cells are also seen in the oncocytic variant of papillary thyroid carcinoma, which is a rare subtype of papillary thyroid carcinoma. Variable biological behaviors have been reported for the oncocytic variant of papillary thyroid carcinoma, which is histologically defined by the presence of oncocytes (at least 75% of the entire tumor) seen along with the classic nuclear features of papillary thyroid carcinoma.2–5
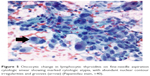
  | Figure 1 Oncocytes (Hürthle cells) showing characteristic abundant, granular eosinophilic cytoplasm and prominent nucleoli (hematoxylin and eosin, ×40). |
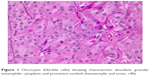
  | Figure 2 Prominent oncocytic change in chronic lymphocytic thyroiditis. Abundant lymphocytes with germinal center formation are present (hematoxylin and eosin, ×10). |
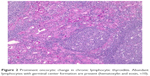
  | Figure 3 Vascular invasion in a Hürthle cell carcinoma (hematoxylin and eosin, ×2). |
HCC was first described by Ewing in 1928; it represents only 3% of all thyroid cancers6 and is currently classified as a variant of follicular carcinoma according to the World Health Organization (WHO).7 However, recent data have called into question whether HCC is indeed a variant of follicular carcinoma or a distinct entity with a different mutational profile, unique pathologic signature, and clinical behavior. A recent study of 27 Hürthle cell tumor samples (8 Hürthle cell adenomas, 9 minimally invasive HCCs, and 10 widely invasive HCCs) used mass spectrometry-based genotyping to interrogate hot spot point mutations in the most common thyroid oncogenes, and real-time polymerase chain reaction was used to assess for common oncogenic fusions.8 Transcriptomes for widely invasive HCC were compared with follicular carcinoma expression array profiles obtained from the Gene Expression Omnibus. The authors reported only 11% RAS mutations and no Pax8-PPARγ rearrangements in HCC tumor samples; in contrast, follicular carcinomas had a 45% RAS mutation rate and rearrangements of Pax8-PPARγ in 25%–60% of cases. This study also showed that the chromosomal copy number profiles of HCCs were distinct from follicular carcinomas, as large regions of gain on chromosomes 5, 7, 12, and 17 are observed in HCC, but they are not seen in follicular carcinoma.
HCC is subdivided based on histology into either minimally invasive or widely invasive subgroups. Minimally invasive carcinomas are fully encapsulated tumors with microscopically identifiable foci of capsular or vascular invasion (<4 foci), in contradistinction to widely invasive tumors, which have extensive vascular invasion (>4 foci) and extrathyroidal invasion. However, there is a lack of consensus in the literature, as some centers consider encapsulated tumors with only microscopic capsular invasion to be minimally invasive HCCs, whereas encapsulated tumors with minimal vascular invasion (<4 foci) are classified separately. Patients with a minimally invasive encapsulated HCC (microscopic capsular invasion with no vascular invasion) often experience a good prognosis.9
Studies have reported that HCC with focal vascular invasion (<4 foci) can also be associated with less aggressive behavior similar to that of HCCs with minimal capsular invasion and no vascular invasion. In a retrospective single-institutional study by Xu et al, the experience of 276 patients with encapsulated differentiated thyroid cancers (224 encapsulated papillary thyroid cancers, 34 encapsulated follicular carcinomas, and 16 encapsulated HCCs) showed that extensive vascular invasion (≥4 foci of vascular invasion) is an independent predictor of recurrence in patients with encapsulated differentiated thyroid cancers; indeed, the risk of recurrence was 42% in patients with extensive vascular invasion compared to 1% in patients with no vascular invasion or focal vascular invasion (<4 sites).10 In a retrospective, single-institution study of 50 patients with encapsulated HCC, Ghossein et al also reported that the pathologic finding of >4 foci of vascular invasion is the most powerful predictor of recurrence and compromised recurrence-free survival (P<0.001) among patients with encapsulated HCC. In this study, patients with no vascular invasion or <4 foci of vascular invasion had 100% recurrence-free survival at 5 years compared to 20% recurrence-free survival among patients with >4 foci of vascular invasion.11
Factors other than vascular invasion have been reported to be associated with overall survival in patients with HCC. Goffredo et al identified 3,311 patients >18 years of age with a diagnosis of HCC and 59,585 patients with papillary and follicular thyroid cancers from the Surveillance, Epidemiology and End Result (SEER) database from 1988 to 2009 and determined that older age, larger tumor size, extrathyroidal tumor extension, and not undergoing surgery were independently associated with reduced overall survival among patients with HCC. HCC was more common in older men, and it appeared to be more aggressive compared to other differentiated thyroid cancers.12 In another study, Chindris et al identified 173 patients with HCC between 2001 and 2012; male gender and American Joint Committee on Cancer TNM (AJCC-TNM) Stages III–IV were independent risk factors for recurrence or death among patients with widely invasive HCC. The cumulative risk of recurrence or death within 5 years of diagnosis was 91% in men with Stages III–IV disease compared to 74% in women with the same stages of disease; the 5-year cumulative probability of recurrence or death in patients with AJCC-TNM Stages I–II was 0% among women compared to 17% among men.13
Diagnosis
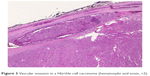
One of the fundamental obstacles to expedited treatment is our inability to establish the diagnosis of HCC preoperatively, either radiologically or with cytology based on fine needle aspiration (FNA). In the absence of identifiable invasive or metastatic disease on imaging studies, it is usually impossible to distinguish between a benign Hürthle cell process or neoplasm and HCC; for this reason, histologic evaluation based on a surgical specimen is generally necessary. Ultrasound alone is unable to distinguish HCC from other histologic variants, as it can clinically demonstrate a spectrum of sonographic findings from hypoechogenicity to hyperechogenicity14 (Figure 4).
