Back to Journals » Research and Reports in Tropical Medicine » Volume 11
Human Fascioliasis: Current Epidemiological Status and Strategies for Diagnosis, Treatment, and Control
Authors Caravedo MA, Cabada MM
Received 21 September 2020
Accepted for publication 12 November 2020
Published 26 November 2020 Volume 2020:11 Pages 149—158
DOI https://doi.org/10.2147/RRTM.S237461
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Mario Rodriguez-Perez
Maria Alejandra Caravedo,1 Miguel Mauricio Cabada1,2
1Division of Infectious Diseases Department of Internal Medicine, University of Texas Medical Branch, Galveston, TX, USA; 2Cusco Branch – Tropical Medicine Institute, Universidad Peruana Cayetano Heredia, Cusco, Peru
Correspondence: Miguel Mauricio Cabada
Division of Infectious Disease, Department of Internal Medicine, University of Texas Medical Branch, 301 University Boulevard RT 0435, Galveston, TX 77555, USA
Tel +1 409 747 0223
Email [email protected]
Purpose of the Review: This review aims to critically assess current knowledge about the epidemiology, diagnosis, and management of Fasciola infection in humans.
Recent Findings: Fascioliasis is an emerging neglected zoonotic infection affecting the health and wellbeing of human populations. The burden of infection is unclear, and studies have shown the geographic expansion of fascioliasis in human and livestock likely related to climate change. The infection can be asymptomatic or present in acute or chronic forms. Regardless of the presentation, fascioliasis can be associated with long-term complications such as anemia and malnutrition. Early in the infection, antibody testing is the only tool available for diagnosis confirmation. In the chronic forms serology and stool microscopy are helpful. Other tests such as antigen detection and PCR-based methods including isothermal tests have shown promising results. Triclabendazole is the only drug available to treat Fasciola infection. However, reports of resistant infections in livestock and human threaten the clinical care and control of the infection in endemic areas.
Summary: Fascioliasis is an emerging infection around the world with an uncertain burden. Lack of standardization of diagnostic testing and treatment alternatives hinder treatment and control of the infection.
Keywords: Fasciola hepatica, Fasciola gigantica, diagnosis, treatment, epidemiology, triclabendazole
Introduction
Fascioliasis is a foodborne trematode infection with a worldwide distribution. Fasciola infection has been reported in 81 countries.1 Fascioliasis is considered a neglected zoonotic disease by the World Health Organization. Two accepted species, Fasciola hepatica and Fasciola gigantica, infect a wide range of mammals including livestock and humans. The current burden of human infection is not well known. The lack of supporting information from large epidemiologic studies and the particularities of the distribution of fascioliasis significantly hinder the estimation of the number of infected people and disease burden. It was estimated that between 2.4 million and 17 million people were infected around the world.2,3 However, these estimates date back more than 25 years and the data used for their calculation are not provided. The more recent estimate of 2.6 million infections by Fürst et al was based on expert opinion.4 Given the emerging character of fascioliasis and reports of its expanding endemic areas,5–8 it is likely that the number of infections around the world surpasses previous estimates.
Fasciola has a complex lifecycle that involves intermediate snail and definitive mammal hosts, including humans. Eggs shed in the stool of the definitive host embryonate in fresh water releasing a single miracidium. The miracidium penetrates the tegument of the snail intermediate host to cause infection. Susceptible snails belong to the Lymnaeidae family. There are 30 snail species shown to be competent hosts in nature or in vitro but with different transmission efficiencies.9 Snails from the Galba and Radix genus, particularly Galba truncatula, are responsible for most of Fasciola transmission around the world.9 The distributions of Fasciola risk and infection follow the distribution of the intermediate host which is key to sustain the parasite in endemic areas.10 Importantly, the snail infection amplifies Fasciola production by generating large numbers of cercariae from a single miracidium. Free swimming cercariae disperse a few meters away from the snail host usually following water currents. These encyst into metacercariae that stay in the water or attach to aquatic plants.11 The mammal definitive host acquires the infection by ingesting metacercariae in water or leafy vegetables that grow near water. After ingestion, metacercariae excyst in the intestine and release the immature parasites that migrate through the wall of the intestine, the abdominal cavity, and the liver parenchyma to reach the biliary tree. Once in the bile ducts, the parasites mature and start producing eggs.
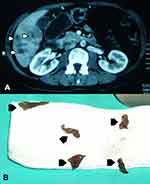
The migratory phase can last more than 12 weeks and causes acute symptoms characterized by fever and right upper quadrant pain associated with high eosinophil counts and hypodense track like lesions on liver imaging studies (Figure 1). Sexually mature parasites established in the bile ducts cause the chronic phase of the infection that can last more than 10 years and is characterized by inflammation of the biliary tree and obstructive symptoms such as intermittent abdominal pain and jaundice. Importantly, at the community level in endemic areas, both phases can have an indolent course with no or mild symptoms that fail to prompt a diagnostic workup.12 Thus, patients can remain undiagnosed for prolonged periods of time causing adverse health impacts. Ectopic infections involving the subcutaneous tissue and other organs inside and outside the abdomen are reported and have been summarized by Taghipour et al.13 However, their prevalence has not been established in endemic areas and are not common at the community level.
Human infection with Fasciola has been associated with short- and long-term complications. During the acute phase, patients may develop an incapacitating prolonged febrile illness that may remain undiagnosed for weeks even in endemic areas.14,15 The migrating parasites may erode into blood vessels causing large and sometimes life-threatening subcapsular liver hematomas.15–17 In the acute and chronic phases, patients can present with cholangitis or liver abscesses associated with bacteria carried by the parasites or obstruction of bile flow.16 The association of chronic fascioliasis with liver fibrosis, cirrhosis and biliary tree disease has been reported in the literature and studied in animals but has not been characterized in humans.18 Most importantly, patients with asymptomatic, acute, or chronic fascioliasis can experience significant weight loss and anemia.14–16,19 Young children are especially vulnerable to the devastating long-term complications associated with undernutrition and anemia such as stunting and poor neurocognitive development.
