Back to Journals » Clinical Ophthalmology » Volume 14
Human Amniotic Membrane Plug for Macular Holes Coexisting with Rhegmatogenous Retinal Detachment
Authors Abouhussein MA , Elbaha SM, Aboushousha M
Received 14 July 2020
Accepted for publication 4 August 2020
Published 24 August 2020 Volume 2020:14 Pages 2411—2416
DOI https://doi.org/10.2147/OPTH.S272060
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Mahmoud Alaa Abouhussein, Samir Mohamed Elbaha, Mohsen Aboushousha
Ophthalmology, Alexandria University, Alexandria, Egypt
Correspondence: Mahmoud Alaa Abouhussein
Ophthalmology, Alexandria University, 8 Hassan Allam Street, Kasr Elmoltazem Building, Smouha, Alexandria 1103, Egypt
Tel +20 1001026075
Email [email protected]
Purpose: To evaluate the efficacy of human amniotic membrane in promoting closure of macular holes coexisting with rhegmatogenous retinal detachment.
Methods: This is a retrospective case series of 14 eyes (14 patients) with macula off retinal detachment. These patients had a macular hole coexistent with peripheral retinal breaks. A human amniotic membrane plug was used to close the macular hole during vitrectomy without ILM peeling.
Results: The mean preoperative BCVA (logMAR value) was 1.87 ± 0.31. At the 6-month follow-up visit, the mean LogMAR best-corrected visual acuity was 0.67 ± 0.17. At the 6-month follow-up, all patients showed complete retinal reattachment with macular hole closure.
Conclusion: The use of human amniotic membrane is a valid option in surgery for macular holes coexisting with rhegmatogenous retinal detachment.
Keywords: human amniotic membrane, macular hole, rhegmatogenous retinal detachment
Introduction
The prevalence of macular hole coexistent with rhegmatogenous retinal detachment with peripheral break ranges from 2% to 8%.1,2 It is not always detected in the initial evaluation of rhegmatogenous retinal detachment cases and it may be not as rare as it is thought to be.3
This is different from the detachment caused by macular holes in high myopia, where the retinal detachment is mostly in the posterior pole with no associated peripheral breaks.4–6
The cause of the macular hole in cases of macula off rhegmatogenous retinal detachment is not certain. It may be caused by posterior vitreous detachment like the peripheral breaks. Another hypothesis is tangential traction by vitreoretinal interface abnormalities or proliferative vitreoretinopathy.1,2
The best treatment strategy for this group of patients is not known. Historically, the aim was to repair the rhegmatogenous retinal detachment and ignore the macular hole.3,7 Then, trials to improve the visual outcome by tackling the problem of macular hole started, either in the same setting or sequentially.8
The main problem in this clinical situation is the difficulty to peel the internal limiting membrane over a detached macula which can lead to macular hole enlargement or macular trauma. There is also a risk of dyes getting in the subretinal space leading to toxic effects. Also, comparing vitrectomy with or without internal limiting membrane peeling in this group of patients; it was shown that anatomic and visual outcomes were better in the group without peeling.9
The use of human amniotic membrane is already established in the treatment of corneal and conjunctival disorders. Amniotic membrane supports epithelialization and has anti-fibrotic, anti-inflammatory, antiangiogenic, and anti-microbial properties.10–17
The idea of using human amniotic membrane in retinal disorders began in 2012 when it was shown in animal studies that amniotic membrane has good growth support on retinal pigment epithelium cells.18 Recently, the use of human amniotic membrane to treat retinal breaks and macular holes was reported with good results.19
Our aim in this study was to evaluate the efficacy of subretinal human amniotic membrane in patients with macular holes coexisting with rhegmatogenous retinal detachment.
Methods
This study was a retrospective, interventional, consecutive case series. Alexandria University ethics review board approval was obtained and the research followed the tenants of the declaration of Helsinki. Written informed consent was obtained from all patients before surgery.
The study included 14 eyes of 14 patients with macula off retinal detachment. These patients had a macular hole coexistent with peripheral retinal breaks. The macular hole was observed either preoperatively or intraoperatively during vitrectomy. Some patients had overhanging detachment that prevented preoperative detection of the macular hole. Peripheral causal retinal tears were detected during preoperative examination in all patients. Minimum follow-up period was 6 months.
The exclusion criteria consisted of posterior pole detachment caused by high myopic holes, proliferative vitreoretinopathy worse than Grade B, previous intraocular surgery (except uneventful cataract surgery), and other retinal degenerative or vascular diseases that might affect visual prognosis.
Preoperative complete ophthalmic evaluation was done including history taking, duration of symptoms, best-corrected visual acuity, intraocular pressure measurement, slit-lamp biomicroscopy, and binocular indirect ophthalmoscopy. Visual acuity measurement was converted to logMAR for statistical analysis.
