Back to Journals » Cancer Management and Research » Volume 14
Human Papillomavirus-Associated Head and Neck Cancers. Where are We Now? A Systematic Review
Authors Pinkiewicz M , Dorobisz K , Zatoński T
Received 19 June 2022
Accepted for publication 19 October 2022
Published 25 November 2022 Volume 2022:14 Pages 3313—3324
DOI https://doi.org/10.2147/CMAR.S379173
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Sanjeev K. Srivastava
Miłosz Pinkiewicz, Karolina Dorobisz, Tomasz Zatoński
Department of Otolaryngology, Head and Neck Surgery, Wroclaw Medical University, Wroclaw, Poland
Correspondence: Karolina Dorobisz, Department of Otolaryngology, Head and Neck Surgery, Wroclaw Medical University, Borowska 213, Wroclaw, 50-529, Poland, Email [email protected]
Background: Human papillomavirus targets the skin and mucous membranes, producing benign hyperplastic lesions and precancerous and cancerous lesions. An increasing number of head and neck cancersin particular, oropharyngeal squamous cell carcinoma, laryngeal squamous cell carcinoma, and oral squamous cell carcinoma, are attributable to HPV infection. HPV-induced HNCs typically affect younger, nonsmoking patients with no prior history of heavy alcohol use, more extensive sexual history, and higher socioeconomic status.
Aim: The purpose of the review is to present the most recent and well-established findings concerning HPV-induced head and neck cancers and consequently to provide medical specialists with essential information regarding the epidemiology, the role of HPV in HNC cancerogenesis, prevention, diagnosis, and treatment.
Material and Methods: All authors independently have searched The EMbase, Medline/Pubmed, and Cochrane databases by using the following keywords “head and neck cancer”, “human papillomavirus”, “HPV”, “HPV biology”, “oropharyngeal squamous cell carcinoma”, “carcinogenesis”, “transoral surgery”, “robotic surgery”. The last search was conducted in March 2022. The references of the publications of interest were also screened for relevant papers. There were no limitations in regard to the publication date.
Conclusion: Aiming to avoid the epidemic of HPV-induced HNC, it is paramount to improve the access to vaccination as well as resolve parental concerns regarding vaccine safety. Physicians should rely on reduced-dose radiation and aim to reduce the overall treatment time. Thanks to a more elaborate understanding of the genomic background of HPV-induced HNC, precision medicine could become a relevant part of patients’ management. In comparison to traditional techniques and non-operative treatment, transoral robotic surgery (TORS) offers similar oncologic and functional outcomes, with a possible benefit on long-term quality of life. However, more research is needed to establish clear guidelines indicating when TORS resections should be supported with adjuvant therapy.
Keywords: head and neck cancer, human papillomavirus, HPV, HPV biology, oropharyngeal squamous cell carcinoma, carcinogenesis, transoral surgery, robotic surgery
Introduction
In the past, studies focused on the role of human papillomaviruses (HPV) in the pathology of the genital tract, describing high-risk types that lead to the development of cervical cancer and other anogenital cancers, with low-risk types causing condylomas. However, after 1983 when Syrjänen first described the potential association between HPV infection and head and neck cancer (HNC), numerous studies evaluating HPV as an etiologic agent followed.1 Roughly 5% of HNC is linked to alcohol consumption, 34% to tobacco use, whereas 36% of cases are associated with the combined use of alcohol with tobacco.2,3 The remaining 25% of HNC is attributable to human papillomavirus infection (HPVs).2,4 Historically, estimating the true prevalence of HPV-induced HNC was hampered by insufficient details on anatomical tumor localization and distinct methods of HPV detection. However, a more elaborate understanding of the molecular landscape of HPV-induced HNC has improved the detection. Thanks to the reliable data on epidemiology, as well as the mounting evidence of the role of HPV in HNC cancerogenesis, the International Agency for Research on Cancer (IARC) has acknowledged HPV as another carcinogen for head and neck anatomical subsitesin particular, oropharyngeal cancer.5
During the last decade, the medical community has been witnessing a growing trend of HPV-induced HNC, with studies reporting a 36.5% increase in the incidence worldwide.6,7 The potential epidemics and the growing awareness of the role of HPV in the HNC pathogenesis resulted in a concerted effort to improve vaccination access and establish an effective treatment regimen for these clinically and molecularly distinct cancers.
In this review, we focus solely on HPV-associated head and neck cancers. Although Head and neck squamous cell carcinomas are routinely categorized as HPV-negative (HPV–ve) and HPV-positive, this classification is mostly applicable to oropharyngeal cancer.5 We provide an overview of emerging findings with respect to epidemiology, the role of HPV in HNC pathogenesis, prevention, diagnosis, and treatment to elucidate gaps in knowledge and improve patient management.
Methodological Approach
A systematic literature review was carried out to review all available relevant data. During the article selection process, the authors followed the recommendations made by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). All authors independently have searched The EMbase, The Medline/Pubmed, and Cochrane databases by using the following keywords “head and neck cancer”, “human papillomavirus”, “HPV”, “HPV biology”, “oropharyngeal squamous cell carcinoma”, “carcinogenesis”, “transoral surgery”, “robotic surgery”. The last search was conducted in March 2022. The references of the publications of interest were also screened for relevant papers. We have excluded single case reports. Only papers written in English have been considered. All of the selected articles were read in full text. Non-peer-reviewed papers and records not available in the full text have not been included. Also, studies were excluded if there was incomplete or missing data. The eligibility and quality of publications have been independently evaluated by three reviewers. Each author extracted data independently using a pre-established data extraction form. Data were extracted on the following items: first author, publication year, journal of publication, study country, study population, case selection methods, study period, mean age of study population, sex distribution, tobacco smoking (ever/never), and alcohol consumption (ever/never). We have chosen articles for inclusion on the grounds of study quality and design. The initial search resulted in 1690 research articles. The judgments concerning the risk of bias were formed by a single reviewer and subsequently double-checked by another two reviewers. The flow Diagram represents our process of article selection (Figure 1).
 |
Figure 1 The flow Diagram represents our process of article selection. Note: adapted from |
Results
Data Set
A total of 1690 articles were retrieved from The EMbase (n = 530), Medline/Pubmed (n = 1158), and Cochrane databases (n = 2). After screening the reference lists of the included papers, 9 more publications were added to the search results, resulting in a total of 1699 articles. Screening for duplicates and their removal resulted in a total of 1102 articles. We have excluded 419 papers due to language and study design. Subsequently, we screened titles and abstracts, excluding 467 articles. A total of 216 full-text articles were screened. After the exclusion of 147 articles, we have included 69 articles for our systematic review.