Fascioliasis has a large impact on the livestock industry. It is estimated that 10–80% of dairy and meat cattle herds are infected in developed and developing countries.20–23 The global economic burden to the industry was estimated at USD 3 billion per year in 1994.24 More recent estimates from developed countries take into account losses in meat, milk, and wool production, as well as, treatment costs suggesting a much higher burden. Charlier et al estimated the costs associated with helminth infections in the ruminant livestock industry from 18 countries in the European Union. In this study, fascioliasis caused losses of USD 750 million a year, with the largest impact on the dairy and meat cattle industries.25 The estimated cost of nematode resistance to medications accounted for an additional USD 45 million, but no estimate was provided for resistance in Fasciola. Triclabendazole resistance is a significant problem in some European, Latin American and African countries that may increase the economic burden of fascioliasis.26 In Germany, the breakdown of the median annual costs associated with fascioliasis in each farm included treatment of cattle (USD 100), decreased efficiency of calf production (USD 2100), and decreased milk production (USD 735).27 In developing countries, the burden of fascioliasis in livestock is less well studied. Small scale farming in rural communities poses challenges to perform accurate estimates. Studies in slaughterhouses provide a rough estimate of the infection and economic burdens. A study in a single slaughterhouse from the Andes Mountains of Peru reported a 55% prevalence of fascioliasis in cattle causing an estimated annual loss of USD 400,000 from liver condemnation and decreased carcass weight.28 Similar studies in Ethiopia and Cameroon showed a lower Fasciola prevalence with estimated losses from liver condemnations of USD 43,000 and USD 76,000 per year, respectively.29,30 In these settings, Fasciola not only inflicts an economic burden on small-scale producers but also threatens food security among subsistence farmers that raise livestock to feed their families.
This manuscript aims to provide an updated critical review of recent data published on Fasciola epidemiology, diagnosis, treatment, and control. In addition, the authors provide some insights regarding knowledge gaps and the management of fascioliasis stemming from ongoing research studies in the Cusco region of Peru.
Epidemiology
Fascioliasis is a global emerging infection. Influences from climate change may increase rainfall in some areas and decrease freezing temperatures in others, creating new habitats where Fasciola and its hosts can emerge. Man-made modifications of the environment such as new irrigation projects may also increase the availability of suitable habitats. Migration, globalization, and import/export of livestock can introduce the parasites and its vectors to new regions. All these factors together can potentially cause the expansion of endemic areas and increase the prevalence of Fasciola in livestock and humans.31–35 Populations in developing countries will likely be the most affected while their health systems are impacted by the COVID 19 pandemic and Fasciola control efforts decline. In addition, sporadic cases of Fasciola infection in developed countries, travelers, expatriates, and migrants will likely be seen more often.
Fascioliasis predominantly affects human populations living in poverty.36 Small rural communities in the Andes Mountains of Bolivia, Ecuador, and Peru bear a significant part of the world’s infection burden.1,4,37 The adaptation of Fasciola and the intermediate snail host to the Andean high altitude environment is remarkable. Some of the highest prevalence of human infection in the world have been documented above 12,500 ft around Lake Titicaca in the Bolivian-Peruvian Altiplano.38 Other important endemic countries include Egypt, Turkey, Iran, China, and Vietnam. There are reports of emerging and reemerging fascioliasis in countries of Africa, Asia, and the Middle East.5,6,39 Some European countries such as Portugal, Spain, and France still report locally acquired human fascioliasis.40,41 Infection is uncommon in North America where most of the reported cases are diagnosed in travelers and immigrants.42–46 Reports of fascioliasis emerging in new areas within endemic countries suggest that significant work is still needed to describe the distribution and burden of the infection.47,48
An important characteristic of Fasciola’s epidemiology is its patchy geographic distribution. The prevalence of Fasciola infection can vary widely within small geographic areas.49–51 Esteban et al studied 2700 subjects in 24 communities located in a small area between La Paz and Lake Titicaca in the Bolivian Altiplano and reported rates of infection varying between 0% and 68%.52 Similarly, Cabada et al studied 2500 children in 26 neighboring communities of the Anta province in Peru and found infection rates between 0% and 20%.36 This variability is explained in part by the spatial distribution of the snail hosts and their habitats.53 In addition, proximity between the snail host and livestock is crucial to maintain Fasciola’s lifecycle. In the Bolivian Altiplano, where infections rates can exceed 50% in human and livestock, there is a spatial correlation between animal and human infection.38 However, studies in areas with lower prevalence may fail to show a correlation between livestock and human infection rates.54–56
The distribution of fascioliasis by sex shows variable results. A large study by Parkinson et al in the Bolivian Altiplano involving almost 8000 subjects of all ages failed to find a significant association with sex.49 However, studies in other areas have suggested a higher prevalence in females especially among children.57 Curtale et al in a study including over 21,000 children in Egypt reported that females had a significantly higher prevalence of fascioliasis and passed more eggs in the stool than males.58
School-age children are disproportionately affected by Fasciola infection in highly endemic countries. The highest chronic infection rate documented was 70% in the 8–11 years age group in the Bolivian Altiplano.49 An epidemiologic study in the highlands of Cusco in Peru showed that older age of the child was an independent risk factor for fascioliasis with infected children having a mean age of 11 years.36 De van et al recently reported a case series of Fasciola infection among infants and toddlers in Vietnam.59 This report documented acute and chronic fascioliasis in 5 very young children and reviewed 38 additional cases of children under 4-years old reported in the literature between the years 1856 and 2016.59 The same group published a report on the seroprevalence of fascioliasis among 1120 patients referred to Hanoi Medical University Hospital. The seropositivity was significantly lower in the group between 5 and 15 years compared to the other age groups (4.9% vs >8.9%, p < 0.05).60 In the Cusco region of Peru, we identified 253 children ages 3 to 16 years old with positive serology and/or stool microscopy for fascioliasis among 2513 children studied. The prevalence on Fasciola infection (6.1% versus 10.6%, p = 0.017), chronic fascioliasis (2.5% versus 6.5%, p = 0.02), and positive serology (5.1% versus 8.8%, p = 0.03) were significantly lower among children under 5 years compared to children 5 years and older.36 Although these data suggest that infection in young children is less common than in older age groups, children under 5 years old are especially vulnerable to Fasciola complications such as chronic anemia and malnutrition and must be included in epidemiologic studies and control programs.
Dietary habits are important factors associated with an increased risk of Fasciola infection. The consumption of wild watercress that grows near livestock grazing areas is reported in most cases of sporadic transmission.15,40,41,43,44,61 However, other leafy vegetables and vegetables that grow at the ground level such as radish have also been associated with transmission.61,62 Drinking untreated water has been associated with a higher likelihood of Fasciola infection in several studies from the highlands of Peru.21,62 Drinks prepared with contaminated water or raw vegetables may lead to inadvertent transmission.