All cases were operated using the Constellation vision system (Alcon-USA) 23-gauge system. Complete 23-gauge transconjunctival vitrectomy with removal of posterior hyaloid face and complete vitreous base shaving was done. Peripheral breaks were identified and marked with endodiathermy. Perfluorocarbon liquid was then injected to push the subretinal fluid through peripheral breaks. Drainage of subretinal fluid was done through retinal breaks. Perfluorocarbon liquid was also used to flatten the macula and facilitate human amniotic membrane insertion inside the macular hole.
Amniotic membrane was obtained from placenta of women undergoing elective cesarean who were seronegative for hepatitis B, hepatitis C, syphilis, and human immunodeficiency virus. Under a lamellar flow hood, the placenta was cleaned of blood clots with sterile Phosphate buffered saline (PBS) containing 50 μg/mL penicillin, 50 μg/mL streptomycin, 100 μg/mL neomycin, and 2.5 μg/mL amphotericin B.20 The amnion was then separated from the rest of the chorion by blunt dissection. It was subsequently rinsed in 4%, 8%and 10% dimethylsulfoxide (DMSO) phosphate-buffered saline for 5 minutes each. The isolated human amniotic membrane was then flattened on a nitrocellulose paper with the epithelium side up. It was then stored in a sterile vial containing 10% DMSO medium and frozen at −70°C. The amniotic membrane was defrosted before use.
A patch of human amniotic membrane was prepared using a cutaneous punch (Disposable Biopsy Punch, Kai Medical, Solingen, Germany) of 2 mm. The amniotic membrane patch was inserted in the eye through the trocar. The amniotic membrane patch was then positioned in the macular hole under perfluorocarbon. The chorion layer was facing the retinal pigment epithelium and the epithelial side facing the vitreous. The patch was spread inside the hole so that it would be covered by the edges of the macular hole all around. Perfluorocarbon liquid air exchange was then done and endolaser was done around peripheral retinal breaks. Silicone oil was then injected. All surgeries were done by the same experienced surgeon (S.M.E).
The minimum follow-up period was 6 months after surgery. Patients were evaluated on the first postoperative day, week1, after 1, 3, and 6 months. Complete ophthalmic examination was done each visit. OCT was performed at the 1-, 3-, and 6-month visits. Silicone oil was removed 3 months postoperatively.
Anatomical and functional results at 6-month visit were used for statistical analysis. Statistical analysis was done using Statistical Package for Social Sciences (SSP/Version 20, LEAD Technology, USA) software. Probability value of less than 0.05 was considered to be significant.
Results
This study included 14 eyes of 14 patients with rhegmatogenous retinal detachment with coexistent macular hole. The baseline characteristics of the included patients are presented in Table 1. There were ten females and four males. The mean age was 63.58 years and standard deviation was 5.69 years (ranging from 52 to 71 years). Four patients were phakic and ten patients were pseudophakic. The mean duration of symptoms was 3.4 ± 2.49 weeks (ranging from 1 to 8 weeks). The mean baseline intraocular pressure was 15.71 ± 1.66 mmHg. All patients had peripheral retinal breaks apart from the macular hole. All the included patients showed PVR grade B. The mean number of peripheral breaks was 2.32 ± 0.94 (range, 1–4). The mean duration of follow up was 9.28 ± 2.37 months, ranging from 6 to 13 months.
 |
Table 1 Baseline Characteristics of the 14 Included Patients |
We were able to detect the coexisting macular hole preoperatively in six patients, while in the other eight patients it was detected during surgery.
The baseline mean log MAR best-corrected visual acuity was 1.87 ± 0.31 (ranging from 1.3 to 2.3 log MAR). Snellen baseline visual acuity ranged from 20/4000 to 20/400.
We used silicone oil as a tamponade in all cases. Silicone oil removal was done 3 months after surgery. The four phakic patients had cataract surgery combined with silicone oil removal.
At the 6-month follow-up visit, the mean intraocular pressure was 15 ± 1.19 mmHg. The mean final best-corrected visual acuity improved to 0.67 ± 0.17 log MAR (ranging from 0.4 to 1 log MAR). This improvement was statistically significant (p < 0.001) compared to the baseline values. Postoperative Snellen visual acuity ranged from 20/100 to 20/50. All patients had improvement in their visual acuity with no cases of diminution of vision after surgery.
Figures 1–4 show OCT scans of a case at baseline, at 1 month, 3 months, and 6 months postoperatively.