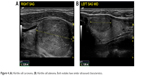  | Figure 4 (A) Hürthle cell carcinoma. (B) Hürthle cell adenoma. Both nodules have similar ultrasound characteristics. |
FNA diagnosis of a Hürthle cell neoplasm (HCN) can typically be classified as a follicular lesion (FLUS) or atypia of undetermined significance (AUS) with Hürthle cell features (AUS/FLUS/Hürthle cell lesion of undetermined significance [HLUS], Bethesda III). Both benign and malignant oncocytic lesions can demonstrate marked cytologic atypia, complicating the process of diagnosis on biopsy alone (Figure 5). Overall, the projected risk of malignancy for this diagnostic category is 5%–15%. A cytologic diagnosis of suspicious for HCN (Bethesda IV) carries a projected 15%–30% risk of malignancy.15 However, the true risk of malignancy for each Bethesda diagnostic cytologic category varies by institution, and such institutional data should be examined regularly and made available to clinicians.16,17 In patients with a cytologic finding of a HLUS, possible management strategies include repeat ultrasound-guided FNA (potentially paired with molecular testing) and close surveillance if the molecular testing is reassuring, or surgical excision for the purpose of definitive diagnosis. In patients with FNA cytology demonstrating a HCN, diagnostic surgical excision should be performed if molecular testing is not done or if it is inconclusive.9
Several studies have examined clinical variables that may be more indicative of malignancy in patients with FNA results that are suspicious for a HCN (Bethesda IV). In a 2010 retrospective single-institution study, Kim et al reviewed clinical features, preoperative imaging, and pathology reports of 57 patients with an FNA cytology consistent with HCN who underwent thyroidectomy. This study reported that on multi-logistic regression, patients with nodules >1.5 cm have a higher risk of being associated with a thyroid malignancy compared to those with nodules <1.5 cm (odds ratio 8.00; 95% confidence interval [CI] 1.92–33.37). This is even more pronounced in older patients, as the predicted probability of malignancy in patients >45 years of age with a tumor >1.5 cm reached 65% in this study.18 It should be noted that this study examined the risk of any thyroid malignancy and included 9 papillary carcinomas and 7 follicular carcinomas in addition to 10 HCCs. A similar retrospective single-institution study by Giorgadze et al reviewed 169 FNAs that were associated with a cytologic diagnosis of a HCN and reported that the overall risk of thyroid malignancy was 55% for nodules ≥2 cm compared to 45% for nodules <2 cm (P<0.0001). Malignancy risk was also higher in older patients, as 82% of nodules among patients >40 years of age represented a thyroid cancer vs 18% in those <40 years of age (P<0.0001).19 Again, the specificity of these findings for HCC is limited, as this study included 53 cases of HCC, 19 cases of papillary carcinoma, 3 follicular carcinomas, and 1 medullary carcinoma.
Examination of factors related specifically to the likelihood of HCC is limited, as many of the studies examining this question have been small, single-institution, retrospective reviews. A 1998 study by Chen et al found that nodule size is the most significant predictor of malignancy. Of 57 patients examined, 20 had HCC, and their tumors were significantly larger (4.0±0.4 cm) than those with Hürthle cell adenomas (2.4±0.2 cm).20 A summary of similar studies is presented in Table 1. Tumor size consistently has been shown to be associated with the increased probability that an HCN on FNA will prove to be a HCC. It should be noted that most of these studies were done prior to formalization of FNA cytology criteria according to the Bethesda schema, and it is unclear as to how these findings would translate into the current Bethesda lexicon. More robust data are needed before clear criteria can be defined for establishing the likelihood of HCC in the preoperative setting.
Molecular marker analysis in thyroid cytology has been studied predominantly in thyroid nodules with indeterminate cytology. The 3 commercial tests for which the most data are available in validation trials are the Afirma Gene Expression Classifier (GEC, mRNA expression of 167 genes),21 a 7-gene panel of genetic mutation and rearrangement testing,22 and a newer multiplexed next-generation sequencing (NGS) panel examining >400 known drivers of oncogenesis in thyroid cancer (ThyroSeq).9
Alexander et al employed the GEC to evaluate 265 nodules with Bethesda III, IV, and V cytology results over 19 months in a prospective, multicenter blinded study. All thyroid nodules were ≥1 cm, and all study subjects had corresponding histological specimens along with fine needle biopsies. The negative predictive value (NPV) and positive predictive value (PPV) of the GEC were 95% and 38%, respectively, for aspirates classified as AUS or FLUS (Bethesda III), and 94% and 37% for aspirates classified as follicular neoplasm/HCN or suspicious for follicular neoplasm (Bethesda IV). However, of the 21 patients with Hürthle cell adenomas in this study, 17 (81%) were classified as “suspicious” on the GEC. In this setting, use of the GEC in Hürthle cell lesions may well be associated with a relatively high false-positive result rate.21 McIver et al employed the GEC to evaluate 60 nodules that were reported to be either suspicious for follicular neoplasm/HCN or AUS/FLUS in patients who were not at high risk for malignancy (no history of head and neck irradiation, no family history of thyroid cancer, no prior history of thyroid cancer, and no worrisome imaging characteristics). There were 31 patients with cytologies suspicious for follicular neoplasm/HCN who underwent surgery; 27 of these patients had suspicious GEC results, and 4 were found to be malignant. PPV and NPV of the GEC were 15% and 75%, respectively, for aspirates classified as suspicious for follicular neoplasm/Hürthle call neoplasm.23 In another study, Brauner et al studied 72 patients with cytologies suspicious for HCN or AUS/FLUS with a predominance of Hürthle cells who underwent GEC testing between 2010 and 2014. Forty-five (63%) GEC results were classified as suspicious for malignancy; 43 (96%) of these patients underwent surgery, and 6 (14%) were found to be malignant.24 Similarly, Lastra et al found that only 15% (2 of 13) of patients with a cytology suspicious for HCN and suspicious GEC harbored a malignancy.25 Wu et al also found an increased rate of “suspicious” GEC results in nodules with Hürthle cell predominance (77.4% vs 50.5% for nodules without Hürthle cell predominance, P<0.01), but there was no difference in the rate of malignancy (25.8% vs 25.3%).26 Together, these data raise concern that there is an increased rate of suspicious GEC results in Hürthle cell lesions despite there being a relatively low risk of malignancy on surgical histology.
Nikiforov et al studied a panel of common thyroid cancer mutations, including BRAFV600E, NRAS, HRAS, and KRAS, as well as RET/PTC and Pax8/PPARγ rearrangements, in 1,056 consecutive thyroid FNA samples with Bethesda III, IV, and V cytology results and reported a PPV of 87%–95%. The NPV of the same mutational panel was 86% for Bethesda IV cytology and 94% for Bethesda III cytology.22 However, there were only 11 follicular carcinomas in the study cohort, and the published results did not specify whether any of these were oncocytic in nature and consistent with a HCC. Mutations were detected in only 5 of 11 follicular carcinoma samples (4 RAS and 1 Pax8-PPARγ), yielding a sensitivity of 45% for this histology subgroup. As RAS mutations are less common (and Pax8-PPARγ mutations nonexistent) in HCC compared to follicular carcinoma,8 it would follow that this limited mutation panel testing is less reliable in detecting HCC.