Diagnosis
The complex pathophysiology of fascioliasis may create some diagnostic challenges for clinicians. The presence of risk factors such as exposure to endemic areas, raw water plants, or untreated water may raise the clinical suspicion.63 The signs and symptoms of fascioliasis are not specific but certain combinations of these may suggest the infection. Subacute onset of fever and right upper quadrant pain with a history of exposure should make the clinician suspect fascioliasis. Eosinophilia, especially when over 1000 cells/μL with or without the symptoms above, should raise concerns for tissue invasive parasite infections including Fasciola. Intermittent long-standing right upper quadrant pain, anemia, and/or stunting in children from endemic areas should also make the clinician think about Fasciola infection.
To confirm the diagnosis of fascioliasis, it is important to differentiate the tissue from the biliary phase of the infection. During tissue migration, the parasites do not produce eggs and diagnosis relies on antibody detection. Antibodies against Fasciola may take 2 to 4 weeks to become detectable after the initial infection and should remain positive through the chronic phase. Once the biliary tree is reached, parasites start producing eggs that can be detected in stool. Of note, parasite egg output may not be consistent and the distribution of eggs within the stool sample is not uniform. This makes testing several parts of the sample or several samples necessary to increase the diagnostic yield of microscopy. Similar considerations are necessary when obtaining the stool aliquot for molecular tests.
There is no gold standard test to diagnose fascioliasis. Stool microscopy tests are probably the most used in endemic countries. Finding eggs in the stool provides diagnostic certainty. However, most microscopy techniques have a low sensitivity and require repeated testing and egg concentration. The sensitivity is further reduced when egg counts are low which may be observed in long-term infections, treatment failure, or infections with hybrid parasites (F. hepatica/F. gigantica).64,65 The Kato Katz test is a quantitative microscopy test recommended by WHO in areas with a high prevalence and intensity of infection.66 The Kato Katz test alone may miss a third of the infections and combining it with methods that concentrate eggs provides a better sensitivity. Sedimentation tests such as spontaneous sedimentation and Lumbreras rapid sedimentation have higher sensitivity than Kato Katz.67 These methods require less training and have a lower cost than ethyl acetate or formalin-ether-based concentration methods. Device-based concentration techniques achieve the highest sensitivity but are expensive, difficult to use in large surveys, and not widely available. The FLOTAC and mini-FLOTAC devices (University of Naples, Naples, Italy) combined with flotation solutions with specific gravities adjusted for Fasciola eggs are considered more sensitive than direct smear, Kato Katz, formalin-ether, and simple flotation techniques.68 The Flukefinder® (Soda Springs, ID) device consists of a series of sieves to concentrate fluke eggs and is commercialized in the veterinary market. Flukefinder® demonstrated a higher overall sensitivity (90%) than mini-FLOTAC (67.5%) and Kato Katz tests (32.5%) in human stool spiked with different numbers of Fasciola eggs.64
Antibody testing by ELISA is a widely used diagnostic tool in fascioliasis especially helpful during the acute phase. Most commercially available tests are based on antigens from the excretory/secretory products or tegument of F. hepatica.69,70 The sensitivity and specificity of most of the modern ELISA tests are higher than 90% when used in serum from selected subjects passing Fasciola egg in the stool.70 However, the diagnostic performance of these tests in field conditions in endemic areas has seldom been evaluated. Some manufacturers of commercial ELISA kits still rely on parasite produced crude antigens which hinders the specificity, batch to batch reliability, and production scalability.69 The availability of commercial Fasciola serologic testing is an issue in developed and developing countries. In the United States, the Center for Diseases Control and Prevention offers an immunoblot test based on a recombinant Fasciola antigen (FhSAP2) with a reported sensitivity and specificity of 94% and 98%, respectively.71 Antibody testing is limited by the poor predictive value to differentiate past from current infection and to evaluate treatment response even after the infection has been eradicated. An IgM ELISA based on the Fas2 antigen was studied as a potential test to differentiate between acute and chronic infection. This study had a limited number of samples and found a sensitivity of 43% and specificity of 100%.72 Further evaluation of the Fas2 IgM ELISA targeting subjects with acute or recent infection may provide a useful diagnostic tool in the future.
Stool antigen ELISA tests for the detection of excretory/secretory products can detect the infection weeks before microscopy. The copro-antigen ELISA has a sensitivity of 94% and specificity of up to 98%. The Fasciola MM3-Coproantigen ELISA is a highly sensitive test commercialized in veterinary medicine that can detect very low burdens of infection (one parasite) in cattle.73
Detection of Fasciola DNA in stool samples using polymerase chain reaction (PCR) tests have an overall high sensitivity and specificity. Some PCR tests can detect Fasciola DNA weeks before eggs are apparent in the stool. However, some authors have reported inconsistent results. Arifin et al compared a conventional PCR test and a loop-mediated isothermal amplification (LAMP) test with stool microscopy and serology to diagnose fascioliasis in human.74 Despite their very good analytical sensitivity, the PCR and LAMP tests had a sensitivity of 10% and 18%, respectively.74 These inconsistencies between studies may be related to differences in DNA extraction protocols which in the case of the hardy Fasciola eggs is a critical step.75
The lack of training and laboratory capacity in endemic areas where molecular testing is most needed is a significant barrier for implementation. In these settings, the ideal diagnostic method should be easily deployable to areas without much laboratory capacity, require minimal training of personnel, and have a high accuracy. Several PCR isothermal DNA detection techniques that do not require a thermocycler or other expensive equipment have been tested for Fascioliasis. Recombinase polymerase amplification (RPA) has a high potential for field deployment in developing countries. Cabada et al in a study testing a limited number of stool samples reported that a single RPA had a sensitivity of 88% and specificity of 100% when tested in stool samples of children with and without fascioliasis and with other parasites. In addition, the RPA was able to detect DNA in 47% of samples from patients with fascioliasis and negative microscopy testing.76 Ghodsian et al reported on LAMP tests with a limit of detection of five eggs per gram of stool using spiked stool samples.77 Despite the promising results of these isothermal techniques, none of these tests have been evaluated in field conditions and most of these studies do not consider issues related to sample preparation and manipulation as a potential source for false negative and false-positive results, respectively.
