Back to Journals » Journal of Inflammation Research » Volume 13
Human Monocytes/Macrophage Inflammatory Cytokine Changes Following in vivo and in vitro Schistomam manoni Infection
Authors Wolde M, Laan LC, Medhin G, Gadissa E, Berhe N, Tsegaye A
Received 4 October 2019
Accepted for publication 6 December 2019
Published 16 January 2020 Volume 2020:13 Pages 35—43
DOI https://doi.org/10.2147/JIR.S233381
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Video abstract presented by Mistire Wolde.
Views: 419
Mistire Wolde, 1, 2 Lisa C Laan, 3 Girmay Medhin, 1 Endalemaw Gadissa, 4 Nega Berhe, 1, 5 Aster Tsegaye 2
1Aklilu Lemma Institute of Pathobiology, Addis Ababa University, Addis Ababa, Ethiopia; 2Department of Medical Laboratory Sciences, College of Health Science, Addis Ababa University, Addis Ababa, Ethiopia; 3Department of Molecular Cell Biology and Immunology, VU University Medical Center, Amsterdam, the Netherlands; 4Armauer Hansen Research Institute (AHRI), Addis Ababa, Ethiopia; 5Oslo University Hospital-Ulleval, Centre for Imported and Tropical Diseases, Oslo, Norway
Correspondence: Mistire Wolde
Aklilu Lemma Institute of Pathobiology, Addis Ababa University, Addis Ababa, Ethiopia
Email [email protected]
Introduction: Epidemiological and animal studies indicate that helminth infections have positive effects due to their potential to protect against autoimmune diseases. Here, we aim to assess the effect of S. mansoni infection on immune modulation of human monocytes and their potential protection against autoimmune disease development both in vivo and in vitro.
Materials and Methods: Monocytes were isolated from helminth-infected Ethiopians (MHIE), and from Dutch healthy volunteers (MHV). The MHV were stimulated in vitro with S. mansoni soluble egg antigens (SEA) or soluble worm antigens (SWA). In addition, phenotypical changes were studied directly, as well as after culturing for 6 days in the presence of human serum to obtain macrophages. Q-PCR, flow cytometry, multiplex bead immunoassay, and live-cell imaging were employed during analysis.
Results: MHIE showed elevated transcripts of SOCS-1 and TNF-α compared to MHV. Similarly, MHV that were stimulated with SEA demonstrated enhanced levels of SOCS-1, IL-10, and IL-12 mRNA, compared to control MHV. Remarkably, the SEA-treated monocytes showed a much higher motility than control monocytes, a hallmark of a patrolling phenotype. Furthermore, in vitro cultured macrophages that were stimulated by SEA exhibited enhanced mRNA levels of SOCS-1, IL-10, TNF-α, IL-12 and TGF-β, compared to control macrophages.
Conclusion: Macrophages from MHIE as well as SEA-treated MHV show an intermediate activation phenotype with both pro-inflammatory and anti-inflammatory characteristics in vitro. The observed pro-inflammatory properties might reflect a recent response of the cells due to contact with a pathogen, whereas the anti-inflammatory properties might contribute to helminth-induced protection against inflammatory diseases. Large-scale study is recommended to consolidate the findings of the present study.