More recently, Nikiforov et al applied NGS technology to greatly expand the simultaneous testing of thyroid cancer-related genetic markers (ThyroSeq V2) in thyroid FNA samples. In a series of 143 nodules (39 malignant) with the cytologic diagnosis of follicular neoplasm/suspicious for follicular neoplasm, the test performance yielded a 90% sensitivity, 93% specificity, 96% NPV, and 83% PPV27 for detection of any thyroid malignancy based on final surgical pathology. The study included only 3 HCC cases, of which 2 had a mutation detected, and the lesion with a false-negative result was reported as a minimally invasive HCC. Therefore, use of molecular testing in the evaluation of thyroid nodules with Hürthle cell cytologic features should be understood to have limitations, as HCC has had a limited representation in most clinical studies. Molecular testing should not replace clinical judgment and consideration should be given to the pretest probability of malignancy based on clinical risk factors, ultrasound imaging, and cytologic findings in interpreting the results of any molecular marker testing of FNA specimens.
Stratification of risk
HCC is currently designated by the WHO as a histopathologic variant of follicular carcinoma, and this is echoed in the American Thyroid Association (ATA) and National Comprehensive Cancer Network (NCCN) treatment guidelines, with HCC following the same risk stratification as that of follicular carcinoma. For the ATA, intrathyroidal encapsulated tumors with minor capsular or vascular invasion (<4 foci) or ≤5 metastatic lymph nodes where the foci of metastases are <0.2 cm are considered to be low risk. Intermediate risk of recurrence is defined by vascular invasion, minimal extrathyroidal extension, or >5 metastatic lymph nodes (0.2–3 cm). High-risk patients include those with macroscopic extrathyroidal extension, incomplete tumor resection, distant metastases, or metastatic lymph nodes >3 cm.9 The NCCN guidelines for HCC (Version 1.2016) describe minimal vascular invasion as a few microscopic foci (≤4) of invasion in an intrathyroidal HCC. The NCCN guidelines assign these patients to a low-risk group.28
Surgical management of HCC
The mainstay of treatment for all differentiated thyroid cancers is surgical resection, and HCC is no exception. Indeed, due to the relatively reduced avidity of HCC for radioactive iodine (RAI) and the resultant compromised efficacy of treatment, the completeness of surgical resection is of paramount importance. Since it is impossible to make the cytologic diagnosis of HCC preoperatively, the 2 key decision points in surgical management are that of planning the initial extent of surgery and whether further surgical resection (completion thyroidectomy) is warranted once the diagnosis of HCC is histologically confirmed.
In general, thyroid surgery is approached with preference for diagnostic thyroid lobectomy unless clinical features prompt considering removal of the entire thyroid gland at the primary surgery. A personal history of craniocervical radiation does not increase the risk of HCC; however, it is an independent risk factor for developing papillary thyroid cancer, and significant exposure (particularly in childhood or adolescence) warrants upfront total thyroidectomy.9 Other clinical considerations that would prompt initial total thyroidectomy include presence of dominant contralateral nodules or preexisting diminished hormone production necessitating thyroxine replacement.9
As previously discussed, the predominant clinical feature associated with an increased risk of HCC in those patients with indeterminate Hürthle cell findings on FNA is the size of the nodule (Table 1). A nodule ≥4 cm is associated with an increased risk of HCC, and the ATA guidelines accordingly prescribe upfront total thyroidectomy.
A thorough history and physical examination are critical in the initial evaluation of patients with the potential diagnosis of HCC, since dysphagia, dyspnea, and voice change as well as the physical examination finding of a palpable hard, immobile mass are concerning for an invasive process. Preoperatively, these patients need laryngoscopy to evaluate vocal cord function and the integrity of the recurrent laryngeal nerves. Cross-sectional imaging such as computed tomography (CT) and magnetic resonance imaging (MRI) is essential in patients with a suspicion for locally advanced disease, including concern for invasion of the aerodigestive tract and/or major vessels of the neck, as it can visualize deeper anatomic compartments not readily imaged by ultrasound. In the past, clinicians were advised against CT performed with intravenous contrast, as the iodine load was felt to delay treatment with RAI; however, more recent studies have clarified adequate clearance of this iodine, with return of urinary iodine levels to baseline within 1 month for most patients with normal renal function.29
HCC is classified as a variant of follicular carcinoma; as a result, many believe that it spreads hematogenously, with rare lymph node metastasis as is the case for follicular carcinoma.30 However, several studies have documented lymph node metastasis in 5.3%–13% of HCC cases.12,13,31,32 Therefore, the finding of a HCN should prompt thorough ultrasound evaluation of all compartments of the neck prior to surgery. In a study of 39 cases of HCC, 3 (8%) were found to have lymph node metastasis at presentation (1 ipsilateral central compartment, 2 ipsilateral central and lateral neck compartments) and warranted neck dissections.31 All 3 cases of node-positive disease were associated with a primary tumor >5 cm in size. Another study examining 173 HCCs treated from 2001 to 2012 found a 9.2% incidence of node-positive disease; all cases were associated with a widely invasive or poorly differentiated phenotype.13 Seven of 173 cases (4.0%) were limited to the central compartment, while 9 (5.2%) involved the lateral compartments. The largest study to date examined 3,311 cases of HCC, with documentation of node-positive disease in 5.3%.12 Implications of this study are limited, since only 73.9% of patients had any lymph nodes pathologically examined.
Once an HCC is histologically confirmed following diagnostic thyroid lobectomy, consideration should be given to performing a completion thyroidectomy. Minimally invasive HCC is considered to be at a low risk of recurrence by both the ATA and NCCN guidelines, and is very often treated with lobectomy alone. In one of the large clinical studies examining outcome for minimally invasive HCC, all 39 cases (100%) were found to be without evidence of recurrence or disease-specific mortality, with a median follow-up of 69.8 months.13 However, most patients were indeed treated with either total thyroidectomy or lobectomy followed by completion thyroidectomy. Of 23 patients with minimally invasive HCC treated over 60 years, none experienced a recurrence or succumbed to their disease.32 The majority (88%) of patients with minimally invasive HCC were managed conservatively with thyroid lobectomy alone.