Treatment and Control
Patients with fascioliasis can present during the acute or chronic phase of the infection. Treatment is indicated to shorten the duration of symptoms and prevent complications. Asymptomatic or mildly symptomatic subjects in the community should also receive treatment to prevent the potential chronic effects of the infection, especially in children. Triclabendazole is the only drug recommended for the treatment of acute and chronic human fascioliasis.66 This is a benzimidazole drug that interferes with the parasite’s β-tubulin polymerization balance like other drugs in the same class, but the exact mechanism of action is still to be fully elucidated.78 Triclabendazole causes marked disruption of the tegument and inhibition of protein synthesis in Fasciola.78 The spectrum of activity of triclabendazole is narrow with indications for fascioliasis and paragonimiasis only. The WHO recommends treatment with one or two doses at 10 mg/kg per dose separated for 12 to 24 hours. The US Food and Drug Administration approved a two-dose regimen for the treatment of acute and chronic fascioliasis in human 6 years and older in 2019.79 Triclabendazole should be administered with food as this increases plasma concentration 2 to 3 fold. It is considered a safe drug based on decades of experience using it around the world and a limited set of safety studies. Drug toxicity evaluation in dogs found the potential for QTc interval prolongation at high doses. The package insert suggests caution in human known to have or taking other medications that prolong the QTc interval.79
Nitazoxanide has been proposed as an alternative medication for the treatment of Fasciola infection. Two small clinical trials showed an efficacy between 40% and 60%.80 However, the results in observational studies are inconsistent. Zumaquero-Rios et al reported 94% efficacy among children treated in Mexico.81 In Egypt, Ramadan et al prescribe nitazoxanide to 30 subjects with acute fascioliasis and reported an efficacy of 36%.82 Our group in Cusco has used nitazoxanide in subjects with acute and chronic fascioliasis that failed triclabendazole with no success.83 In light of the conflicting evidence about the effectiveness of nitazoxanide a recommendation about its use cannot be made. Antiparasitic drugs used to treat other trematode parasites such as praziquantel are not effective against Fasciola infection.
Subjects with fascioliasis should have a test of cure after treatment. In acute fascioliasis, symptoms and eosinophilia usually start improving within a few days after treatment, but the latter can take several weeks to return to normal. If symptoms of the acute infection resolve, stool microscopy testing for Fasciola eggs should be considered 1 or 2 months after treatment. The resolution of symptoms in some of those patients could be related to the development of chronic infection. Similarly, patients with chronic infection should have multiple stool samples examined by microscopy and using an egg concentration method to ascertain cure between 1 and 2 months after treatment. The role of serology in the clinical follow up of subjects with acute and chronic fascioliasis is unclear and may depend on the method. Although ELISA titers of available tests may decrease over time after successful treatment, the clinically relevant time and level of titer reduction have not been established. In addition, the proportion of subjects that revert to negative ELISA over time has not been well described.84 The family members of patients diagnosed with fascioliasis in endemic areas should be screened for the infection using stool microscopy, as cases tend to cluster in families.57,85
The control of fascioliasis in endemic countries may focus on some of the critical points in the lifecycle of Fasciola. Snail control strategies could significantly decrease transmission. However, the use of molluscicides causes only a transient reduction in snail populations and affects many other bystander species. Draining of water bodies may have similar effects on other living organisms and is difficult to perform in endemic areas.86 Treatment of livestock has been a major control strategy applied widely in developed and developing countries. Several drugs such as triclabendazole, albendazole, clorsulon, and closantel are available for treatment in animals. However, triclabendazole is one of the most widely used flukicides due to its high efficacy and effects on very young and adult parasites. Importantly, sheep exhibit high morbidity and mortality in acute infection and triclabendazole is the only alternative to prevent losses caused in early disease.87 However, this strategy is likely associated with the significant reduction in triclabendazole effectiveness in livestock around the world and should be substituted by drug replacement and stewardship programs to target infected and vulnerable animals more specifically.
The WHO promotes mass drug administration to decrease the prevalence of fascioliasis in human populations in high burden countries. Bolivia, Egypt, Peru, and Vietnam have tried different approaches to control in human populations.66 These included diagnose and treat, school-based administration, and mass drug administration programs that for the most part are inactive now.66,88 Egypt launched a screen and treat program in schools and villages in endemic areas that decreased the prevalence of fascioliasis from 6% to 1% in 7 years. In Peru, initial efforts for a school-based mass drug administration program was piloted in the Northern highlands but was not fully implemented in the rest of the country.66 Bolivia applied a mass drug administration program that consistently provided yearly triclabendazole doses to populations living in hyperendemic areas near Lake Titicaca. This program treated all the population for more than 10 years with a fixed 250 mg dose independently of age or weight.89 A recent study by Mollinedo et al documented a reduction in Fasciola prevalence to <1% compared with 12–27% from historical data in the area where the program was implemented.89 This experience has not been replicated in other areas and further evaluation of its effectiveness and sustainability are probably warranted.
Depending on a single drug for treatment and control of fascioliasis is problematic. Overreliance on triclabendazole originated the emergence of triclabendazole resistance in F. hepatica and, less often, F. gigantica. Cattle and sheep infections with triclabendazole resistance parasites have been reported in more than 17 endemic countries around the world.87 Inconsistent farming practices, lack of quality control in veterinary products, and underdosing of triclabendazole are likely associated with the emergence of resistance in developing countries.90 A detailed review of resistant infections in livestock has been recently published by Fairweather et al and the readers are referred to that publication for additional details.87 Importantly, triclabendazole resistance in human with fascioliasis is also emerging and is likely associated with resistance in livestock. Case series from Chile,91 The Netherlands,92 Peru,83 Portugal,93 and Turkey94 have documented treatment failures despite multiple doses of triclabendazole. Our group documented treatment failures after multiple courses of treatment in 7/19 (37%) patients referred to our center for treatment.83 Ramadan et al reported on 67 Egyptian patients with acute fascioliasis treated with a single triclabendazole dose. Only 37 (55%) of them responded to the first round of treatment.82 Our group studied a series of 146 children with chronic fascioliasis in Peru treated with triclabendazole. In this study, only 38% of the children that failed one round of triclabendazole treatment cured after a second round. (Cabada personal communication)
Efforts are being made to develop an effective vaccine for livestock. Some of the candidate antigens include FhCL1, FhCL2, FhPrx, FhLAP and FhHDM.95 FhLAP is one of the better studied antigens inducing protection in between 83% and 90% of livestock.96–98 However, no vaccine is now commercially available and further research is needed to achieve better and sustained responses.
Conclusions
Fascioliasis is a neglected zoonotic infection with a global distribution lacking a precise estimation of infection and disease burden. Fascioliasis in the asymptomatic, acute, and chronic forms is associated with short- and long-term impacts on human health. The complex pathophysiology of Fasciola infection in human may pose challenges for the diagnosis and management of the disease. However, once diagnosed, all the forms of the infection warrant treatment. The only drug recommended for treatment and control of fascioliasis in humans is triclabendazole. The emerging Fasciola resistance to triclabendazole in livestock and human constitutes a threat to the management of infected patients and the control in affected human populations. Further research is needed to understand the relationships of susceptible and resistant parasites with the intermediate, livestock, and human hosts. In addition, research on alternative drugs to treat human infection and/or overcome triclabendazole resistance is urgently needed.