Epidemiology
Worldwide, approximately 4.5% of all cancers diagnosed are attributable to HPV infection.8 Given the multifactorial etiology of HNCs, the quantitative evaluation of the etiological involvement of HPVs in HNCs was a considerable challenge, with past studies and meta-analyses evaluating the quantitative role of HPV in HNCs using the presence and detection of HPV-DNA in the tumor as the sole criterion to categorize the tumor as HPV-driven. This has most probably led to an overestimation of the real contribution of HPV to head and neck carcinogenesis.9 Given that the sole presence of HPV DNA in HNCs is not tantamount to its involvement in cancerogenesis, it is recommended to check the presence of E6 and E7 mRNA to precisely assess the biological and oncogenic involvement of HPVs in HNC, as well as to establish the true prevalence of HPV-related HNC.9
According to the International Agency for Research on Cancer, in the head and neck, HPV-attributable cancers represent 38,000 cases of which 21,000 are oropharyngeal cancers reported in more developed countries.8 A 2016 study that involved samples from 29 countries found that the proportion attributable to HPV by subsite was 18% for oropharyngeal cancers, 3% for oral cavity, and 1% for laryngeal cancers.9 The highest HPV prevalence was reported in the oropharynx (24.9%), followed by pharynx unspecified (21.4%), nasopharynx (7.9%), oral cavity (7.4%), larynx (5.7%), and hypopharynx (3.9%).9 South and Central America, as well as northern Europe, demonstrated the highest HPV attributable fractions of both oral and laryngeal cancers.9
A more recent study involving HNC cases mainly from Europe and South America attributed 22.4% oropharynx, 4.4% oral cavity cancers, and 3.5% laryngeal cancers to HPV.10 A large epidemiologic study assessing the USA population has found that the incidence of HPV-attributable oropharyngeal cancer varies by sex, age, and race.11 Within 2015 alone, there was a more significant increase in the incidence of oropharyngeal squamous cell carcinoma (OPSCC) attributable to HPV in White men of all ages (18 per 100,000 people) in comparison to Hispanic men (6 per 100,000 people) and Black men (4 per 100,000 people).11 The incidence in white women was almost double the incidence reported in Hispanic or Black women.11 Another study has found that the US incidence of HPV-positive OPSCC was 4.62 per 100,000 persons—250% of the incidence rate of HPV-negative OPSCC.12 On the global scale, HPV attributable fractions of OPSCC rose from 7.2% in the period 1990–1994 to 32.7% from 2010 to 2012.13 Aiming to evaluate the association of HPV vaccination with future OPC incidence in the US, Zhang et al project a decrease in OPC incidence in younger individuals (36–45 years of age) through 2045 and a continued increase among older individuals (70–83 years of age).14 During the coming decades, we will experience an increase in HPV-induced oropharyngeal cancer incidence, with a shift toward an older population.14 Given the significant influence on the quality of life of patients and the burden on healthcare, the incoming epidemic will constitute a formidable challenge for healthcare workers.
As much as literature is abundant with epidemiological data from Europe and North and South America, there is an evident need for precise data from Asia pacific and Africa. According to a recent review and meta-analysis, HPV16 was the most common type detected in all the HPV DNA-positive cases from the Asia Pacific, accounting for approximately 90% overall, with HPV18 being the second most common oncogenic type detected.8 In South Asia, the HPV prevalence in oral cavity squamous cell carcinoma is equal to 48.61%. In East Asia, it is 42.84%, with Australia reporting a prevalence of only 5.6%.8 With respect to oropharyngeal cancer, studies show an HPV prevalence of 49.32%, with 38.70% reported for East Asia.8 Although South Asia has the lowest HPV prevalence in oropharyngeal cancer, it has the highest HPV prevalence in laryngeal cancer (48.05%).8 Oceania reports a prevalence of HPV-associated laryngeal cancer of 6%.8 There is no data on HPV-associated oropharyngeal or laryngeal cancer from Southeast Asia.8 In regard to Africa, according to a retrospective cross-sectional study in Ghana, HPV was prevalent in 50% of oropharyngeal cancers, 27% of laryngeal cancers, and 23% of oral cavity cancers.15 HPV E6/E7 oncogenic DNA was found in 18% of the HNC cases, with HPV-16 being the predominant genotype present.15 Likewise, a study of the South-African cohort found HPV DNA in 57/780 (7.3%) samples, with the highest prevalence being in the sinonasal tract (16.0%) and oropharynx (10.8%).16 HPV16 was the most commonly detected type, being found in 26/57 (45.6%) positive samples.16
The Human Papillomavirus (HPV)
Known for its ability to infect and replicate in epithelial cells in the skin and mucosal regions, HPVs constitute a large family of non-enveloped double-stranded DNA viruses, with over 220 types having been identified.3,17 Its circular genome of ~8000 base pairs encodes two structural genes necessary for viral assembly (late genes: L1 and L2) and six nonstructural genes, ie, E1, E2, and E4, responsible for viral replication and its regulation, with E5, E6, and E7 being involved in HPV-associated cellular transformation.3,18
Given that E6 and E7 genes are the only viral genes that are always retained and expressed in HPV-positive cancer cells, they are recognized as the most definitive marker for transforming HPV infections in regard to their oncogenic activity.18,19 E6 binds to p53 via E6AP and subsequently degrades it via a proteasome-mediated pathway.20 In order to support viral replication, E7 induces S phase-related molecular changes by binding and degrading the retinoblastoma pocket proteins RB, p130 (also called RBL2), and p107 (also called RBL1).21 The E6 oncoprotein is capable of inhibiting apoptosis by blocking the Fas/Fas ligand death-induced pathway or by binding to the tumor necrosis factor receptor 1 (TNFR1).19–22 Another function of E6 involves reducing the immune response against HPV antigens through suppressing Interferon Regulatory Factor 3 (IRF-3), which is a transcription factor of Interferon.22 Furthermore, because the E6 oncoprotein inhibits the phosphorylation of Tyrosine Kinase 2 (TYK2) via the STAT/TYK2 pathway, it prevents the binding between IFNα and its receptor.23 Human telomerase reverse transcriptase (hTERT) is a catalytic unit of telomerase, which normally is repressed in somatic cells.24 E6, and to some degree E7, remove transcriptional suppressors from the promoter of hTERT through hypomethylation and acetylation of the promoter, leading to the expression of hTERT, which subsequently elongates telomeres via replication.2,24 Consequently, an end-to-end fusion of chromosomes and apoptosis is prevented from occurring, allowing the cell to continue to divide and become