Keywords: Schistosoma mansoni, immune modulation, monocytes, macrophages
Introduction
Schistosomiasis, mainly caused by S. mansoni, S. haematobium, and S. japonicum, affects more than 200 million people in many tropical and subtropical areas worldwide. In Ethiopia, S. mansoni is common in most parts of the country and the prevalence in endemic areas, such as Kemisse administrative zone in northeastern Ethiopia, reaches up to 69.9%.1
The infection process by S. mansoni has two clinical conditions: an acute stage and a chronic stage. The acute stage, which occurs between 6–8 weeks after infection and before the appearance of S. mansoni eggs in the stool, is characterized by febrile illness (katayama fever) and production of inflammatory cytokines, such as TNF-α, IL-1 and IL-6. The chronic stage, which starts after egg-laying at around 12 weeks of infection, may last up to 40 years. This stage is characterized by the production of Th2 cytokines, such as IL-4, IL-5 and IL-13, as well as by long-lasting anti-inflammatory responses by macrophages, which also have a potential to reduce tissues damage caused by the parasite.2
Previous studies by our group and others showed that soluble products of S. mansoni and Trichurissuis can modulate the function of dendritic cells (DCs), and polarize T cell responses towards a T cell helper 2 response.3–5 However, also innate immune cells like monocytes and macrophages are expected to play a major role in the interaction with helminths, as monocytes are the first cells to recognize foreign particles in the blood. Monocyte subsets include classical monocytes, which are regarded to be more pro-inflammatory compared to the non-classical monocytes which display an enhanced patrolling behaviour including a higher motility, and are regarded as anti-inflammatory cells.6 These monocyte subsets can differentiate into specific DC and macrophage subsets that act as antigen-presenting cells and coordinate the innate and adaptive immune reactions. Macrophages display a variety of phenotypes, ranging from a cytotoxic, pro-inflammatory subset (M1) on one side of the spectrum and a wound-healing, anti-inflammatory subset (M2) on the other side.7,8 Pathogenic compounds such as bacterial lipo-polysaccharide (LPS) can initiate the development of M1 macrophages that produce inflammatory cytokines including TNFα, IL-1β, IL-6, IL-12.7 On the other hand, stimuli such as helminthic compounds, or cytokines such as IL-4 or IL-13 can initiate the development of anti-inflammatory (M2) macrophages that express the mannose receptor (MR) and secrete anti-inflammatory mediators including IL-10 and TGF-β.9
At the nuclear levels also, molecules such as SOCS-1 (suppressor of cytokine signalling), also called SSI-1 (STAT-induced STAT inhibitor-1), initiated by various stimuli, including LPS, IFN-, IL-4, IL-6, and LIF;10–12 through JAK-STAT pathways. These, in turn, stimulate the expression of nucleic acids and have a negative regulator effect that protects the host from hyperinflammatory reactions, such as endotoxin-induced fatal syndrome which sometimes occurs following infection.13
Here we report for the first time the effect of helminth infection on monocytes of individuals recruited from Ethiopia, a country where S. mansoni is endemic. To validate these observations, we describe the immunological changes that are induced in blood monocytes of healthy individuals, stimulated with S. mansoni egg antigens (SEA) and soluble worm compounds (SWA), and the effect of these compounds on the monocyte-to-macrophage differentiation. The changes in monocyte/macrophage phenotype in relation to the suppression of inflammation are discussed.
Materials and Methods
Isolation of Human Monocyte and Their Differentiation to Macrophages
Collection of Monocytes from Ethiopian Study Participants
The study participants from S. mansoni endemic area kemisse (north-eastern Ethiopia) were recruited by employing a systemic random sampling method. After getting written informed consent, each study participant eligibility for the study assessed by interviewing standardized question (for Inclusion criteria’s such as have DM family history, previous GDM; and exclusion criteria’s including known DM patient, took anthelminthic treatment before 3 months, have immune-suppressive diseases, such as Tuberculosis, Cancer, and HIV) as well as other risk factors associated questioners.