Thyroid cancers <1 cm usually do not prompt aggressive surgical treatment or completion thyroidectomy. However, it is important to note that not all sub-centimeter thyroid cancers have the same excellent prognosis. Hürthle cell histology is reported to be an independent risk factor for reduced survival even among tumors that are <1 cm. Studying 564 cases of follicular carcinomas (N=371) and HCCs (N=193) <1 cm compared to 22,174 cases of papillary thyroid microcarcinomas from the SEER database between 1988 and 2009, Kuo et al reported that follicular and Hürthle cell histologies remain independent risk factors for reduced 10-year disease-specific survival even after adjustment for patient age, type of surgery, RAI treatment, extrathyroidal extension, and nodal and distant metastases (hazard ratio 5.3, 95% CI 2.78–10.10).33
In cases of widely invasive HCC, the risk of recurrence is 73%, and therefore, they should be placed in the ATA intermediate- or high-risk categories, prompting adjuvant treatment with RAI.32 For these patients, completion thyroidectomy is mandated.
Adjuvant treatment
Thyroxine suppression
Differentiated thyroid cancer cells express the thyroid-stimulating hormone (TSH) receptor and respond to TSH by promoting cell division and tumor growth. Employing supra-physiological doses of levothyroxine can suppress TSH levels, potentially decreasing the risk of recurrence.9 The goal for the degree of TSH suppression following surgery is determined by the patient’s overall risk of recurrence, incorporating the patient’s response to therapy as well as any comorbid conditions that could be associated with an increased risk of complications with prolonged TSH suppression. Completely resected minimally invasive HCC is categorized by both the ATA and NCCN as being at low risk of recurrence, and it does not require TSH-suppressive therapy; serum TSH may be kept within the low-normal reference range (0.5–2 mU/L). In patients with structurally incomplete response to therapy, serum TSH should be kept at <0.1 mU/L indefinitely, unless there is a contraindication. In patients with an incomplete biochemical response to therapy and those with high-risk HCC and an excellent or indeterminate response to therapy, TSH should be maintained at between 0.1 and 0.5 mU/L, taking into account thyroglobulin levels, trends in those levels over time, and the risks of TSH suppression.9
Radioiodine
Overall, treatment with RAI is not routinely recommended in patients at a low risk of recurrence; it should be reserved for patients at an intermediate or high risk of recurrence. Examining the utility of RAI in minimally invasive follicular carcinoma and HCC, Goffredo et al identified 617 minimally invasive follicular carcinomas in the National Cancer Data Base (NCDB); 333 had only capsular invasion, and 284 had minor vascular invasion with or without focal capsular invasion. They found that 75% of the minimally invasive follicular carcinoma group with only capsular invasion underwent total thyroidectomy, and 52.6% received RAI; in comparison, 72.9% of the minimally invasive follicular carcinoma group with only vascular invasion underwent total thyroidectomy, and 62.1% received RAI. These findings demonstrate that US health care providers have been managing minimally invasive follicular carcinoma more aggressively than suggested by guidelines, which could be due to the absence of consensus across pathologists, experts, and guidelines regarding criteria for the diagnosis of minimally invasive follicular carcinoma.34
There is controversy with regard to RAI avidity of HCC and the effect of RAI treatment on survival of patients with HCC. Most studies that have evaluated the efficacy of RAI for HCC are small, retrospective, single-institutional series, likely due to the rarity of HCC.
In an old study, Lopez-Penabad et al reviewed 127 patients with HCN (89 patients with HCC and 38 patients with Hürthle cell adenoma) who were treated from 1944 to 1955 and reported 38% RAI avidity in patients with HCC. This study showed that RAI therapy is associated with survival benefit in patients with HCC when it is used primarily for ablation rather than when residual disease is believed to be present.35 In a retrospective single-institution study, Besic et al reviewed 16 patients with HCC and distant metastases; all these patients underwent thyroid hormone withdrawal. Scanning demonstrated uptake (range 0.1%–12%) in 11 of 16 patients (69%).36 In another study, Besic et al evaluated 30 HCC patients with distant metastases and reported RAI uptake in metastases in 16 of 30 patients (53%), with uptake of ≥0.5% in 9 of 30 patients (30%).37 At a nationwide level, Jillard et al identified 1,909 patients with HCC who underwent total thyroidectomy in the NCDB between 1998 and 2006. Patients were included if they had AJCC-TNM pT1 tumors with N1or M1 disease, and pT2–4 tumors with any N or M disease status. This study showed an association between use of adjuvant RAI and improved survival among patients with HCC. Five- and 10-year survival for patients with tumors measuring 2–4 cm who received RAI were improved compared with those who did not receive RAI (97.8% and 87.8% vs 94.1% and 71.9%, respectively; P<0.001). Five- and 10-year survival were also improved for patients with tumors >4 cm who received RAI compared to those who did not receive RAI (82.3% and 77.6% vs 62.3% and 56.9%, respectively; P<0.001). Therefore, the authors concluded that RAI is indicated in HCC patients who have tumors >2 cm and and/or nodal and distant metastases (Figure 6).38
  | Figure 6 Overall survival according to RAI use in patients with Hürthle cell carcinoma in the National Cancer Data Base, 1998–2006. Copyright © 2016. Adapted from Jillard Christa L, Youngwirth Linda, Scheri Randall P, Roman Sanziana, and Sosa Julie A. Thyroid. 2016;26(7):959–964.38 |
Cervical radiation
External beam radiation therapy (EBRT) is another treatment option in patients with clinically evident gross extrathyroidal extension that is incompletely resected. However, it is important to balance the potential benefit of radiation with potential complications, including dental decay, tracheal stenosis, esophageal stricture, osteonecrosis, fibrosis, and xerostomia.39 Intensity-modulated radiation therapy (IMRT) uses 3-dimensional (3D) CT to map the tumor and also computerizes dose calculations. It allows higher radiation doses to be delivered to the tumor and minimizes the dose received by surrounding tissues.40 In a study using the NCDB, 106,374 patients with differentiated thyroid cancer were evaluated; they included patients >18 years of age, without distant metastases, who underwent cervical radiation therapy to assess the impact of IMRT vs 3D-conformal radiotherapy (3D-CT). Although not statistically significant, comparison of overall survival between patients undergoing IMRT vs 3D-CT demonstrated a hazard ratio of 0.67 (95% CI 0.4–1.1; P=0.115) and trend toward improved overall survival with IMRT.41
The role of radiation as an adjuvant therapy in patients who have had complete resection of all locally invasive disease is controversial.42–46 In the absence of persistent gross residual disease, radiation is usually reserved for older patients (>50 years) with tumors that are more likely to be iodine-refractory (including HCC) and who are at high risk of early cervical recurrence not amenable to surgical resection. Radiation is seldom recommended as an adjuvant therapy in young patients, and especially those with iodine-avid tumors.