immortal.2,24 The tumor suppressor gene CDKN2A encodes the p16INK4A protein, a cyclin-dependent kinase inhibitor, which attaches and disrupts the cyclin D–CDK4 and cyclin D–CDK6 complexes.25 Loss of CDKN2A and the commonly seen amplification of cyclin D1 (CCND1) on 11q13 drive cells through the G1–S checkpoint of the cell cycle and promote unscheduled DNA replication25 (Figure 2). The expression of p16 is a consequence of functional inactivation of the retinoblastoma protein (pRb) by the HPV E7 protein.19 Furthermore, E7 promotes cell division by increasing the activity of cyclin-dependent kinase 2, at the same time inactivating cyclin-dependent kinase inhibitors: p21CIP1 and p27Kip1.3,26 E7 supports E6 in reducing the immune response by binding and inhibiting the Toll-like receptor-9 (TLR9).27 By skipping cell-cycle checkpoints through E6- and E7-mediated degradation of p53 and Rb proteins, respectively, high-risk HPV types, such as HPV16, HPV18, HPV31, HPV33, HPV35, HPV39, HPV45, HPV51, HPV52, HPV56, HPV58, HPV59, and HPV68, can stimulate the carcinogenic transformation of the infected mucosal epithelium.28 E6/E7 expression is commonly linked to the integration of the viral genome into DNA regions of genomic instability and subsequent disruption of the E2 coding region and dysregulation of E6/E7 themselves.28 Thanks to this, HPV can provoke persistent infections and continue to replicate since the infected epithelial cells are terminally differentiated.28 However, even though viral proteins are synthesized, no viral particles are produced by the virus.28 According to Michaud, this nonproductive infection by HPV is crucial for the induction of tumorigenesis.28 Low-risk HPV types cause mucosal infections that, generally, are cleared by the host immune response.28 In case they evade innate immunity, low-risk HPVs can generate benign lesions, such as genital warts (HPV1, HPV6, and HPV11), and in some cases, recurrent respiratory papillomatosis (HPV11) with associated cancer risk.28 The smaller carcinogenicity of low-risk HPVs is attributable to the low affinity of E6 and E7 towards p53 and Rb, given that they are committed to other functions, including viral episomal maintenance and induction of apoptosis.
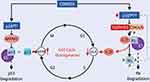 |
Figure 2 The scheme demonstrates the HPV-induced cell cycle disruption. HPV E6 oncoprotein binds to p53 via E6AP and subsequently degrades it via a proteasome-mediated pathway. The retinoblastoma protein (pRb) is functionally inactivated by the HPV E7 oncoprotein. The tumor suppressor gene CDKN2A encodes the p16INK4A protein, a cyclin-dependent kinase inhibitor, which attaches and disrupts the cyclin D–CDK4 and cyclin D–CDK6 complexes. Loss of CDKN2A and the commonly seen amplification of cyclin D1 (CCND1) drive cells through the G1–S checkpoint of the cell cycle and promote unscheduled DNA replication. Notes: Reprinted from Faraji F, Zaidi M, Fakhry C, Gaykalova DA. Molecular mechanisms of human papillomavirus-related carcinogenesis in head and neck cancer. Microbes Infect. 2017 Sep-Oct;19(9-10):464-475. Copyright (2017), with permission from Elsevier.29 |
Recent research has highlighted the significant role of E5 oncoprotein in cancerogenesis. The overactivation of the EGFR pathway by E5 stimulates proliferation and, consequently, transformation.29
According to global estimates, approximately 85% of HPV-HNC associated are caused by HPV16 and HPV18, whereas the remaining ~15% of HPV-HNC are attributable to HPV33, HPV35, HPV52, HPV45, HPV39, HPV58, HPV53, and HPV56.30 A systematic review involving12163HNC cases from all around the world has found that HPV16 was the most common type in all sites and regions, with the exception of oral cavity squamous cell carcinoma in Africa, where HPV18 was the most common type.4 A study from Syria showed that the most frequent HPV types in HNC are 33, 16, 18, 45, 52, 58, 35, 51, and 31, correspondingly.31
Mounting evidence from numerous studies supports the notion that infection with at least one type of high-risk HPV alone is not sufficient to provoke neoplastic transformation.31,32 High-risk HPV-infected cells have to undergo further genetic alteration and/or co-infection with another oncovirus to obtain total cellular transformation and lead to tumor development.31,32 It is hypothesized that high-risk HPVs and Epstein–Barr virus (EBV) may cooperate in the initiation as well as the progression of head and neck cancer. According to one study, high-risk HPVs and EBV were present in 35/80 (43.7%) and 41/80 (51.2%) of the HNC samples, respectively. Future studies should involve a larger number of samples to adequately confirm these findings.
Education and Prevention
Seeing how HPV infection has become the most frequently sexually transmitted disease in the USA, with the incidence of HPV-associated oropharyngeal cancer alone growing by more than 225% in incidence during the last 3 decades, a concerted effort has been made to prevent HPV infection.33 Consequently, three prophylactic vaccines have been approved (Cervarix, Gardasil-4, and Gardasil-9)33 Aiming to prevent HPV-related cervical cancer in women, in 2006, Merck and Co. introduced Gardasil-4, a quadrivalent HPV vaccine protecting against HPV 6, 11, 16, and 18. Subsequently, a nine-valent vaccine (Gardasil 9) has been approved for use. Because it provides protection against HPV types 6, 11, 16, 18, 31, 33, 45, 52, 58 Gardasil 9 covers strains that cause over 90% of HPV-related cancers including HPV-related OPC.34,35 Shown to be effective over a 5-year period, Cervarix is a bivalent vaccine protecting against HPV16 and HPV18.36 Given that there is some amount of cross-protection with the HPV types 31 and 45, Cervarix protects against approximately 80% of cervical cancers.36 Although it has been fully recognized that persistent infection with oncogenic HPV types can lead to non-cervical cancer, vaccination campaigns have been mainly aimed at preventing cervical and anogenital cancers. This resulted in scarce awareness in regard to the correlation between HPV vaccination and prevention of OPC among physicians and the general population. A recent study determining the opinions of parents of children aged 9 to 18 on HPV-related oropharyngeal cancers (OPC) has found that out of 150 respondents, only 12.0% of participants had heard about HPV-related oropharyngeal cancers, whereas only 12.9% of participants considered the HPV vaccine to effective in providing immunity for HPV-related oropharyngeal cancers.37 According to a 2020 review, the knowledge of HPV-associated OPSCC among health care providers ranged from 22% to 100%, whereas a range from 7% to 57% was reported in the general population.38 These evident gaps in knowledge in regard to HPV-associated OPSCC, together with parental concerns regarding vaccine safety have to be adequately approached to reduce the increasing incidence of HPV-associated OPSCC.