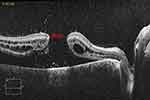 |
Figure 1 OCT scan of the right eye of a patient showing the preoperative macular hole measuring 1065 microns and the retinal detachment. |
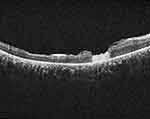 |
Figure 2 OCT scan of the same patient at 1 month showing the human amniotic membrane plug filling the macular hole and the retina is attached. |
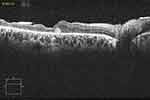 |
Figure 3 OCT scan of the same patient at 3 month, before silicone oil removal, showing retinal layers growing over the amniotic membrane plug which decreased in size. |
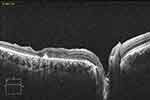 |
Figure 4 OCT scan of the same patient at 6 month, after silicone oil removal, showing a small remnant of the amniotic membrane patch. |
Optical coherence tomography showed a fixed pattern in all patients. Initially, the human amniotic membrane patch filled the macular hole. Then, the neurosensory retina gradually grew over the patch. By the 6-month visit, the human amniotic membrane patch decreased in size, but it was still detected in some of the OCT scans in all patients. Also at the 6-month follow-up, all patients showed complete retinal reattachment with macular hole closure.
No postoperative complications such as increased intraocular pressure, intraocular inflammation, or amniotic membrane displacement were recorded during the study follow-up period.
Discussion
Few studies addressed the problem of rhegmatogenous retinal detachment caused by peripheral breaks with a concomitant noncausal macular hole. There is no consensus as to the preferred surgical treatment for this clinical situation.
One study compared two treatment strategies in dealing with rhegmatogenous retinal detachment and coexisting macular holes.8 Patients in the combined surgery group had vitrectomy, internal limiting membrane peel, retinopexy to the peripheral breaks, and gas tamponade. Patients in the sequential group had vitrectomy, retinopexy to the peripheral breaks, and gas tamponade with macular hole surgery as a secondary procedure. The results of this study suggested that good anatomic and visual outcome can be achieved using either approach.
There are contradictory data regarding the role of internal limiting membrane peeling in this clinical situation. Ryan et al showed that internal limiting membrane peeling increased the rate of macular hole closure. They also pointed out the risks and technical difficulties associated with internal limiting membrane peeling in this group of patients.21 While Shukla et al found that ILM peeling did not result in higher closure rates for macular hole.9 In fact, the eyes that did not undergo ILM peeling not only achieved better closure rates, but the hole edges were also better apposed, and the final visual acuity was significantly better. This controversy is probably related to the difficulty and risks of removing ILM over a mobile retina.
We describe a new method of dealing with macular hole coexistent with rhegmatogenous retinal detachment. We used human amniotic membrane plug to close the macular hole. Human amniotic membrane acts as a basement membrane substrate allowing for cellular growth over it.22 It also expresses cytokines and growth factors, such as fibroblastic growth factor and hepatocyte growth factor, promoting epithelisation.23
Recently, Rizo et al reported the use of human amniotic membrane subretinal implantation to treat recurrent macular holes, especially in highly myopic eyes, with good functional and anatomical outcomes. They also showed that human amniotic membrane implantation seems to promote retinal break repair and retinal regeneration.19
Our study presents a case series of using a human amniotic membrane patch to treat macular holes coexistent with rhegmatogenous retinal detachment. Macular hole closure and retinal reattachment were achieved in all patients. We used silicone oil tamponade in all cases because of the multiple pathologies involved in these cases. The human amniotic membrane is readily available in the operating room. The manipulations to insert it in the macular hole are technically relatively easy leading to shortened operative time. This surgery also avoids dealing with the internal limiting membrane over a mobile retina which is an inherently delicate and hazardous procedure.
All of our patients had parts of the human amniotic membrane detected in some OCT scans at the end of follow up. It is still not known whether these remnants might affect the full recovery of neurosensory retinal functions.
Limitations of our study are retrospective nature and relatively small sample size. To the best of our knowledge, this is the first study that shows the beneficial effect of the human amniotic membrane in patients having a non-causal macular coexisting with rhegmatogenous retinal detachment. Prospective studies are needed to further evaluate the most effective surgical treatment for this group of cases.
Disclosure
The authors report no grants, funds proprietary interest, or conflicts of interest for this work.