Directed therapy for distant metastasis
Targeted therapies in patients with advanced thyroid cancer are valuable tools to address malignant foci that arise in critical locations and that are at high risk of causing significant morbidity or mortality secondary to tumor infiltration or compression of vital structures if they are not treated expeditiously.9 Such critical locations include the brain, spine, and bone (at risk of pathological fracture). Cervical and mediastinal disease places key airway structures (eg, trachea, larynx, major bronchi), as well as the digestive tract and great vessels, at risk. These lesions may require treatment prior to RAI therapy to prevent morbidity related to swelling that can occur in some of these metastatic foci. The choice of specific targeted therapy is dependent on tumor size and location, patient preference, and discussion between all members of the transdisciplinary disease management team. Frequently, this communication is best accomplished in a multidisciplinary tumor board setting.
The risk of bone metastases is likely increased in HCC patients. Bone metastases in patients with differentiated thyroid cancer including HCC are associated with significant morbidity, including pain and pathologic fracture.47 Surgery is the preferred treatment option for bone metastases that are associated with structural instability or that are in critical locations. A retrospective study of 109 patients with bone metastases from thyroid cancer (71% with follicular carcinoma) reported that complete resection of bone metastases in young patients is associated with a significant improvement in survival.48 Radiotherapy plays an important role in treatment of bone lesions, since it can be used in isolation or to complement surgery in cases of incomplete resection.9 In a retrospective study of 32 patients with metastatic HCC, Besic et al reported that the effect of EBRT on bone metastases lasted from 13 to 165 months (median, 93 months).37 Another treatment option is embolization of tumor vasculature; this can be used for pain control, and it can also be used in a neoadjuvant fashion prior to surgical resection, as these bony metastases tend to be vascular and prone to intraoperative bleeding. Radiofrequency ablation has also been reported to reduce pain from thyroid cancer skeletal metastases.48–51
Localized therapies can also be effective treatment options for HCC that involves the respiratory tract and airways. Options include bronchoscopy with laser therapy for intraluminal disease, endo-bronchial stenting to maintain patency of airways that are compressed or the subject of local invasion, surgical resection of the metastatic lesion, and focused EBRT.
Overall, brain metastases are uncommon; they are usually found in the setting of widespread metastatic disease and are associated with a very poor overall prognosis. High-dose glucocorticoids are usually recommended to decrease the surrounding edema once brain metastases are identified. Surgical resection is the preferred treatment option, unless the lesions are very small and/or mutifocal. Stereotactic radiation can be effective if <3–5 small lesions are present, and whole-brain radiation is required when there are numerous lesions.52–56
Systemic therapy
Some patients with widely metastatic HCC can survive for many years with only minimal disease progression, and they can remain relatively asymptomatic; thus, it is important to identify which patients will likely benefit from a wait-and-watch approach vs systemic therapy. Factors related to the tumor, patient comorbidities, patients’ tolerance of treatment-related morbidity and its impact on quality of life, and patient preference, all should be incorporated into these complex management decisions. It is unusual to initiate systemic therapy for lesions that are <1 cm; however, not all metastatic lesions >1 cm require systemic therapy. Systemic therapy should be initiated sooner if metastatic lesions are in locations where continued growth would result in compromise of critical surrounding structures. Systemic therapy should be likely considered in the following cases: when patients have symptoms of weight loss, muscle wasting, or fatigue, or other constitutional symptoms attributable to their significant disease burden; when the rate of progression of structural disease is rapid enough that the disease is likely to cause morbidity; or when additional metastatic foci are identified.
Currently, sorafenib and lenvatinib are the only US Food and Drug Administration-approved multi-kinase inhibitors available for RAI-refractory differentiated thyroid cancer. Due to the relatively small number of such cases, there are no published studies examining outcomes of systemic therapy specifically for HCC. However, most published studies do report the response rate of HCC to treatment. A prospective randomized trial of lenvatinib vs placebo in the treatment of RAI-refractory thyroid cancer included 70 HCC cases (lenvatinib N=48, placebo N=22). Lenvatinib demonstrated a progression-free survival hazard ratio of 0.22 (95% CI 0.10–0.51) in favor of treatment when compared to placebo.57 The DECISION trial was a multicenter, randomized, double-blind, placebo-controlled Phase III trial of sorafenib in 417 RAI-refractory thyroid cancers; it included 74 HCC cases. The precise hazard ratio and CI for HCC were not published as part of the DECISION trial; however, sorafenib was reported to be associated with overall progression-free survival advantage among the subset of patients with HCC.58
Vandetanib has also been studied in a European Phase II trial in RAI-refractory differentiated thyroid cancer. Unfortunately, the study included only 2 patients categorized as having follicular carcinoma with oncocytic features, and both were randomized to the placebo control group.59 A multicenter, open-label, single-arm, Phase II study of axitinib in 52 advanced thyroid cancers included 8 HCCs, of which 2 demonstrated a partial response to treatment.60 There are currently several studies with active recruitment exploring the role of small-molecule inhibitors for iodine-refractory differentiated thyroid cancer, including HCC. Based on Category 2A evidence, the NCCN guidelines recommend that axitinib, everolimus, pazopanib, and sunitinib be considered in patients with advanced HCC if clinical trials are not available or appropriate.61 In general, these small-molecule inhibitors can sometimes provide progression-free survival advantages with a side-effect profile that is tolerable in properly selected patients. However, they have not been shown to improve either disease-specific or overall survival, and specific drug activity in HCC is unlikely to be known due to the very small number of HCC cases included in most trials.