Then, blood samples were collected from S. mansoni is endogenous (15 positive for S. mansoni with and without other helminths, 12 positive for one or more intestinal helminths other than S. mansoni, and 9 helminth-negative samples, see Table 1). The type of infection was determined by examination of the stool on the presence of helmitheseggs. Approximately 5 g of stool was collected from each individual and two slides prepared using the kato-katz method for microscopic examination of ova at the study site. Subsequently, the slides were analysed at Aklilu Lemma institute of pathobiology (ALIPB) for quality control and establishment of diagnosis. In addition, blood samples were collected from each study participants and transported at room temperature from the collection site to Armauer Hansen Research Institute (AHRI) laboratory in Addis Ababa, and monocytes were isolated within 8 hrs14 as described by Kuijk et al4 In short, peripheral blood mononuclear cells, PBMCs were isolated from total blood by ficoll density gradient centrifugation, and monocytes were isolated from the PBMCs using human CD14 Micro Beads (MACS MilteniBiotec, Germany) according to the manufacturer’s manual. The extracted monocytes were snap-frozen and stored at −80°C until analysis.
 |
Table 1 Overview of Groups of Ethiopian Individuals ± Helminth Infections |
Extraction and Activation of Monocytes from Healthy Donors
Monocytes were isolated from buffy coats of healthy blood donors (Sanquin, Amsterdam, the Netherlands), as described above by Kuijk et al.4 The monocytes were incubated (5×106 cells/well) with and without SEA or SWA (20 μg/mL) in 0.5 mL/well RPMI 1640 medium (Invitrogen, USA), supplemented with 10% fetal calf serum (FCS, BioWhittacker, USA) plus glutamine (2 mM, Lonza, Switzerland), penicillin and streptomycin (100 U/mL, BioWhittacker, USA) in 48 well culture plates, in a CO2 incubator at 37°C for 24 hrs.
Macrophages were obtained by culturing monocytes (5×106 cells/well) with and without SEA or SWA (20 μg/mL) in 2 mL DMEM medium (Invitrogen, USA), supplemented with 10% FCS (BioWhittacker, USA) plus glutamine (2 mM, Lonza, Switzerland), penicillin and streptomycin (100 U/mL, BioWhittacker, USA), and 5% human serum, in 6-well plates, in a CO2 incubator at 37°C for 6 days.
Schistosomamansoni Soluble Egg Antigen (SEA) and Worm Compounds (SWA)
S. mansoni adult worms were kindly provided by Dr. A. Deelder (Leiden, the Netherlands). The S. mansoni soluble egg antigens (SEA), and worm compounds (SWA) were prepared and stored at −80°C, as described by Kuijk et al.4 Endotoxin contamination was determined by Limulus Amoebocyte Lysate assay (Charles River Laboratories, Leiden, NL). Contamination was lower than 1 ng/mL endotoxin for both preparations.
mRNA Extraction, cDNA Synthesis and Quantitative Real-Time PCR (Q-PCR)
Monocytes were collected at the time points indicated, and snap-frozen at −80°C. mRNA was extracted using the mRNA capture kit (Roche, Switzerland), and subsequently transcribed into cDNA using the Reverse Transcriptase System Kit (Promega, USA), as described by Garcia-Vallejo et al.15 The synthesized cDNA was stored at −20°C until further analysis. Then, Q-PCR reactions were performed with the SYBR Green method as described by Garcia-Vallejo et al. Oligonucleotides were prepared by using Primer express 2.0 (Applied bioscience, USA) computer software, as shown in Table 2. The Ct value of GADPH was taken as a household reference, and expression of genes of interest was determined by comparing mean fold Ct values of control samples with those of test samples, or relative Ct values were given as indicated.
 |
Table 2 Oligonucleotide Primers, Designed and Used in the Study |
Multiplex Bead Immunoassay
Multiplex bead immunoassay was employed to measure IL-6, TNFα and IL-10 production from monocytes extracted from healthy donors stimulated with and without SEA/SWA. Cytokines were measured according to Invitrogen’s user manual “Human Inflammatory 5-Plex Panel” as previously described.4 In brief, experimental samples were added to 50 µL incubation buffer and a mix of cytokinecoupled beads was added to the plate and incubated for 2 h at room temperature (RT) in the dark while shaking. A mix of biotin-coupled antibodies was prepared according to the protocol and added at 100µL/well followed by 1 h incubation at RT in the dark while shaking. After washing, 100 µL/well streptavidin-PE (SA-PE) was added to the plate and incubated for 15 min at RT while shaking in the dark. Following two washes, the samples were measured by Luminex® 200TM (Bio-Rad, Hercules, CA) and data were analyzed using Bio-plexManagerTM 6.0 software.