Disclosure
The authors report no conflicts of interest for this work.
References
1. Fürst T, Duthaler U, Sripa B, Utzinger J, Keiser J. Trematode infections. liver and lung flukes. Infect Dis Clin North Am. 2012;26(2):399–419. doi:10.1016/j.idc.2012.03.008
2. Rim H-J, Farag HF, Sornmani S, Cross JH. Food-borne trematodes: ignored or emerging? Parasitol Today. 1994;10(6):207–209. doi:10.1016/0169-4758(94)90111-2
3. Hopkins DR. Homing in on helminths. Am J Trop Med Hyg. 1992;46(6):626–634. doi:10.4269/ajtmh.1992.46.626
4. Fürst T, Keiser J, Utzinger J. Global burden of human food-borne trematodiasis: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12(3):210–221. doi:10.1016/S1473-3099(11)70294-8
5. Qureshi AW, Zeb A, Mansoor A, Hayat A, Mas-Coma S. Fasciola hepatica infection in children actively detected in a survey in rural areas of Mardan district, Khyber Pakhtunkhawa province, northern Pakistan. Parasitol Int. 2019;69:39–46. doi:10.1016/j.parint.2018.11.003
6. Sah R, Khadka S, Khadka M, et al. Human fascioliasis by Fasciola hepatica: the first case report in Nepal. BMC. 2017;10(1):10–13. doi:10.1186/s13104-017-2761-z
7. Outa JO, Sattmann H, Köhsler M, Walochnik J, Jirsa F. Diversity of digenean trematode larvae in snails from Lake Victoria, Kenya: first reports and bioindicative aspects. Acta Trop. 2020;206:105437. doi:10.1016/j.actatropica.2020.105437
8. Carolus H, Muzarabani KC, Hammoud C, et al. A cascade of biological invasions and parasite spillback in man-made Lake Kariba. Sci Total Environ. 2019;659:1283–1292. doi:10.1016/j.scitotenv.2018.12.307
9. Vázquez Perera A, Alda P, Lounnas M, et al. Lymnaeid snails hosts of Fasciola hepatica and Fasciola gigantica (Trematoda: digenea): a worldwide review. CAB Rev Perspect Agric Vet Sci Nutr Nat Resour. 2018;13:1–15. doi:10.1079/PAVSNNR201813062
10. Rondelaud D, Hourdin P, Vignoles P, Dreyfuss G. The contamination of wild watercress with Fasciola hepatica in central France depends on the ability of several lymnaeid snails to migrate upstream towards the beds. Parasitol Res. 2005;95(5):305–309. doi:10.1007/s00436-004-1283-5
11. Rondelaud D, Vignoles P, Dreyfuss G. Fasciola hepatica: the dispersal of cercariae shed by the snail Galba truncatula. Parasite. 2020;27:17. doi:10.1051/parasite/2020013
12. Moazeni M, Ahmadi A. Controversial aspects of the life cycle of Fasciola hepatica. Exp Parasitol. 2016;169:81–89. doi:10.1016/j.exppara.2016.07.010
13. Taghipour A, Zaki L, Rostami A, et al. Highlights of human ectopic fascioliasis: a systematic review. Infect Dis. 2019;51(11–12):785–792. doi:10.1080/23744235.2019.1663362
14. Marcos LA, Maco V, Castillo M, Terashima A, Zerpa R, Gotuzzo E. Report of cases of human fascioliosis in the specialized Children’s Health Institute, Lima, Peru (1988ʹ–2003). Rev Gastroenterol Peru. 2005;25(2):198–205.
15. Torres GB, Iwashita AT, Vargas CM, Luján LV, Bianchi HA, Casanova RT. Human fasciolasis and gastrointestinal compromise: study of 277 patients in the Cayetano Heredia National Hospital (1970–2002). Rev Gastroenterol Peru. 2004;24(2):143–157.
16. Chang Wong MR, Pinto Elera JOA, Guzman Rojas P, Terashima Iwashita A, Samalvides Cuba F. Demographic and clinical aspects of hepatic fascioliasis between 2013–2010 in National Hospital Cayetano Heredia, Lima, Peru. Rev Gastroenterol Peru. 2016;36(1):23–28.
17. Krsak M, Patel NU, Poeschla EM. Case report: hepatic fascioliasis in a young afghani woman with severe wheezing, high-grade peripheral Eosinophilia, and liver lesions: a brief literature review. Am J Trop Med Hyg. 2019;100(3):588–590. doi:10.4269/ajtmh.18-0625
18. Machicado C, Machicado JD, Maco V, Terashima A, Marcos LA, Garcia HH. Association of Fasciola hepatica infection with liver fibrosis, cirrhosis, and cancer: a systematic review. PLoS Negl Trop Dis. 2016;10(9):1–11. doi:10.1371/journal.pntd.0004962
19. Lopez M, White AC, Cabada MM. Burden of Fasciola hepatica infection among children from Paucartambo in Cusco, Peru. Am J Trop Med Hyg. 2012;86(3):481–485. doi:10.4269/ajtmh.2012.11-0448
20. May K, Bohlsen E, König S, Strube C. Fasciola hepatica seroprevalence in Northern German dairy herds and associations with milk production parameters and milk ketone bodies. Vet Parasitol. 2020;277:109016. doi:10.1016/j.vetpar.2019.109016
21. Barbosa R, Pinto C, Garcia P, Rodrigues A. Prevalence of fasciolosis in slaughtered dairy cattle from São Miguel Island, Azores, Portugal. Vet Parasitol. 2019;17:100319. doi:10.1016/j.vprsr.2019.100319
22. Rinca KF, Prastowo J, Widodo DP, Nugraheni YR. Trematodiasis occurrence in cattle along the Progo River, Yogyakarta, Indonesia. Vet World. 2019;12(4):593–597. doi:10.14202/vetworld.2019.593-597
23. Arenal A, García Y, Quesada L, et al. Risk factors for the presence of Fasciola hepatica antibodies in bulk-milk samples and their association with milk production decreases, in Cuban dairy cattle. BMC. 2018;14(1):336. doi:10.1186/s12917-018-1654-2
24. Mehmood K, Zhang H, Sabir AJ, et al. A review on epidemiology, global prevalence and economical losses of fasciolosis in ruminants. Microb Pathog. 2017;109:253–262. doi:10.1016/j.micpath.2017.06.006
25. Charlier J, Rinaldi L, Musella V, et al. Initial assessment of the economic burden of major parasitic helminth infections to the ruminant livestock industry in Europe. Prev Vet Med. 2020;182:105103. doi:10.1016/j.prevetmed.2020.105103
26. Gandhi P, Schmitt EK, Chen CW, Samantray S, Venishetty VK, Hughes D. Triclabendazole in the treatment of human fascioliasis: a review. Trans R Soc Trop Med Hyg. 2019;113(12):797–804. doi:10.1093/trstmh/trz093
27. Fanke J, Charlier J, Steppin T, von Samson-himmelstjerna G, Vercruysse J, Demeler J. Economic assessment of Ostertagia ostertagi and Fasciola hepatica infections in dairy cattle herds in Germany using Paracalc(®). Vet Parasitol. 2017;240:39–48. doi:10.1016/j.vetpar.2017.03.018