Prognosis and follow-up
HCC is associated with more aggressive clinical behavior compared to other differentiated thyroid cancers; for example, it is more often seen with a higher rate of distant metastases.12,33,35,62,63 In a retrospective study of 32 patients with metastatic HCC, Besic et al reported estimated 5- and 10-year disease-specific survival rates of 81% and 60%, respectively.37
During initial follow-up of patients with HCC, serum thyroglobulin/thyroglobulin antibody (Tg/Tg Ab) levels on levothyroxine therapy should be measured every 6–12 months. In the ATA high-risk patients, more frequent measurements may be appropriate. Time intervals between serum Tg/Tg Ab measurement in the ATA low- to intermediate-risk HCC patients can be lengthened to 12–24 months. The ATA high-risk HCC patients and all patients with biochemically or structurally incomplete or indeterminate response to therapy should have Tg/Tg Ab levels measured every 6–12 months for several years.9
Cervical ultrasound to evaluate the thyroid bed, central, and lateral cervical nodal compartments should be performed every 6–12 months in patients with HCC initially following surgery, and then periodically depending on each patient’s risk of recurrence and unique response to therapy.9 RAI scanning has a high false-negative rate in patients with HCC due to the lack of iodine avidity in a majority of patients; therefore, it is not recommended as the principle imaging modality for surveillance.13 The ATA guidelines recommend that fluorine-18-fluorodeoxyglucose positron emission tomography (FDG PET) be considered in patients with high-risk differentiated thyroid cancers, and those cancers with elevated Tg levels and negative RAI scans. FDG PET may be considered as part of initial staging in patients with invasive HCC.9 Multiple studies have shown a high sensitivity of FDG PET for HCC. In a retrospective single-institution study of 44 patients with HCC, Pryma et al reported a sensitivity of 95.8% and specificity of 95% for FDG PET scans in patients with HCC.64 In a study of 12 patients with HCC, Lowe et al found that FDG PET had a sensitivity of 92%; they also noticed that half of the PET scans detected tumors not seen on conventional imaging, which resulted in a change in disease staging and management.65 In a retrospective study of 17 HCC patients, Plotkin et al reported a sensitivity of 92%, specificity of 80%, PPV of 92%, and NPV of 80% for FDG PET/CT for identification of HCC.66
Cross-sectional imaging including CT and MRI of the neck and chest should be considered in the setting of bulky recurrent cervical disease, when potential aerodigestive tract invasion requires complete assessment, and in high-risk HCC patients with concern for lung metastases. Cross-sectional imaging of other organs including the brain, skeleton, and abdomen should be considered in high-risk HCC patients with elevated serum Tg and negative neck and chest imaging, and patients who have symptoms referable to those organs.9
Conclusion
There remains significant controversy with regard to optimal management of patients with HCC; it is more pronounced than the management of other forms of differentiated thyroid cancer, likely due to the relative rarity of the disease and scant high-quality evidence. Large multi-institutional studies are needed; until that time, utilization of national and nationwide data to assemble an adequate number of cases to inform valid conclusions will need to serve as a surrogate. Important questions that are outstanding include identification of the effect of minimal vascular invasion (1–4 foci) on the risk of recurrence and survival among patients with HCC, as well as measurement of the efficacy of adjuvant RAI in these patients. There also continues to be a need for consensus regarding the criteria needed to establish a diagnosis of minimally invasive HCC in order to avoid potential overtreatment.
Disclosure
JAS is a member of the Data Monitoring Committee of the Medullary Thyroid Cancer Consortium Registry, sponsored by NovoNordisk, GlaxoSmithKline, Astra Zeneca, and Eli Lilly. The authors report no other conflicts of interest in this work.
References
Hürthle, K. Beiträge zur Kenntniss des Secretionsvorgangs in der Schilddrüse [Contributions to the knowledge of the secretion process in the thyroid]. Pflüger Arch. 1894;56(1):1–44. doi:10.1007/BF01662011. | ||
Montone KT, Baloch ZW, LiVolsi VA. The thyroid Hurthle (oncocytic) cell and its associated pathologic conditions: a surgical pathology and cytopathology review. Arch Pathol Lab Med. 2008;132(8):1241–1250. | ||
Gross M, Eliashar R, Ben-Yaakov A, Weinberger JM, Maly B. Clinicopathologic features and outcome of the oncocytic variant of papillary thyroid carcinoma. Ann Otol Rhinol Laryngol. 2009;118(5):374–381. | ||
Berho M, Suster S. The oncocytic variant of papillary carcinoma of the thyroid: a clinicopathologic study of 15 cases. Hum Pathol. 1997;28(1):47–53. | ||
Hong JH, Yi HS, Yi S, Kim HW, Lee J, Kim KS. Implications of oncocytic change in papillary thyroid cancer. Clin Endocrinol (Oxf). Epub 2016 May 28. | ||
Hundahl SA, Fleming ID, Fremgen AM, Menck HR. A National Cancer Data Base report on 53,856 cases of thyroid carcinoma treated in the U.S., 1985–1995 [see comments]. Cancer. 1998;83(12):2638–2648. | ||
DeLellis RA, Lloyd RV, Heitz PU, Eng C, editors. Pathology and Genetics of Tumors of the Endocrine Organs. Lyon: IARC Press; 2004. | ||
Ganly I, Ricarte Filho J, Eng S, et al. Genomic dissection of Hurthle cell carcinoma reveals a unique class of thyroid malignancy. J Clin Endocrinol Metab. 2013;98(5):E962–E972. | ||
Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1–133. | ||