Measurement of Cell Movement
Cell movement of monocytes with and without SEA and SWA (40 μg/mL) was determined by adding 300.000 monocytes in 250 μL RPMI/PSG/10% FCS per well (Ibidi slides 8 wells). Monocytes were allowed to settle for 5 mins and images were taken, at a temperature of 37°C, humidity 60%, and 5% CO2 every 5 mins for 10 hrs, by using an Olympus IX 81 motorized inverted microscope (Olympus, Hamburg, Germany) and Cell® software. Then, the video image was analysed using the Image J software from the National Institutes of Health (Bethesda, MD, US).
Statistical Analysis
Statistical analysis was carried out by applying Shapiro–Wilk test of normality and one way ANOVA using SPSS version 20 software with post hoc multiple comparisons tests, and data were considered significant if p≤0.05. Moreover, Graph pad Prism was used to plot graphs with mean and SEM. When using monocytes from healthy donors, data were derived from independent experiments using at least five separate donors.
Ethical Considerations
This study was conducted in accordance with the Declaration of Helsinki. Accordingly, the aim of the study was explained and informed consent obtained from all Ethiopian participants. The study protocol was reviewed and ethical approval obtained from the Institute review board of Addis Ababa University, Institute of Pathobiology, and from the National Ethical Clearance committee, Ethiopia.
Results
Monocytes from Helminth-Infected Individuals Show an Intermediate Activation Phenotype
The effect of helminth infection on gene expression in monocytes was studied by extracting monocytes directly from blood samples of Ethiopian study participants with or without a positive egg stool for S. mansoni, or other helminths (Table 1). Analysis of the mRNA levels of these monocytes by Q-PCR indicated that individuals with helminth infections showed elevated mRNA levels of SOCS-1, TNF-α, IL-10 and IL-12 as compared with monocytes from the helminth-negative study participants (Figure 1).
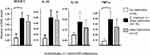 |
Figure 1 Gene expression in monocytes isolated from blood samples of Ethiopian individuals either infected or not infected by helminths (see Table 1), as determined by real-time PCR (Q-PCR) using GAPDH as reference gene; helminth infection was established by stool examination. *Significance is p<0.05. |
Monocytes from Healthy Donors Stimulated with S. mansoni SEA Show an Intermediate Activation Phenotype and Enhanced Motility
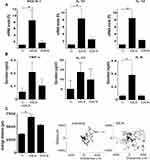
The effect of S. mansoni SEA and SWA on monocytes was studied by incubation of monocytes derived from blood samples of Dutch healthy donors. As this study was a continuation investigation on previous studies done in our group on S. mansoni and T. suis parasites,4,5,16 here also used the effect of the already identified cytokine on monocytes as well in vivo analysis. Accordingly, analysis of the mRNA of these monocytes by Q-PCR indicated that SEA was much more potent than SWA in inducing elevated SOCS-1, IL-10, and IL-12 mRNA levels compared with monocyte control samples (Figure 2A). Furthermore, the SEA-stimulated monocytes showed enhanced secretion of IL-6, IL-10 and TNF-α as compared to control monocyte samples (Figure 2B). These data indicate that SEA confers both pro- and anti-inflammatory properties to the monocytes, whereas the SWA induced effects were much more restricted.
The properties of both SEA and SWA-stimulated monocytes were further defined by investigating their live motility, which can be regarded as a monocyte activation marker associated with a patrolling phenotype.14 Using a live cell imaging approach, we showed that the SEA-treated cells showed both a higher undirected motility and more distance travelled compared to SWA-treated cells and untreated cells (Figure 2C), suggesting the induction of a patrolling monocyte phenotype by SEA.