28. Arias-Pacheco C, Lucas JR, Rodríguez A, Córdoba D, Lux-Hoppe EG. Economic impact of the liver condemnation of cattle infected with Fasciola hepatica in the Peruvian Andes. Trop Anim Health Prod. 2020;52(4):1927–1932. doi:10.1007/s11250-020-02211-y
29. Zewde A, Bayu Y, Wondimu A. Prevalence of bovine fasciolosis and its economic loss due to liver condemnation at Wolaita Sodo Municipal Abattoir, Ethiopia. Vet Med Int. 2019;2019:9572373. doi:10.1155/2019/9572373
30. Takang EE, LeBreton M, Ayuk CE, MacLeod ET. A socio-economic study of Fasciola infections in cattle and sheep at the Etoudi slaughterhouse, Yaoundé, Cameroon. J Helminthol. 2019;94:e92. doi:10.1017/S0022149X19000890
31. Beesley NJ, Caminade C, Charlier J, et al. Fasciola and fasciolosis in ruminants in Europe: identifying research needs. Transbound Emerg Dis. 2018;65(April 2017):199–216. doi:10.1111/tbed.12682
32. Haydock LAJJ, Pomroy WE, Stevenson MA, Lawrence KE. A growing degree-day model for determination of Fasciola hepatica infection risk in New Zealand with future predictions using climate change models. Vet Parasitol. 2016;228:52–59. doi:10.1016/j.vetpar.2016.05.033
33. Sabourin E, Alda P, Vázquez A, Hurtrez-Boussès S, Vittecoq M. Impact of human activities on fasciolosis transmission. Trends Parasitol. 2018;34(10):891–903. doi:10.1016/j.pt.2018.08.004
34. Charlier J, Ghebretinsae AH, Levecke B, Ducheyne E, Claerebout E, Vercruysse J. Climate-driven longitudinal trends in pasture-borne helminth infections of dairy cattle. Int J Parasitol. 2016;46(13–14):881–888. doi:10.1016/j.ijpara.2016.09.001
35. Pozio E. How globalization and climate change could affect foodborne parasites. Exp Parasitol. 2020;208(August):107807. doi:10.1016/j.exppara.2019.107807
36. Cabada MM, Morales ML, Webb CM, et al. Socioeconomic factors associated with Fasciola hepatica infection among children from 26 communities of the cusco region of Peru. Am J Trop Med Hyg. 2018;99(5):1180–1185. doi:10.4269/ajtmh.18-0372
37. Carmona C, Tort JF. Fasciolosis in South America: epidemiology and control challenges. J Helminthol. 2017;91(2):99–109. doi:10.1017/S0022149X16000560
38. Parkinson M, O’neill SM, Dalton JP. Controlling fasciolosis in the bolivian altiplano. Trends Parasitol. 2007;23(6):238–239. doi:10.1016/j.pt.2007.04.002
39. Zoghi S, Emami M, Shahriarirad S, et al. Human fascioliasis in nomads: a population-based serosurvey in southwest Iran. Infez Med. 2019;27(1):68–72.
40. Temido H, Oliveira-Santos M, Parente F, Santos L. Fascioliasis—a rare cause of hepatic nodules. BMJ Case Rep. 2017;
41. Remacha MA, Goñi MP, Espinel J. Obstructive jaundice of a parasitic etiology. Rev Esp Enferm Dig. 2019;111(2):165–166. doi:10.17235/reed.2018.5827/2018
42. Fried B, Abruzzi A. Food-borne trematode infections of humans in the United States of America. Parasitol Res. 2010;106(6):1263–1280. doi:10.1007/s00436-010-1807-0
43. Micic D, Oto A, Charlton MR, Benoit J-L, Siegler M, Solomon CG. Hiding in the water. N Engl J Med. 2020;382(19):1844–1849. doi:10.1056/NEJMcps1902741
44. Kain D, Mukkala AN, Boggild AK. Prolonged antibiotic use leading to clostridium difficile colitis in an ill returned traveller with acute fascioliasis. J Travel Med. 2018;25(1):1. doi:10.1093/jtm/tay012
45. Kwok J, Buxbaum JL. Liver fluke. N Engl J Med. 2019;381(19):e34. doi:10.1056/NEJMicm1903220
46. Weisenberg SA, Perlada DE. Case report: domestically acquired fascioliasis in northern california. Am J Trop Med Hyg. 2013;89(3):588–591. doi:10.4269/ajtmh.13-0069
47. Badirzadeh A, Sabzevari S. Hepatic fascioliasis in Mashhad, Northeast Iran: first report. Rev Soc Bras Med Trop. 2017;50(4):571–574. doi:10.1590/0037-8682-0526-2016
48. Cabada MM, Castellanos-Gonzalez A, Lopez M, Caravedo MA, Arque E, White AC. Fasciola hepatica infection in an indigenous community of the peruvian jungle. Am J Trop Med Hyg. 2016;94(6):1309–1312. doi:10.4269/ajtmh.15-0769
49. Parkinson M, O’Neill SM, Dalton JP. Endemic human fasciolosis in the bolivian altiplano. Epidemiol Infect. 2007;135(4):669–674. doi:10.1017/S095026880600728X
50. Cabada MM, Goodrich MR, Graham B, et al. Fascioliasis and eosinophilia in the highlands of Cuzco, Peru and their association with water and socioeconomic factors. Am J Trop Med Hyg. 2014;91(5):989–993. doi:10.4269/ajtmh.14-0169
51. Silva AEP, Freitas CDC, Dutra LV, Molento MB. Correlation between climate data and land altitude for Fasciola hepatica infection in cattle in Santa Catarina, Brazil. Rev Bras Parasitol Vet. 2020;29(3):e008520. doi:10.1590/s1984-29612020065
52. Esteban JG, Flores A, Angles R, Mas-Coma S. High endemicity of human fascioliasis between Lake Titicaca and La Paz valley, Bolivia. Trans R Soc Trop Med Hyg. 1999;93(2):151–156. doi:10.1016/s0035-9203(99)90289-4
53. Owiny MO, Obonyo MO, Gatongi PM, Fèvre EM. Prevalence and spatial distribution of Trematode cercariae in vector snails within different Agro-Ecological Zones in Western Kenya, 2016. Pan Afr Med J. 2019;32:142. doi:10.11604/pamj.2019.32.142.14418
54. Khademvatan S, Majidiani H, Khalkhali H, Taghipour A, Asadi N, Yousefi E. Prevalence of fasciolosis in livestock and humans: a systematic review and meta-analysis in Iran. Comp Immunol Microbiol Infect Dis. 2019;65:116–123. doi:10.1016/j.cimid.2019.05.001
55. Squire SA, Yang R, Robertson I, Ayi I, Squire DS, Ryan U. Gastrointestinal helminths in farmers and their ruminant livestock from the Coastal Savannah zone of Ghana. Parasitol Res. 2018;117(10):3183–3194. doi:10.1007/s00436-018-6017-1
56. Greter H, Batil AA, Ngandolo BN, et al. Human and livestock trematode infections in a mobile pastoralist setting at Lake Chad: added value of a One Health approach beyond zoonotic diseases research. Trans R Soc Trop Med Hyg. 2017;111(6):278–284. doi:10.1093/trstmh/trx051
57. El-Sahn F, Farghaly A, El-Masry A, Mandil A, Gad A, El-Morshedy H. Human fascioliasis in an Egyptian village: prevalence and some epidemiological determinants. J Egypt Public Health Assoc. 1995;70(5–6):541–557.