Xu B, Wang L, Tuttle RM, Ganly I, Ghossein R. Prognostic impact of extent of vascular invasion in low-grade encapsulated follicular cell-derived thyroid carcinomas: a clinicopathologic study of 276 cases. Hum Pathol. 2015;46(12):1789–1798. | ||
Ghossein RA, Hiltzik DH, Carlson DL, et al. Prognostic factors of recurrence in encapsulated Hurthle cell carcinoma of the thyroid gland: a clinicopathologic study of 50 cases. Cancer. 2006;106(8): 1669–1676. | ||
Goffredo P, Roman SA, Sosa JA. Hurthle cell carcinoma: a population-level analysis of 3311 patients. Cancer. 2013;119(3):504–511. | ||
Chindris AM, Casler JD, Bernet VJ, et al. Clinical and molecular features of Hurthle cell carcinoma of the thyroid. J Clin Endocrinol Metab. 2015;100(1):55–62. | ||
Maizlin ZV, Wiseman SM, Vora P, et al. Hurthle cell neoplasms of the thyroid: sonographic appearance and histologic characteristics. J Ultrasound Med. 2008;27(5):751–757; quiz 759. | ||
Cibas ES, Ali SZ. The Bethesda System for Reporting Thyroid Cytopathology. Thyroid. 2009;19(11):1159–1165. | ||
Bernstein JM, Shah M, MacMillan C, Freeman JL. Institution-specific risk of papillary thyroid carcinoma in atypia/follicular lesion of undetermined significance. Head Neck. 2016;38 Suppl 1:E1210–E1215. | ||
Wang CC, Friedman L, Kennedy GC, et al. A large multicenter correlation study of thyroid nodule cytopathology and histopathology. Thyroid. 2011;21(3):243–251. | ||
Kim TH, Lim JA, Ahn HY, et al. Tumor size and age predict the risk of malignancy in Hurthle cell neoplasm of the thyroid and can therefore guide the extent of initial thyroid surgery. Thyroid. 2010;20(11):1229–1234. | ||
Giorgadze T, Rossi ED, Fadda G, Gupta PK, Livolsi VA, Baloch Z. Does the fine-needle aspiration diagnosis of “Hurthle-cell neoplasm/follicular neoplasm with oncocytic features” denote increased risk of malignancy? Diagn Cytopathol. 2004;31(5):307–312. | ||
Chen H, Nicol TL, Zeiger MA, et al. Hurthle cell neoplasms of the thyroid: are there factors predictive of malignancy? Ann Surg. 1998;227(4):542–546. | ||
Alexander EK, Kennedy GC, Baloch ZW, et al. Preoperative diagnosis of benign thyroid nodules with indeterminate cytology. N Engl J Med. 2012;367(8):705–715. | ||
Nikiforov YE, Ohori NP, Hodak SP, et al. Impact of mutational testing on the diagnosis and management of patients with cytologically indeterminate thyroid nodules: a prospective analysis of 1056 FNA samples. J Clin Endocrinol Metab. 2011;96(11):3390–3397. | ||
McIver B, Castro MR, Morris JC, et al. An independent study of a gene expression classifier (Afirma) in the evaluation of cytologically indeterminate thyroid nodules. J Clin Endocrinol Metab. 2014;99(11): 4069–4077. | ||
Brauner E, Holmes BJ, Krane JF, et al. Performance of the Afirma gene expression classifier in Hurthle cell thyroid nodules differs from other indeterminate thyroid nodules. Thyroid. 2015;25(7):789–796. | ||
Lastra RR, Pramick MR, Crammer CJ, LiVolsi VA, Baloch ZW. Implications of a suspicious afirma test result in thyroid fine-needle aspiration cytology: an institutional experience. Cancer Cytopathol. 2014;122(10):737–744. | ||
Wu JX, Young S, Hung ML, et al. Clinical factors influencing the performance of gene expression classifier testing in indeterminate thyroid nodules. Thyroid. 2016;26(7):916–922. | ||
Nikiforov YE, Carty SE, Chiosea SI, et al. Highly accurate diagnosis of cancer in thyroid nodules with follicular neoplasm/suspicious for a follicular neoplasm cytology by ThyroSeq v2 next-generation sequencing assay. Cancer. 2014;120(23):3627–3634. | ||
National Comprehensive Cancer Network. Available from: http://www.nccn.org/professionals/physician_gls/pdf/thyroid.pdf . Accessed September 29, 2016. | ||
Padovani RP, Kasamatsu TS, Nakabashi CC, et al. One month is sufficient for urinary iodine to return to its baseline value after the use of water-soluble iodinated contrast agents in post-thyroidectomy patients requiring radioiodine therapy. Thyroid. 2012;22(9):926–930. | ||
Sugino K, Kameyama K, Ito K, et al. Does Hurthle cell carcinoma of the thyroid have a poorer prognosis than ordinary follicular thyroid carcinoma? Ann Surg Oncol. 2013;20(9):2944–2950. | ||
Guerrero MA, Suh I, Vriens MR, et al. Age and tumor size predicts lymph node involvement in Hurthle cell carcinoma. J Cancer. 2010;1: 23–26. | ||
Stojadinovic A, Hoos A, Ghossein RA, et al. Hurthle cell carcinoma: a 60-year experience. Ann Surg Oncol. 2002;9(2):197–203. | ||
Kuo EJ, Roman SA, Sosa JA. Patients with follicular and Hurthle cell microcarcinomas have compromised survival: a population level study of 22,738 patients. Surgery. 2013;154(6):1246–1253; discussion 1253–1254. | ||
Goffredo P, Jillard C, Thomas S, Scheri RP, Sosa JA, Roman S. Minimally invasive follicular carcinoma: predictors of vascular invasion and impact on patterns of care. Endocrine. 2016;51(1):123–130. | ||
Lopez-Penabad L, Chiu AC, Hoff AO, et al. Prognostic factors in patients with Hurthle cell neoplasms of the thyroid. Cancer. 2003;97(5):1186–1194. | ||
Besic N, Vidergar-Kralj B, Frkovic-Grazio S, Movrin-Stanovnik T, Auersperg M. The role of radioactive iodine in the treatment of Hurthle cell carcinoma of the thyroid. Thyroid. 2003;13(6):577–584. | ||
Besic N, Schwarzbartl-Pevec A, Vidergar-Kralj B, Crnic T, Gazic B, Marolt Music M. Treatment and outcome of 32 patients with distant metastases of Hurthle cell thyroid carcinoma: a single-institution experience. BMC Cancer. 2016;16:162. | ||
Jillard CL, Youngwirth L, Scheri RP, Roman S, Sosa JA. Radioactive iodine treatment is associated with improved survival for patients with Hurthle cell carcinoma. Thyroid. 2016;26(7):959–964. | ||