Effects of S. mansoni SEA on the Differentiation of Monocytes to Macrophages
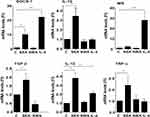
Monocytes were differentiated into macrophages by culturing them for 6 days in the presence or absence of S. mansoni SEA or SWA. In addition, monocytes were not stimulated to differentiate into M0 macrophages, and in some cases stimulated with IL-4 as a control for M2 polarization. The mRNA levels of both pro-inflammatory and anti-inflammatory genes were determined by Q-PCR. The results indicate that SEA, but not SWA, significantly enhanced the mRNA levels of SOCS-1, IL-10, TNF-α, IL-12, and TGF-β, compared to the unstimulated macrophages. These data indicate that the SEA-stimulated macrophages display an intermediate M1-M2 phenotype, as shown in Figure 3.
Discussion
The dual role of parasitic helminths to act as pathogens, and to protect excessive inflammation has raised great interest in the mechanisms by which the worms modulate the human immune system. We here report on the interaction of parasitic helminths with monocytes, which are the initial cells to recognize invading pathogens and upon contact differentiate to dendritic cells and macrophages that orchestrate adaptive responses.16,17
Here we show that helminths induce an intermediate activation phenotype in monocytes derived from helminth-infected individuals. These data were confirmed in vitro using monocytes from healthy donors stimulated with S. mansoni soluble egg antigen (SEA). The most prominent anti-inflammatory properties observed upon stimulation of monocytes with SEA are the upregulation of suppressor of cytokine signalling-1 (SOCS-1), the enhanced expression of IL-10, and their enhanced motility, which reflects a patrolling monocyte phenotype.
SOCS1 is one of the cytokine-inducible SH2-containing, CIS/SOCS family proteins that can down-regulate inflammatory reactions.18,19 SOCS1 negatively regulates JAK/STAT signalling and can also suppress TLR4 signalling via MAPK and NF-κB pathways.18,20 Recently we showed that SEA strongly induces SOCS1 in human dendritic cells.21 In the present study, we demonstrate that monocytes either from Ethiopians infected with helminths (with or without S. mansoni), or from healthy donors stimulated by SEA, have significantly higher expression of SOCS-1 compared to control monocytes. In addition, monocytes from Ethiopian study participants infected by helminths (with or without S. mansoni) showed a tendency for enhanced production of IL-10, a major immune-regulatory cytokine that has potential in protecting the host from autoimmune reactions,22 compared to non-infected groups. In line with this, SEA-stimulated monocytes from healthy donors showed a significant upregulation of IL-10 production compared with untreated monocytes. This indicates that SEA induces monocytes to have anti-inflammatory properties and triggers the production of regulatory cytokines.
Next to anti-inflammatory properties, helminths seem to confer pro-inflammatory properties to monocytes, although expression levels are low as compared to the levels induced by LPS (data not shown here and displayed in our previous work, ref. [4]). Upregulation of TNFα expression was observed in monocytes of helminth-infected individuals, and SEA significantly induced the expression of TNFα, IL-12 and IL-6 in monocytes of healthy donors. The induction of these inflammatory cytokines may reflect the recent interaction of helminth antigens by monocytes. On the other hand, although IL-6 is known as an inflammatory cytokine, IL-6 can act also like IL-10 in promoting an anti-inflammatory response, for example, in the absence of SOCS-3.23 A study conducted in Ghana indicated that during S. haematobium infection, SOCS-3 expression is low;24 thus, we cannot exclude that this may also be the case in monocytes during S. mansoni infections, although it should be noted that SEA is reported to induce SOCS-3 expression in dendritic cells.21,25
During inflammation blood-derived monocytes migrate to the tissues and differentiate into macrophages. To define the phenotype of macrophages derived from S. mansoni stimulated monocytes, we analysed the phenotype of macrophages, which were cultured in vitro from S. mansoni SEA and SWA-stimulated monocytes from healthy donors. Our data indicate that monocytes stimulated in vitro with SEA develop in a macrophage subset with a pro-inflammatory phenotype, as indicated by elevated transcript levels of TNFα and IL-12. In addition, anti-inflammatory/regulatory properties are enhanced as indicated by an upregulated SOCS1, TGFβ and IL-10 expression, whereas a well-known M2 marker, the MR, was hardly upregulated.21 Remarkably, this phenotype is only acquired by the treatment of the monocytes with SEA, since SWA had a lesser effect on the monocyte/macrophage phenotype.