HPV vaccines are capable of preventing HPV infection, with studies demonstrating the reduced prevalence of oral infection, maintained for up to 4 years after vaccination.39 According to estimates, the reported vaccination efficacy is in the range of 88% and 93.3%.39 Immunization may provide direct protection against type-specific HPV infection and HPV-associated head and neck cancer considering that Handisurya et al demonstrated that HPV vaccination produces neutralizing antibodies to the vaccine-targeted types in oral fluids.40 However, there is no clear evidence for vaccine efficacy in the prevention of head and neck cancer as there are no detectable precancerous lesions that could be used as an endpoint in vaccine effectiveness.39 Further research is necessary to provide a more precise evaluation of the HPV vaccines’ potential to prevent oral HPV infections and HPV-associated HNC.
It is paramount to develop therapeutic vaccines against HPV-associated HNC, which improve cell-mediated immunity against HPV antigens rather than produce neutralizing antibodies. New therapeutic anti-HPV vaccines should target E6 and E7 as they constitute primary oncogenes.41 Phase III clinical trial involving patients with human papillomavirus (HPV)-related, high-grade squamous intraepithelial lesions (HSIL) has evaluated the efficacy of nucleic-acid vaccine candidates called MAV E2, which provokes cell-mediated immune responses.41 Out of 1176 female patients, 1051 (89.3%) female patients demonstrated complete elimination of lesions after treatment with MVA E2, with 81% of women cleared oncogenic HPV.42
Wishing to avoid the resulting individual and societal burden, it is essential to effectively approach the evident gaps in knowledge in regard to HPV-associated OPSCC, together with parental concerns regarding vaccine safety.
Clinical Manifestation and Assessment of HPV Involvement in Head and Neck Squamous Cell Carcinoma
HPV-related OPC demonstrates a different clinical manifestation and prognosis than its HPV-negative counterpart. Patients suffering from HPV-related OPC most commonly manifest a neck mass, whereas patients with HPV-negative OPC more frequently report sore throat and dysphagia.43 Cantrell et al showed that HPV-negative tumors were more likely to demonstrate radiographic evidence of invasion into adjacent muscle (26% vs 6%; P = 0.01).44 Furthermore, it is more likely for patients with HPV-positive OPC to demonstrate small primary tumors (T1–2: 75.6% vs 62.2%) and more advanced nodal disease (N2–3: 67.8% vs 56.1%).45 Another study demonstrated that patients with HPV-positive OPSCC demonstrate a higher incidence of lymph node metastasis (P = 0.003).46 By analyzing the immune infiltrate in HNC, studies demonstrated that HPV-positive cancers had greater CD8+ T cell infiltrate and PD-L1 expression than HPV-negative tumors.47,48
Despite having a more extensive nodal disease, patients with HPV-related OPC demonstrate better treatment response and have a more favorable prognosis than patients with HPV-negative OPC.43–45
In regard to genetic differences, HPV-positive tumors are characterized by recurrent mutations of PTEN, TRAF3 (TNF receptor-associated factor 3), and PIK3CA and focal amplification of E2F1, while HPV-negative tumors demonstrate a high rate of TP53 mutations and abrogation of the G1/S checkpoint via CDKN2A/B deletion and/or CCND1 amplification.47
A diagnostic work-up for HPV-associated HNC includes Polymerase Chain Reaction (PCR) or Quantitative Reverse Transcription-PCR (qRT-PCR) for the direct detection of HPV DNA or RNA and immunohistochemistry (IHC) for the detection of P16INK4A (p16) cell protein.46 Given the possibility of detecting viral DNA depositions from other sites or laboratory contamination, the DNA PCR methods alone are not precise enough to correctly assess HPV involvement.5,49 PCR lacks specificity as it is not capable of distinguishing between carcinogenesis invoked by infection and a common oral HPV infection. In comparison, HPV-DNA by in situ hybridization is characterized by good specificity, allowing for the virus to be directly visualized within the tumor cell nuclei, but it lacks sensitivity.5,49 Reaching a precise diagnosis of HPV-driven OPSCC is possible by combining p16INK4A immunohistochemistry and HPV DNA PCR.5,9 However, this combination is not reliable in the case of non-oropharyngeal cancers as it has been demonstrated that in many laryngeal tumors that are positive for HPV E6*I mRNA, there is an absence of p16INK4A overexpression.5,9 Because E6 and E7 are the most important oncogenes driving the transformation of HPV infections into cancer, using reverse transcription PCR to detect the E6 and E7 region E6*I mRNA is the recommended method of confirming the involvement of HPV in cancerogenesis.5,9
Management
Once the diagnosis is reached through clinical examination supported by modern imaging modalities (computed tomography (CT), magnetic resonance imaging (MRI), and 18F-fluorodeoxyglucose (FDG)–positron emission tomography (PET)), appropriate treatment can be introduced. Prior to deciding on which treatment method to rely on (surgery, chemotherapy, radiotherapy), it is paramount to take into consideration factors such as tumor-node-metastasis, anatomical site, as well as patient’s comorbidity, and age.