Schlumberger M, Challeton C, De Vathaire F, et al. Radioactive iodine treatment and external radiotherapy for lung and bone metastases from thyroid carcinoma. J Nucl Med. 1996;37(4):598–605. | ||
Lee N, Puri DR, Blanco AI, Chao KS. Intensity-modulated radiation therapy in head and neck cancers: an update. Head Neck. 2007;29(4):387–400. | ||
Goffredo P, Robinson TJ, Youngwirth LM, Roman SA, Sosa JA. Intensity-modulated radiation therapy use for the localized treatment of thyroid cancer: nationwide practice patterns and outcomes. Endocrine. 2016;53(3):761–773. | ||
Farahati J, Reiners C, Stuschke M, et al. Differentiated thyroid cancer. Impact of adjuvant external radiotherapy in patients with perithyroidal tumor infiltration (stage pT4). Cancer. 1996;77(1):172–180. | ||
O’Connell ME, A’Hern RP, Harmer CL. Results of external beam radiotherapy in differentiated thyroid carcinoma: a retrospective study from the Royal Marsden Hospital. Eur J Cancer. 1994;30A(6):733–739. | ||
Phlips P, Hanzen C, Andry G, Van Houtte P, Früuling J. Postoperative irradiation for thyroid cancer. Eur J Surg Oncol. 1993;19(5):399–404. | ||
Kim TH, Yang DS, Jung KY, Kim CY, Choi MS. Value of external irradiation for locally advanced papillary thyroid cancer. Int J Radiat Oncol Biol Phys. 2003;55(4):1006–1012. | ||
Keum KC, Suh YG, Koom WS, et al. The role of postoperative external-beam radiotherapy in the management of patients with papillary thyroid cancer invading the trachea. Int J Radiat Oncol Biol Phys. 2006;65(2):474–480. | ||
Farooki A, Leung V, Tala H, Tuttle RM. Skeletal-related events due to bone metastases from differentiated thyroid cancer. J Clin Endocrinol Metab. 2012;97(7):2433–2439. | ||
Bernier MO, Leenhardt L, Hoang C, et al. Survival and therapeutic modalities in patients with bone metastases of differentiated thyroid carcinomas. J Clin Endocrinol Metab. 2001;86(4):1568–1573. | ||
Sandri A, Carbognin G, Regis D, et al. Combined radiofrequency and kyphoplasty in painful osteolytic metastases to vertebral bodies. Radiol Med. 2010;115(2):261–271. | ||
Toyota N, Naito A, Kakizawa H, et al. Radiofrequency ablation therapy combined with cementoplasty for painful bone metastases: initial experience. Cardiovasc Intervent Radiol. 2005;28(5):578–583. | ||
Monchik JM, Donatini G, Iannuccilli J, et al. Radiofrequency ablation and percutaneous ethanol injection treatment for recurrent local and distant well-differentiated thyroid carcinoma. Ann Surg. 2006;244(2):296–304. | ||
Henriques de Figueiredo B, Godbert Y, Soubeyran I, et al. Brain metastases from thyroid carcinoma: a retrospective study of 21 patients. Thyroid. 2014;24(2):270–276. | ||
Chiu AC, Delpassand ES, Sherman SI. Prognosis and treatment of brain metastases in thyroid carcinoma. J Clin Endocrinol Metab. 1997; 82(11):3637–3642. | ||
McWilliams RR, Giannini C, Hay ID, Atkinson JL, Stafford SL, Buckner JC. Management of brain metastases from thyroid carcinoma: a study of 16 pathologically confirmed cases over 25 years. Cancer. 2003;98(2):356–362. | ||
Bernad DM, Sperduto PW, Souhami L, Jensen AW, Roberge D. Stereotactic radiosurgery in the management of brain metastases from primary thyroid cancers. J Neurooncol. 2010;98(2):249–252. | ||
Kim IY, Kondziolka D, Niranjan A, Flickinger JC, Lunsford LD. Gamma knife radiosurgery for metastatic brain tumors from thyroid cancer. J Neurooncol. 2009;93(3):355–359. | ||
Schlumberger M, Tahara M, Wirth LJ, et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med. 2015;372(7): 621–630. | ||
Brose MS, Nutting CM, Jarzab B, et al. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet. 2014; 384(9940):319–328. | ||
Leboulleux S, Bastholt L, Krause T, et al. Vandetanib in locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 2 trial. Lancet Oncol. 2012;13(9):897–905. | ||
Locati LD, Licitra L, Agate L, et al. Treatment of advanced thyroid cancer with axitinib: Phase 2 study with pharmacokinetic/pharmacodynamic and quality-of-life assessments. Cancer. 2014;120(17):2694–2703. | ||
NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Thyroid Carcinoma, Version 2.2015. 2015. | ||
Shaha AR, Shah JP, Loree TR. Patterns of nodal and distant metastasis based on histologic varieties in differentiated carcinoma of the thyroid. Am J Surg. 1996;172(6):692–694. | ||
Ruegemer JJ, Hay ID, Bergstralh EJ, Ryan JJ, Offord KP, Gorman CA. Distant metastases in differentiated thyroid carcinoma: a multivariate analysis of prognostic variables. J Clin Endocrinol Metab. 1988;67(3):501–508. | ||
Pryma DA, Schöder H, Gönen M, Robbins RJ, Larson SM, Yeung HW. Diagnostic accuracy and prognostic value of 18F-FDG PET in Hurthle cell thyroid cancer patients. J Nucl Med. 2006;47(8):1260–1266. | ||
Lowe VJ, Mullan BP, Hay ID, McIver B, Kasperbauer JL. 18F-FDG PET of patients with Hurthle cell carcinoma. J Nucl Med. 2003;44(9):1402–1406. | ||
Plotkin M, Hautzel H, Krause BJ, et al. Implication of 2-18fluor-2-deoxyglucose positron emission tomography in the follow-up of Hurthle cell thyroid cancer. Thyroid. 2002;12(2):155–161. | ||
Zhang YW, Greenblatt DY, Repplinger D, et al. Older age and larger tumor size predict malignancy in Hurthle cell neoplasms of the thyroid. Ann Surg Oncol. 2008;15(10):2842–2846. | ||
Sippel RS, Elaraj DM, Khanafshar E, et al. Tumor size predicts malignant potential in Hurthle cell neoplasms of the thyroid. World J Surg. 2008;32(5):702–707. | ||
Pisanu A, Di Chiara B, Reccia I, Uccheddu A. Oncocytic cell tumors of the thyroid: factors predicting malignancy and influencing prognosis, treatment decisions, and outcomes. World J Surg. 2010; 34(4):836–843. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.