SOCS1 has previously been attributed to have an important role in macrophage polarization. It has been shown to be up-regulated in M2 macrophages, thereby contributing to their anti-inflammatory properties.20 In addition, in M1 macrophages, SOCS1 was shown to dampen proinflammatory responses.20 Also, IL-10 is an important regulator; in animal studies, IL-4/IL-10 knock out mice die at the early acute stage of S. mansoni infection due to the development of severe intestinal and liver pathology.22 The helminth-induced regulatory macrophages may control Th1 cells functions through suppression of production of IFN-γ, the principal cytokine in the development of auto-immune diseases.26 This characteristic feature of Schistosome SEA to induce anti-inflammatory/regulatory properties in human monocytes/macrophages may help to suppress inflammation during autoimmune diseases27 and suggest that SEA, or mimics of the active components, may have a potential to inhibit inflammation in individuals with a high risk for the development of the auto-immune disease. Furthermore, large-scale study, which includes assessing monocyte/macrophage cells skewed towards a pro-inflammatory or anti-inflammatory direction, and Phagocytic and cytotoxicity activity of monocyte or macrophages recommended to consolidating findings of the present study.
Limitation of the Study
Due to the high prevalence of SM with other helminths together in the study site, the chance of getting only SM stool positive samples was less. Thus, it makes only SM stool positive samples very small in number. Moreover, the prevalence of DM in the area was scarce; so getting individuals having both SM and DM family members was very challenging.
Acknowledgment
This research was supported by a grant from CIS (VU University Amsterdam, the Netherlands). We thank the MCBI Department (VU University Medical Centre, Amsterdam, the Netherlands), for their hospitality to let MW perform part of the experiments in Amsterdam, and Drs Irma van Die, Christine Dijkstra and Gijs Kooijfor their help and valuable advice during the cell biological experiments. We greatly acknowledge the study participants, the volunteer blood donors, the support provided by Aklilu Lemma Institute of Pathobiology (ALIPB), and Armauer Hansen Research Institute (AHRI) (Ethiopia).
Author Contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
Reference
1. Berhe N, Myrvang B, Gundersen SG. Intensity of Schistosoma mansoni, hepatitis B, age, and sex predict levels of hepatic periportal thickening/fibrosis (PPT/F): a large-scale community-based study in Ethiopia. Am J Trop Med Hyg. 2007;77:1079–1086.
2. Wynn TA, Thompson RW, Cheever AW, Mentink-Kane MM. Immunopathogenesis of schistosomiasis. Immunol Rev. 2004;201:156–167. doi:10.1111/imr.2004.201.issue-1
3. van Liempt E, van Vliet SJ, Engering A, et al. Schistosoma mansoni soluble egg antigens are internalized by human dendritic cells through multiple C-type lectins and suppress TLR-induced dendritic cell activation. Mol Immunol. 2007;44:2605–2615.
4. Kuijk LM, Klaver EJ, Kooij G, et al. Soluble helminth products suppress clinical signs in murine experimental autoimmune encephalomyelitis and differentially modulate human dendritic cell activation. Mol Immunol. 2012;51:210–218.
5. Klaver EJ, Kuijk LM, Laan LC, et al. Trichuris suis-induced modulation of human dendritic cell function is glycan-mediated. Int J Parasitol. 2013;43:191–200.