Surgery for oropharyngeal cancer underwent a vivid transformation over the last decade, advancing from open transcervical and transmandibular techniques to minimally invasive transoral endoscopic techniques. Transoral robotic surgery (TORS) is a minimally invasive technique demonstrating encouraging results as a single modality in the early stage OPSCC allowing to reduce the need for adjuvant therapy. We present a summary of selected oncologic outcomes after surgery for HPV-induced OPSCC in Table 1.50–55
 |
Table 1 Selected Outcomes After Transoral Robotic Surgery for HPV-Induced OPSCC |
Early-stage tumors are treated with surgery or radiotherapy, whereas, in the case of advanced tumors, surgery is supported with postoperative chemoradiotherapy or preoperative chemoradiation.56 A multinational, randomized study comparing radiotherapy alone with radiotherapy plus cetuximab found that the combined treatment drastically increased progression-free survival. The median duration of overall survival was 49.0 months in patients treated with combined therapy and 29.3 months in the radiotherapy alone group.57 Cancerous cells express programmed death-ligand 1 or 2 (PD-L1/2), which binds to the PD receptor on the T-cell, resulting in an inactivation of the cytotoxic response of the T-cell.58 This has led to the evaluation of pembrolizumab, a humanized anti-programmed death receptor 1 (PD-1) antibody, in an open-label, multicentre, phase 1b trial of patients with recurrent or metastatic squamous cell carcinoma.59 Out of 60 patients, 23 were HPV-positive. Overall survival was 13 months, with the duration of response equal to approximately 53 weeks.59 Pembrolizumab demonstrated clinically significant antitumor activity, with 18% of patients demonstrating an overall response by central review and 21% of patients obtaining an overall response by investigator assessment.59 Another study also used pembrolizumab in patients with recurrent/metastatic head and neck squamous cell carcinoma previously treated with platinum and cetuximab.60 Authors reported a 2.1 months median progression-free survival and an 8 months median overall survival in all patients, without differences in regard to HPV status.60 An open-label, Phase 3 study at 97 medical centers in 20 countries compared the efficacy of pembrolizumab with either methotrexate, docetaxel, or cetuximab (standard-of-care therapy). The therapeutic benefit of pembrolizumab compared with standard-of-care therapy was more significant in patients with PD-L1 expression on their tumors or in the tumor microenvironment than in patients without PD-L1 expression.61 Furthermore, pembrolizumab demonstrated a better safety profile than the standard of care, with overall profiles consistent with those previously reported.61 Median overall survival in the intention-to-treat population was 8.4 months in the case of pembrolizumab and 6.9 months with the standard of care.61
Studies have hypothesized that DNA double-strand breaks (DSBs) repair defects underlie the increased radiosensitivity of HPV-positive cancers.61 Although numerous mechanisms for the DSB repair defect, such as non-responsiveness towards the TGF-b pathway and enhanced usage of alternative non-homologous end-joining have been described, the exact mechanism remains unknown.62–64
Given that the standard therapy for locoregionally advanced OPSCC involving a combination of 70 Gy of radiation therapy (RT) with concurrent platinum chemotherapy can be associated with severe short- and long-term toxicities, studies have aimed to evaluate the therapeutic efficacy of reduced RT doses.64–68 According to Phase II clinical trials, the reduced-dose radiation results in decreased toxicity, leading to significantly improved swallowing and nutritional status64 while maintaining therapeutic outcomes, thus offering to improve the therapeutic ratio and optimize long-term function for patients with HPV-induced head and neck cancers.65
DAHANCA 6 and 7 trials demonstrated that reducing overall treatment time improves outcomes in the radiotherapy of HNC66. Authors report that using six fractions of conventionally fractionated radiotherapy per week to a total dose of 66–70 Gy led to superior loco-regional tumor control than conventionally fractionated radiotherapy.66 Chen et al recommend using 69.96 Gy delivered in 33, 2.12 Gy fractions to reduce the increased treatment‐related toxicity reported with the DAHANCA regimen.67
The multi-institutional trial showed that nonsmokers with small-volume HPV-positive OPSCC can obtain high tumor control rates and decreased salivary toxicity after moderately accelerated hypofractionated intensity-modulated radiation therapy without chemotherapy.69
Conclusions
Due to the extensive evidence, infection of human papillomavirus (HPV) has been acknowledged as a distinct risk factor for developing head and neck cancers. Future studies should rely on reverse transcription PCR to detect the E6 and E7 region E6*I mRNA to confirm the involvement of HPV in cancerogenesis. Considering the increasing incidence, with a shift toward an older population, the next decades will see an epidemic of OPC-HPV associated. As much as there is a need for further research assessing the HPV vaccines’ potential to prevent oral HPV infections and HPV-associated HNC, a concerted effort should be dedicated to developing therapeutic vaccines against HPV-associated HNC targeting E6 and E7 oncogenes. With respect to radiotherapy, it is recommended to reduce radiation dose and the overall treatment time as this leads to decreased toxicity and improved swallowing and nutritional status while maintaining therapeutic outcomes. Future research illuminating the exact mechanism behind increased radiosensitivity of HPV-positive cancers could improve radiotherapy outcomes. Pembrolizumab demonstrates encouraging results in patients with recurrent or metastatic squamous cell carcinoma of the head and neck, giving promise to the regular use of immunostimulatory antibodies in head and neck cancer. As has been observed in selected patients, TORS provides similar oncologic and functional outcomes, with a possible benefit on long-term quality of life, in comparison to traditional techniques and non-operative treatment. More research is needed to establish clear guidelines indicating when TORS resections should be supported with adjuvant therapy.
Data Sharing Statement
All data gathered for the systematic review was gathered from articles cited in the paper and listed in the reference section.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests and received no funding.