58. Curtale F, Hassanein YAW, Barduagni P, et al. Human fascioliasis infection: gender differences within school-age children from endemic areas of the Nile Delta, Egypt. Trans R Soc Trop Med Hyg. 2007;101(2):155–160. doi:10.1016/j.trstmh.2006.05.006
59. de Van N, Le TH, Agramunt VH, Mas-Coma S. Early postnatal and preschool-age infection by Fasciola spp.: report of five cases from vietnam and worldwide review. Am J Trop Med Hyg. 2020. doi:10.4269/ajtmh.20-0139
60. Van DN, Minh PN, Bich NN, Chai J-Y. Seroprevalence of tissue and luminal helminths among patients in Hanoi Medical University Hospital, Vietnam, 2018. Korean J Parasitol. 2020;58(4):387–392. doi:10.3347/kjp.2020.58.4.387
61. Mailles A, Capek I, Ajana F, Schepens C, Ilef D, Vaillant V. Commercial watercress as an emerging source of fascioliasis in Northern France in 2002: results from an outbreak investigation. Epidemiol Infect. 2006;134(5):942–945. doi:10.1017/S095026880600611X
62. Rodríguez-Ulloa C, Rivera-Jacinto M, Del Valle-Mendoza J, et al. Risk factors for human fascioliasis in schoolchildren in Baños del Inca, Cajamarca, Peru. Trans R Soc Trop Med Hyg. 2018;112(5):216–222. doi:10.1093/trstmh/try049
63. Mas-Coma S, Bargues MD, Valero MA. Human fascioliasis infection sources, their diversity, incidence factors, analytical methods and prevention measures. Parasitology. 2018;145(13):1665–1699. doi:10.1017/S0031182018000914
64. Zárate-Rendón DA, Vlaminck J, Levecke B, Briones-Montero A, Geldhof P. Comparison of Kato-Katz thick smear, mini-FLOTAC, and Flukefinder for the detection and quantification of fasciola hepatica eggs in artificially spiked human stool. Am J Trop Med Hyg. 2019;101(1):59–61. doi:10.4269/ajtmh.18-0988
65. Calvani NED, Jan Š. Fasciola Species introgression: just a fluke or something more? Trends Parasitol. 2020;1471–4922. doi:10.1016/j.pt.2020.09.008
66. World Health Organization. Report of the WHO Informal Meeting on use of triclabendazole in fascioliasis control. 2007. Available from: https://www.who.int/neglected_diseases/preventive_chemotherapy/WHO_CDS_NTD_PCT_2007.1.pdf.
67. Lopez M, Morales ML, Konana M, et al. Kato-Katz and Lumbreras rapid sedimentation test to evaluate helminth prevalence in the setting of a school-based deworming program. Pathog Glob Health. 2016;110(3):130–134. doi:10.1080/20477724.2016.1187361
68. Cringoli G, Maurelli MP, Levecke B, et al. The mini-FLOTAC technique for the diagnosis of helminth and protozoan infections in humans and animals. Nat Protoc. 2017;12(9):1723–1732. doi:10.1038/nprot.2017.067
69. Article R, Sarkari B, Khabisi SA. Immunodiagnosis of human Fascioliasis: an update of concepts and performances of the serological assays. J Clin Diagn Res. 2017;11(6):OE05. doi:10.7860/JCDR/2017/26066.10086
70. Muñoz Zambrano ME, Placencia Medina M, Del Pozo Muñoz JA, Sevilla Andrade C, Huiza Franco A. Serological diagnosis of Fasciola hepatica infection: a systematic review. Rev Gastroenterol Peru. 2020;40(2):155–161.