6. Cros J, Cagnard N, Woollard K, et al. Human CD14dim monocytes patrol and sense nucleic acids and viruses via TLR7 and TLR8 receptors. Immunity. 2010;33:375–386.
7. Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969.
8. Murray PJ, Allen JE, Biswas SK, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41:14–20.
9. Kreider T, Anthony RM, Urban JF
10. Starr R, Willson TA, Viney EM, et al. A family of cytokine-inducible inhibitors of signalling. Nature. 1997;387:917–921.
11. Endo TA, Masuhara M, Yokouchi M, et al. A new protein containing an SH2 domain that inhibits JAK kinases. Nature. 1997;387(6636):921–924.
12. Naka T, Narazaki M, Hirata M, et al. Structure and function of a new STAT-induced STAT inhibitor. Nature. 1997;387(6636):924–929.
13. Nakagawa R, Naka T, Tsutsui H, et al. SOCS-1 participates in negative regulation of LPS responses. Immunity. 2002;17:677–687.
14. Olson WC, Smolkin ME, Farris EM, et al. Shipping blood to a central laboratory in multicenter clinical trials: effect of ambient temperature on specimen temperature, and effects of temperature on mononuclear cell yield, viability and immunologic function. J Transl Med. 2011;9:26.
15. Garcia-Vallejo JJ, van Liempt E, da Costa Martins P, et al. DC-SIGN mediates adhesion and rolling of dendritic cells on primary human umbilical vein endothelial cells through LewisY antigen expressed on ICAM-2. Mol Immunol. 2008;45:2359–2369.
16. Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327:656–661.
17. Ginhoux F, Jung S. Monocytes and macrophages: developmental pathways and tissue homeostasis. Nat Rev Immunol. 2014;14:392–404.
18. Yoshimura A, Suzuki M, Sakaguchi R, Hanada T, Yasukawa H. SOCS, inflammation, and autoimmunity. Front Immunol. 2012;3:20.
19. Whyte CS, Bishop ET, Ruckerl D, et al. Suppressor of cytokine signaling (SOCS)1 is a key determinant of differential macrophage activation and function. J Leukoc Biol. 2011;90:845–854.
20. Klaver EJ, Kuijk LM, Lindhorst TK, Cummings RD, Van Die I. Schistosoma mansoni soluble egg antigens induce expression of the negative regulators SOCS1 and SHP1 in human dendritic cells via interaction with the mannose receptor. PLoS One. 2015;10(4):e0124089.
21. Ng TH, Britton GJ, Hill EV, Verhagen J, Burton BR, Wraith DC. Regulation of adaptive immunity; the role of interleukin-10. Front Immunol. 2013;4:129.
22. Yasukawa H, Ohishi M, Mori H, et al. IL-6 induces an anti-inflammatory response in the absence of SOCS3 in macrophages. Nat Immunol. 2003;4:551–556.
23. Hartgers FC, Obeng BB, Kruize YC, et al. Lower expression of TLR2 and SOCS-3 is associated with Schistosoma haematobium infection and with lower risk for allergic reactivity in children living in a rural area in Ghana. PLoS Negl Trop Dis. 2008;2:e227.
24. Correale J, Farez MF. Parasite infections in multiple sclerosis modulate immune responses through a retinoic acid-dependent pathway. J Immunol. 2013;191:3827–3837.
25. Vogel DY, Glim JE, Stavenuiter AW, et al. Human macrophage polarization in vitro: maturation and activation methods compared. Immunobiology. 2014;219:695–703.
26. Shachar I, Karin N. The dual roles of inflammatory cytokines and chemokines in the regulation of autoimmune diseases and their clinical implications. J Leukoc Biol. 2013;93:51–61.
27. Raz I, Eldor R, Naparstek Y. Immune modulation for prevention of type 1 diabetes mellitus. Trends Biotechnol. 2005;23:128–134.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.