References
1. Syrjänen K, Syrjänen S, Lamberg M, Pyrhönen S, Nuutinen J. Morphological and immunohistochemical evidence suggesting human papillomavirus (HPV) involvement in oral squamous cell carcinogenesis. Int J Oral Surg. 1983;12(6):418–424. PMID: 6325356. doi:10.1016/s0300-9785(83)80033-7
2. Tumban E. A current update on human papillomavirus-associated head and neck cancers. Viruses. 2019;11(10):922. PMID: 31600915; PMCID: PMC6833051. doi:10.3390/v11100922
3. Hashibe M, Brennan P, Chuang SC, et al. Interaction between tobacco and alcohol use and the risk of head and neck cancer: pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. Cancer Epidemiol Biomarkers Prev. 2009;18(2):541–550. PMID: 19190158; PMCID: PMC3051410. doi:10.1158/1055-9965.EPI-08-0347
4. Ndiaye C, Mena M, Alemany L, et al. HPV DNA, E6/E7 mRNA, and p16INK4a detection in head and neck cancers: a systematic review and meta-analysis. Lancet Oncol. 2014;15(12):1319–1331. PMID: 25439690. doi:10.1016/S1470-2045(14)70471-1
5. Leemans CR, Snijders PJF, Brakenhoff RH. The molecular landscape of head and neck cancer. Nat Rev Cancer. 2018;18(5):269–282. PMID: 29497144. doi:10.1038/nrc.2018.11
6. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. PMID: 30207593. doi:10.3322/caac.21492
7. Sabatini ME, Chiocca S. Human papillomavirus as a driver of head and neck cancers. Br J Cancer. 2020;122(3):306–314. PMID: 31708575; PMCID: PMC7000688. doi:10.1038/s41416-019-0602-7
8. de Martel C, Plummer M, Vignat J, Franceschi S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int J Cancer. 2017;141(4):664–670. PMID: 28369882; PMCID: PMC5520228. doi:10.1002/ijc.30716
9. Castellsagué X, Alemany L, Quer M, et al. HPV involvement in head and neck cancers: comprehensive assessment of biomarkers in 3680 patients. J Natl Cancer Inst. 2016;108(6):djv403. PMID: 26823521. doi:10.1093/jnci/djv403
10. de Sanjosé S, Serrano B, Tous S, et al. Burden of Human Papillomavirus (HPV)-related cancers attributable to HPVs 6/11/16/18/31/33/45/52 and 58. JNCI Cancer Spectr. 2019;2(4):ky045. PMID: 31360870; PMCID: PMC6649711. doi:10.1093/jncics/pky045
11. Tota JE, Best AF, Zumsteg ZS, Gillison ML, Rosenberg PS, Chaturvedi AK. Evolution of the oropharynx cancer epidemic in the United States: moderation of increasing incidence in younger individuals and shift in the burden to older individuals. J Clin Oncol. 2019;37(18):1538–1546. PMID: 31026209; PMCID: PMC6599405. doi:10.1200/JCO.19.00370
12. Mahal BA, Catalano PJ, Haddad RI, et al. Incidence and demographic burden of HPV-associated oropharyngeal head and neck cancers in the United States. Cancer Epidemiol Biomarkers Prev. 2019;28(10):1660–1667. PMID: 31358520. doi:10.1158/1055-9965.EPI-19-0038
13. D’Souza G, Kreimer AR, Viscidi R, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356(19):1944–1956. PMID: 17494927. doi:10.1056/NEJMoa065497
14. Zhang Y, Fakhry C, D’Souza G. Projected association of human papillomavirus vaccination with oropharynx cancer incidence in the US, 2020–2045. JAMA Oncol. 2021;7(10):e212907. PMID: 34473210; PMCID: PMC8414358. doi:10.1001/jamaoncol.2021.2907
15. Aboagye E, Agyemang-Yeboah F, Duduyemi BM, Obirikorang C. Human papillomavirus detection in head and neck squamous cell carcinomas at a tertiary hospital in Sub-Saharan Africa. ScientificWorldJournal. 2019;2019:2561530. PMID: 31061653; PMCID: PMC6466863. doi:10.1155/2019/2561530
16. Bulane A, Goedhals D, Seedat RY, Goedhals J, Burt F. Human papillomavirus DNA in head and neck squamous cell carcinomas in the Free State, South Africa. J Med Virol. 2020;92(2):227–233. PMID: 31347711. doi:10.1002/jmv.25556
17. Graham SV, Faizo AAA. Control of human papillomavirus gene expression by alternative splicing. Virus Res. 2017;231:83–95. PMID: 27867028; PMCID: PMC5335905. doi:10.1016/j.virusres.2016.11.016
18. Roman A, Munger K. The papillomavirus E7 proteins. Virology. 2013;445(1–2):138–168. PMID: 23731972; PMCID: PMC3783579. doi:10.1016/j.virol.2013.04.013
19. Schwarz E, Freese UK, Gissmann L, et al. Structure and transcription of human papillomavirus sequences in cervical carcinoma cells. Nature. 1985;314(6006):111–114. PMID: 2983228. doi:10.1038/314111a0
20. Scheffner M, Werness BA, Huibregtse JM, Levine AJ, Howley PM. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990;63(6):1129–1136. PMID: 2175676. doi:10.1016/0092-8674(90)90409-8
21. Filippova M, Parkhurst L, Duerksen-Hughes PJ. The human papillomavirus 16 E6 protein binds to Fas-associated death domain and protects cells from Fas-triggered apoptosis. J Biol Chem. 2004;279(24):25729–25744. PMID: 15073179. doi:10.1074/jbc.M401172200
22. Shah M, Anwar MA, Park S, Jafri SS, Choi S. In silico mechanistic analysis of IRF3 inactivation and high-risk HPV E6 species-dependent drug response. Sci Rep. 2015;5:13446. PMID: 26289783; PMCID: PMC4542336. doi:10.1038/srep13446
23. Li S, Labrecque S, Gauzzi MC, et al. The human papilloma virus (HPV)-18 E6 oncoprotein physically associates with Tyk2 and impairs Jak-STAT activation by interferon-alpha. Oncogene. 1999;18(42):5727–5737. PMID: 10523853. doi:10.1038/sj.onc.1202960
24. Katzenellenbogen R. Telomerase Induction in HPV Infection and Oncogenesis. Viruses. 2017;9(7):180. PMID: 28698524; PMCID: PMC5537672. doi:10.3390/v9070180
25. Stephen JK, Divine G, Chen KM, Chitale D, Havard S, Worsham MJ. Significance of p16 in site-specific HPV positive and HPV negative head and neck squamous cell carcinoma. Cancer Clin Oncol. 2013;2(1):51–61. PMID: 23935769; PMCID: PMC3736998. doi:10.5539/cco.v2n1p51
26. Moody CA, Laimins LA. Human papillomavirus oncoproteins: pathways to transformation. Nat Rev Cancer. 2010;10(8):550–560. PMID: 20592731. doi:10.1038/nrc2886
27. Hasan UA, Zannetti C, Parroche P, et al. The human papillomavirus type 16 E7 oncoprotein induces a transcriptional repressor complex on the Toll-like receptor 9 promoter. J Exp Med. 2013;210(7):1369–1387. PMID: 23752229; PMCID: PMC3698525. doi:10.1084/jem.20122394
28. Michaud DS, Langevin SM, Eliot M, et al. High-risk HPV types and head and neck cancer. Int J Cancer. 2014;135(7):1653–1661. PMID: 24615247; PMCID: PMC4107082. doi:10.1002/ijc.28811
29. Gutierrez-Xicotencatl L, Pedroza-Saavedra A, Chihu-Amparan L, Salazar-Piña A, Maldonado-Gama M, Esquivel-Guadarrama F. Cellular functions of HPV16 E5 oncoprotein during oncogenic transformation. Mol Cancer Res. 2021;19(2):167–179. PMID: 33106372. doi:10.1158/1541-7786.MCR-20-0491
30. Marur S, D’Souza G, Westra WH, Forastiere AA. HPV-associated head and neck cancer: a virus-related cancer epidemic. Lancet Oncol. 2010;11(8):781–789. PMID: 20451455; PMCID: PMC5242182. doi:10.1016/S1470-2045(10)70017-6
31. Gupta I, Ghabreau L, Al-Thawadi H, et al. Co-incidence of human papillomaviruses and Epstein-Barr virus is associated with high to intermediate tumor grade in human head and neck cancer in Syria. Front Oncol. 2020;10:1016. PMID: 32974123; PMCID: PMC7468388. doi:10.3389/fonc.2020.01016
32. Al-Thawadi H, Gupta I, Jabeen A, et al. Co-presence of human papillomaviruses and Epstein-Barr virus is linked with advanced tumor stage: a tissue microarray study in head and neck cancer patients. Cancer Cell Int. 2020;20:361. PMID: 32774155; PMCID: PMC7397600. doi:10.1186/s12935-020-01348-y
33. Dunne EF, Markowitz LE, Saraiya M, et al.; Centers for Disease Control and Prevention (CDC). CDC grand rounds: reducing the burden of HPV-associated cancer and disease. MMWR Morb Mortal Wkly Rep. 2014;63(4):69–72. PMID: 24476977; PMCID: PMC4584896.