References
1. Najafi M, Brown JS, Rosenberg KI. Increased reoperation rate in surgical treatment of rhegmatogenous retinal detachment with coexistent macular hole. Ophthalmol Retina. 2017;2(3):187–191. doi:10.1016/j.oret.2017.07.005
2. Cunningham MA, Tarantola RM, Folk JC, et al. Proliferative vitreoretinopathy may be a risk factor in combined macular hole retinal detachment cases. Retina. 2013;33(3):579–585. doi:10.1097/IAE.0b013e31826b0c41
3. O’Driscoll AM, Goble RR, Kirkby GR. Vitrectomy for retinal detachments with both peripheral retinal breaks and macular holes. An assessment of outcome and the status of the macular hole. Retina. 2001;21:221–225. doi:10.1097/00006982-200106000-00004
4. Oie Y, Emi K, Takaoka G, Ikeda T. Effect of indocyanine green staining in peeling of internal limiting membrane for retinal detachment resulting from macular hole in myopic eyes. Ophthalmology. 2007;114:303–306. doi:10.1016/j.ophtha.2006.07.052
5. Cheung BT, Lai TY, Yuen CY, et al. Results of high-density silicone oil as a tamponade agent in macular hole retinal detachment in patients with high myopia. Br J Ophthalmol. 2007;91:719–721. doi:10.1136/bjo.2006.111526
6. Li KK, Tang EW, Li PS, Wong D. Double peel using triamcinolone acetonide and trypan blue in the management of myopic macular hole with retinal detachment: a case–control study. Clin Exp Ophthalmol. 2010;38:664–668. doi:10.1111/j.1442-9071.2010.02333.x
7. Ah Kiné D, Benson SE, Inglesby DV, Steel DH. The results of surgery on macular holes associated with rhegmatogenous retinal detachment. Retina. 2002;22(4):429–434. doi:10.1097/00006982-200208000-00006
8. Singh AJ. Combined or sequential surgery for management of rhegmatogenous retinal detachment with macular holes. Retina. 2009;29:1106–1110. doi:10.1097/IAE.0b013e3181a3b8fc
9. Shukla D, Kalliath J, Srinivasan K, et al. Management of rhegmatogenous retinal detachment with coexisting macular hole: a comparison of vitrectomy with and without internal limiting membrane peeling. Retina. 2013;33(3):571–578. doi:10.1097/IAE.0b013e31826b6748
10. Anderson DF, Prabhasawat P, Alfonso E, Tseng SC. Amniotic membrane transplantation after the primary surgical management of band keratopathy. Cornea. 2001;20:354–361. doi:10.1097/00003226-200105000-00004
11. Tseng SC, Prabhasawat P, Barton K, et al. Amniotic membrane transplantation with or without limbal allografts for corneal surface reconstruction in patients with limbal stem cell deficiency. Arch Ophthalmol. 1998;116:431–441. doi:10.1001/archopht.116.4.431
12. Meller D, Pires RT, Mack RJ, et al. Amniotic membrane transplantation for acute chemical or thermal burns. Ophthalmology. 2000;107:980–989. doi:10.1016/S0161-6420(00)00024-5
13. Ucakhan OO, Koklu G, Firat E. Nonpreserved human amniotic membrane transplantation in acute and chronic chemical eye injuries. Cornea. 2002;21:169–172. doi:10.1097/00003226-200203000-00008
14. Gomes JA, Dos Santos MS, Cunha MC, et al. Amniotic membrane transplantation for partial and total limbal stem cell deficiency secondary to chemical burn. Ophthalmology. 2003;110:466–473. doi:10.1016/S0161-6420(02)01888-2
15. Tsubota K, Satake Y, Ohyama M, et al. Surgical reconstruction of the ocular surface in advanced ocular cicatricial pemphigoid and Stevens-Johnson syndrome. Am J Ophthalmol. 1996;122:38–52. doi:10.1016/S0002-9394(14)71962-2
16. Grueterich M, Tseng SC. Human limbal progenitor cells expanded on intact amniotic membrane ex vivo. Arch Ophthalmol. 2002;120:783–790. doi:10.1001/archopht.120.6.783
17. Meller D, Dabul V, Tseng SC. Expansion of conjunctival epithelial progenitor cells on amniotic membrane. Exp Eye Res. 2002;74:537–545. doi:10.1006/exer.2001.1163
18. Kiilgaard JF, Scherfig E, Prause JU, La Cour M. Transplantation of amniotic membrane to the subretinal space in pigs. Stem Cell Int. 2012:716968.
19. Rizzo S, Caporossi T, Tartaro R, et al. A human amniotic membrane plug to promote retinal breaks repair and recurrent macular hole closure. Retina. 2019;39:S95–S103. doi:10.1097/IAE.0000000000002320
20. Prabhasawat P, Tesavibul N. Preserved amniotic membrane transplantation for conjunctival surface reconstruction. Cell Tissue Bank. 2001;2:31–39. doi:10.1023/A:1011597332277
21. Ryan EH
22. Dua HS, Gomes JAP, King AJ, Maharajan S. The amniotic membrane in ophthalmology. Surv Ophthalmol. 2004;49:51–77. doi:10.1016/j.survophthal.2003.10.004
23. Shimazaki J, Shinozaki N, Tsubota K. Transplantation of amniotic membrane and limbal autograft for patients with recurrent pterygium associated with symblepharon. Br J Ophthalmol. 1998;82:235–240. doi:10.1136/bjo.82.3.235
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