71. Shin SH, Hsu A, Chastain HM, et al. Development of two FhSAP2 recombinant-based assays for immunodiagnosis of human chronic Fascioliasis. Am J Trop Med Hyg. 2016;95(4):852–855. doi:10.4269/ajtmh.16-0253
72. Kazantseva L, Lázaro MDPG, Herrera-Velit P, Espinoza JR. Anti-Fas2 IgM antibodies in Fasciola hepatica infected patients with positive IgG serology. Trans R Soc Trop Med Hyg. 2017;111(3):102–106. doi:10.1093/trstmh/trx024
73. Martínez-Sernández V, Orbegozo-Medina RA, González-Warleta M, Mezo M, Ubeira FM. Rapid enhanced MM3-COPRO ELISA for detection of Fasciola Coproantigens. PLoS Negl Trop Dis. 2016;10(7):1–20. doi:10.1371/journal.pntd.0004872
74. Arifin MI, Höglund J, Novobilský A. Comparison of molecular and conventional methods for the diagnosis of Fasciola hepatica infection in the field. Vet Parasitol. 2016;232:8–11. doi:10.1016/j.vetpar.2016.11.003
75. Calvani NED, Windsor PA, Bush RD, Šlapeta J. Scrambled eggs: a highly sensitive molecular diagnostic workflow for Fasciola species specific detection from faecal samples. PLoS Negl Trop Dis. 2017;11(9):e0005931. doi:10.1371/journal.pntd.0005931
76. Cabada MM, Malaga JL, Castellanos-gonzalez A, et al. Recombinase polymerase amplification compared to real-time polymerase chain reaction test for the detection of Fasciola hepatica in human stool. Am J Trop Med Hyg. 2017;96(2):341–346. doi:10.4269/ajtmh.16-0601
77. Ghodsian S, Rouhani S, Fallahi S, Seyyed-Tabaei SJ, Taghipour N. Detection of spiked Fasciola hepatica eggs in stool specimens using LAMP technique. Iran J Parasitol. 2019;14(3):387–393. doi:10.18502/ijpa.v14i3.1477
78. Keiser J, Engels D, Büscher G, Utzinger J. Triclabendazole for the treatment of fascioliasis and paragonimiasis. Expert Opin Investig Drugs. 2005;14(12):1513–1526. doi:10.1517/13543784.14.12.1513
79. FDA. Food and Drug Adminstration. FDA approved drugs labels for NDA 208711. 2020 [Cited September 2, 2020]. Available from: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=BasicSearch.process.
80. Favennec L, Jave Ortiz J, Gargala G, Lopez Chegne N, Ayoub A, Rossignol JF. Double-blind, randomized, placebo-controlled study of nitazoxanide in the treatment of fascioliasis in adults and children from northern Peru. Aliment Pharmacol Ther. 2003;17(2):265–270. doi:10.1046/j.1365-2036.2003.01419.x
81. Zumaquero-Ríos JL, Sarracent-Pérez J, Rojas-García R, et al. Fascioliasis and intestinal parasitoses affecting schoolchildren in Atlixco, Puebla State, Mexico: epidemiology and treatment with nitazoxanide. PLoS Negl Trop Dis. 2013;7(11):e2553. doi:10.1371/journal.pntd.0002553
82. Ramadan HK-A, Hassan WA, Elossily NA, et al. Evaluation of nitazoxanide treatment following triclabendazole failure in an outbreak of human fascioliasis in Upper Egypt. PLoS Negl Trop Dis. 2019;13(9):e0007779. doi:10.1371/journal.pntd.0007779
83. Cabada MM, Lopez M, Cruz M, Delgado JR, Hill V, White ACJ. Treatment failure after multiple courses of triclabendazole among patients with Fascioliasis in Cusco, Peru: a case series. PLoS Negl Trop Dis. 2016;10(1):e0004361. doi:10.1371/journal.pntd.0004361
84. Espinoza JR, Maco V, Marcos L, et al. Evaluation of FAS2-ELISA for the serological detection of Fasciola hepatica infection in humans. Am J Trop Med Hyg. 2007;76(5):977–982. doi:10.4269/ajtmh.2007.76.977
85. Marcos L, Maco V, Terashima A, Samalvides F, Espinoza JR, Gotuzzo E. Fascioliasis in relatives of patients with Fasciola hepatica infection in Peru. Rev Inst Med Trop Sao Paulo. 2005;47(4):219–222. doi:10.1590/s0036-46652005000400008
86. Roberts JA. Approaches to the control of fasciolosis in ruminants. Int J Parasitol. 1996;26(8–9):971–981. doi:10.1016/s0020-7519(96)80074-9
87. Fairweather I, Brennan GP, Hanna REB, Robinson MW, Skuce PJ. Drug resistance in liver flukes. Int J Parasitol Drugs Drug Resist. 2020;12:39–59. doi:10.1016/j.ijpddr.2019.11.003
88. McManus DP. Recent progress in the development of liver fluke and blood fluke vaccines. Vaccines. 2020;8(3):1–15. doi:10.3390/vaccines8030553
89. Mollinedo S, Gutierrez P, Azurduy R, et al. Mass drug administration of Triclabendazole for Fasciola Hepatica in Bolivia. Am J Trop Med Hyg. 2019;100(6):1494–1497. doi:10.4269/ajtmh.19-0060
90. Ortiz P, Castope N, Cabrera M, et al. Pharmacokinetic evaluation of different generic triclabendazole formulations in heifers. N Z Vet J. 2014;62(5):279–285. doi:10.1080/00480169.2014.925411
91. Gil LC, Díaz A, Rueda C, Martínez C, Castillo D, Apt W. Resistant human fasciolasis: report of four patients. Rev Med Chil. 2014;142(10):1330–1333. doi:10.4067/S0034-98872014001000014
92. Winkelhagen AJS, Mank T, de Vries PJ, Soetekouw R. Apparent triclabendazole-resistant human Fasciola hepatica infection, the Netherlands. Emerg Infect Dis. 2012;18(6):1028–1029. doi:10.3201/eid1806.120302
93. Branco EA, Ruas R, Nuak J, Sarmento A. Treatment failure after multiple courses of triclabendazole in a Portuguese patient with fascioliasis. BMJ Case Rep. 2020;13:3. doi:10.1136/bcr-2019-232299
94. Belgin G, Kanık Yüksek S, Tezer H, et al. Partial hepatectomy for the resistant Fasciola Hepatica Infection in a Child. APSP J Case Rep. 2015;6(3):27.
95. Dominguez MF, González-Miguel J, Carmona C, et al. Low allelic diversity in vaccine candidates genes from different locations sustain hope for Fasciola hepatica immunization. Vet Parasitol. 2018;258(May):46–52. doi:10.1016/j.vetpar.2018.06.011
96. Piacenza L, Acosta D, Basmadjian I, Dalton JP, Carmona C, Moore RN. Vaccination with cathepsin L proteinases and with leucine aminopeptidase induces high levels of protection against fascioliasis in sheep. Infect Immun. 1999;67(4):1954–1961. doi:10.1128/IAI.67.4.1954-1961.1999
97. Acosta D, Cancela M, Piacenza L, Roche L, Carmona C, Tort JF. Fasciola hepatica leucine aminopeptidase, a promising candidate for vaccination against ruminant fasciolosis. Mol Biochem Parasitol. 2008;158(1):52–64. doi:10.1016/j.molbiopara.2007.11.011
98. Maggioli G, Acosta D, Silveira F, et al. The recombinant gut-associated M17 leucine aminopeptidase in combination with different adjuvants confers a high level of protection against Fasciola hepatica infection in sheep. Vaccine. 2011;29(48):9057–9063. doi:10.1016/j.vaccine.2011.09.020
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.