34. Timbang MR, Sim MW, Bewley AF, Farwell DG, Mantravadi A, Moore MG. HPV-related oropharyngeal cancer: a review on burden of the disease and opportunities for prevention and early detection. Hum Vaccin Immunother. 2019;15(7–8):1920–1928. PMID: 31050595; PMCID: PMC6746516. doi:10.1080/21645515.2019.1600985
35. Markowitz LE, Dunne EF, Saraiya M, et al.; Centers for Disease Control and Prevention (CDC). Human papillomavirus vaccination: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2014;63(RR–05): 1–30. PMID: 25167164.
36. Harper DM, Franco EL, Wheeler CM, et al.; HPV Vaccine Study group. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised control trial. Lancet. 2006;367(9518):1247–1255. PMID: 16631880. doi:10.1016/S0140-6736(06)68439-0
37. Dundar Y, Eldem I, Schwartz C, et al. Screening awareness of HPV-related oropharyngeal cancers and attitudes and concerns towards HPV vaccination among parents: HPV and oropharyngeal cancer. J Cancer Educ. 2022;37(4):1152–1160. PMID: 33411252. doi:10.1007/s13187-020-01932-w
38. Parsel SM, Barton BM, Beatty S, Friedlander PL. Knowledge gaps among patients and providers in HPV-related oropharyngeal cancer: a systematic review. Otolaryngol Head Neck Surg. 2020;162(5):612–621. PMID: 32122242. doi:10.1177/0194599820908596
39. Diana G, Corica C. Human Papilloma Virus vaccine and prevention of head and neck cancer, what is the current evidence? Oral Oncol. 2021;115:105168. PMID: 33730628. doi:10.1016/j.oraloncology.2020.105168
40. Handisurya A, Schellenbacher C, Haitel A, Senger T, Kirnbauer R. Human papillomavirus vaccination induces neutralising antibodies in oral mucosal fluids. Br J Cancer. 2016;114(4):409–416. PMID: 26867163; PMCID: PMC4815771. doi:10.1038/bjc.2015.462
41. Tang J, Li M, Zhao C, et al. Therapeutic DNA vaccines against HPV-related malignancies: promising leads from clinical trials. Viruses. 2022;14(2):239. PMID: 35215833; PMCID: PMC8874761. doi:10.3390/v14020239
42. Rosales R, López-Contreras M, Rosales C, et al. Regression of human papillomavirus intraepithelial lesions is induced by MVA E2 therapeutic vaccine. Hum Gene Ther. 2014;25(12):1035–1049. PMID: 25275724; PMCID: PMC4270165. doi:10.1089/hum.2014.024
43. McIlwain WR, Sood AJ, Nguyen SA, Day TA. Initial symptoms in patients with HPV-positive and HPV-negative oropharyngeal cancer. JAMA Otolaryngol Head Neck Surg. 2014;140(5):441–447. PMID: 24652023. doi:10.1001/jamaoto.2014.141
44. Cantrell SC, Peck BW, Li G, Wei Q, Sturgis EM, Ginsberg LE. Differences in imaging characteristics of HPV-positive and HPV-Negative oropharyngeal cancers: a blinded matched-pair analysis. AJNR Am J Neuroradiol. 2013;34(10):2005–2009. PMID: 23660291; PMCID: PMC3951375. doi:10.3174/ajnr.A3524
45. Stenmark MH, Shumway D, Guo C, et al. Influence of human papillomavirus on the clinical presentation of oropharyngeal carcinoma in the United States. Laryngoscope. 2017;127(10):2270–2278. PMID: 28304083; PMCID: PMC6615732. doi:10.1002/lary.26566
46. Paz IB, Cook N, Odom-Maryon T, Xie Y, Wilczynski SP. Human papillomavirus (HPV) in head and neck cancer. An association of HPV 16 with squamous cell carcinoma of Waldeyer’s tonsillar ring. Cancer. 1997;79(3):595–604. PMID: 9028373. doi:10.1002/(sici)1097-0142(19970201)79:3<595::aid-cncr24>3.0.co;2-y
47. Sastre-Garau X, Harlé A. Pathology of HPV-associated head and neck carcinomas: recent data and perspectives for the development of specific tumor markers. Front Oncol. 2020;10:528957. PMID: 33312940; PMCID: PMC7701329. doi:10.3389/fonc.2020.528957
48. Gameiro SF, Ghasemi F, Barrett JW, et al. Treatment-naïve HPV+ head and neck cancers display a T-cell-inflamed phenotype distinct from their HPV- counterparts that has implications for immunotherapy. Oncoimmunology. 2018;7(10):e1498439. PMID: 30288365; PMCID: PMC6169583. doi:10.1080/2162402X.2018.1498439
49. Mirghani H, Casiraghi O, Amen F, et al. Diagnosis of HPV-driven head and neck cancer with a single test in routine clinical practice. Mod Pathol. 2015;28(12):1518–1527. PMID: 26403782. doi:10.1038/modpathol.2015.113
50. Frederiksen JG, Channir HI, Larsen MHH, et al. Long-term survival outcomes after primary transoral robotic surgery (TORS) with concurrent neck dissection for early-stage oropharyngeal squamous cell carcinoma. Acta Otolaryngol. 2021;141(7):714–718. PMID: 34191671. doi:10.1080/00016489.2021.1939147
51. Cannon RB, Houlton JJ, Patel S, et al. Patterns of cervical node positivity, regional failure rates, and fistula rates for HPV+ oropharyngeal squamous cell carcinoma treated with transoral robotic surgery (TORS). Oral Oncol. 2018;86:296–300. PMID: 30409315. doi:10.1016/j.oraloncology.2018.10.001
52. Meccariello G, Montevecchi F, D’Agostino G, et al. Trans-oral robotic surgery for the management of oropharyngeal carcinomas: a 9-year institutional experience. Acta Otorhinolaryngol Ital. 2019;39(2):75–83. PMID: 31097824; PMCID: PMC6522856. doi:10.14639/0392-100X-2199
53. Carey RM, Brody RM, Shimunov D, et al. Locoregional recurrence in p16-positive oropharyngeal squamous cell carcinoma after TORS. Laryngoscope. 2021;131(12):E2865–E2873. PMID: 34076275. doi:10.1002/lary.29659
54. Moore EJ, Van Abel KM, Price DL, et al. Transoral robotic surgery for oropharyngeal carcinoma: surgical margins and oncologic outcomes. Head Neck. 2018;40(4):747–755. PMID: 29327784. doi:10.1002/hed.25055
55. O’Hara J, Warner L, Fox H, et al. Primary transoral robotic surgery +/- adjuvant therapy for oropharyngeal squamous cell carcinoma-A large observational single-centre series from the United Kingdom. Clin Otolaryngol. 2021;46(5):1005–1012. PMID: 33754476. doi:10.1111/coa.13769
56. Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354(6):567–578. PMID: 16467544. doi:10.1056/NEJMoa053422
57. Kaidar-Person O, Gil Z, Billan S. Precision medicine in head and neck cancer. Drug Resist Updat. 2018;40:13–16. PMID: 30466712. doi:10.1016/j.drup.2018.09.001
58. Seiwert TY, Burtness B, Mehra R, et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. Lancet Oncol. 2016;17(7):956–965. PMID: 27247226. doi:10.1016/S1470-2045(16)30066-3
59. Bauml J, Seiwert TY, Pfister DG, et al. Pembrolizumab for platinum- and cetuximab-refractory head and neck cancer: results from a single-arm, Phase II study. J Clin Oncol. 2017;35(14):1542–1549. PMID: 28328302; PMCID: PMC5946724. doi:10.1200/JCO.2016.70.1524
60. Cohen EEW, Soulières D, Le Tourneau C, et al.; KEYNOTE-040 investigators. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. Lancet. 2019;393(10167):156–167. PMID: 30509740. doi:10.1016/S0140-6736(18)31999-8
61. Zech HB, Berger J, Mansour WY, et al. Patient derived ex vivo tissue slice cultures demonstrate a profound DNA double-strand break repair defect in HPV-positive oropharyngeal head and neck cancer. Radiother Oncol. 2022;168:138–146. PMID: 35093407. doi:10.1016/j.radonc.2022.01.017
62. Leeman JE, Li Y, Bell A, et al. Human papillomavirus 16 promotes microhomology-mediated end-joining. Proc Natl Acad Sci U S A. 2019;116(43):21573–21579. PMID: 31591214; PMCID: PMC6815166. doi:10.1073/pnas.1906120116
63. Liu Q, Ma L, Jones T, et al. Subjugation of TGFβ signaling by human papilloma virus in head and neck squamous cell carcinoma Shifts DNA repair from homologous recombination to alternative end joining. Clin Cancer Res. 2018;24(23):6001–6014. PMID: 30087144. doi:10.1158/1078-0432.CCR-18-1346
64. Marur S, Li S, Cmelak AJ, et al. E1308: Phase II trial of induction chemotherapy followed by reduced-dose radiation and weekly cetuximab in patients with HPV-associated resectable squamous cell carcinoma of the oropharynx- ECOG-ACRIN cancer research group. J Clin Oncol. 2017;35(5):490–497. PMID: 28029303; PMCID: PMC5455313. doi:10.1200/JCO.2016.68.3300
65. Chen AM, Felix C, Wang PC, et al. Reduced-dose radiotherapy for human papillomavirus-associated squamous-cell carcinoma of the oropharynx: a single-arm, Phase 2 study. Lancet Oncol. 2017;18(6):803–811. PMID: 28434660; PMCID: PMC6488353. doi:10.1016/S1470-2045(17)30246-2
66. Lassen P, Eriksen JG, Krogdahl A, et al.; Danish Head and Neck Cancer Group (DAHANCA). The influence of HPV-associated p16-expression on accelerated fractionated radiotherapy in head and neck cancer: evaluation of the randomised DAHANCA 6&7 trial. Radiother Oncol. 2011;100(1):49–55. PMID: 21429609. doi:10.1016/j.radonc.2011.02.010
67. Chen L, Riaz N, Lee N, McBride S. Current considerations for radiotherapy in HPV-associated head and neck cancer. J Surg Oncol. 2021;124(6):945–951. PMID: 34617275. doi:10.1002/jso.26689
68. Yom SS, Torres-Saavedra P, Caudell JJ, et al. Reduced-dose radiation therapy for HPV-associated oropharyngeal carcinoma (NRG oncology HN002). J Clin Oncol. 2021;39(9):956–965. PMID: 33507809; PMCID: PMC8078254. doi:10.1200/JCO.20.03128
69. Eisbruch A, Harris J, Garden AS, et al. Multi-institutional trial of accelerated hypofractionated intensity-modulated radiation therapy for early-stage oropharyngeal cancer (RTOG 00-22). Int J Radiat Oncol Biol Phys. 2010;76(5):1333–1338. PMID: 19540060; PMCID: PMC2846217. doi:10.1016/j.ijrobp.2009.04.011
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
