Back to Journals » Journal of Blood Medicine » Volume 14
Howell-Jolly Body-Like Inclusions in Coronavirus Disease 2019 (COVID-19): Possible Novel Findings
Authors Oehadian A, Huang I, Kartikasari A, Alisjahbana B , Prihatni D
Received 7 December 2022
Accepted for publication 17 March 2023
Published 29 March 2023 Volume 2023:14 Pages 233—238
DOI https://doi.org/10.2147/JBM.S399596
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Martin H Bluth
Amaylia Oehadian,1 Ian Huang,2,3 Andini Kartikasari,4 Bachti Alisjahbana,5 Delita Prihatni6
1Division of Hematology and Oncology Medic, Department of Internal Medicine Faculty of Medicine, Universitas Padjadjaran/Dr. Hasan Sadikin General Hospital, Bandung, Indonesia; 2Department of Internal Medicine Faculty of Medicine, Universitas Padjadjaran/Dr. Hasan Sadikin General Hospital, Bandung, Indonesia; 3Faculty of Medicine, Universitas Pelita Harapan, Tangerang, Banten, Indonesia; 4Bandung City Regional General Hospital, Bandung, Indonesia; 5Division of Infectious and Tropical Diseases, Department of Internal Medicine Faculty of Medicine, Universitas Padjadjaran/Dr. Hasan Sadikin General Hospital, Bandung, Indonesia; 6Department of Clinical Pathology Universitas Padjadjaran/Dr. Hasan Sadikin General Hospital, Bandung, Indonesia
Correspondence: Amaylia Oehadian, Department of Internal Medicine, Padjadjaran University/Dr. Hasan Sadikin General Hospital, Jl. Pasteur No. 38, Bandung, West Java, 40161, Indonesia, Email [email protected]
Background: During COVID-19 pandemic, it is difficult to distinguish febrile patient infected by SARS-CoV-2 or bacterial causes. Howell-Jolly bodies are a well-known entity found in red blood cells. They are nuclear fragments, composed of deoxyribonucleic acid, commonly observed in the peripheral blood smears of hyposplenic or asplenic patients. Recently, similar inclusions often referred to as Howell-Jolly body-like inclusions (HJBLIs) have been reported in the neutrophils of patients with acquired immune deficiency syndrome (AIDS) and COVID-19 patient.
Aim: To explore whether HJBLIs in peripheral blood smear could differentiate between patients with confirmed SARS-CoV-2 and bacterial pneumonia.
Methods: We performed cross-sectional study using secondary data from COVID-19 database and re-evaluated peripheral blood smears to identify HJBLIs. We included confirmed COVID-19 adults age > 18 years who were hospitalized in Dr. Hasan Sadikin General Hospital, Bandung, Indonesia from March 1st 2020–May 31st 2020. We also examined peripheral blood smears in patients with confirmed bacterial pneumonia as a control group. Clinical characteristics including disease severity, CURB-65 score, comorbidity, and the present of HJBLIs in peripheral blood smears were evaluated.
Results: Overall, 33 patients were included: 22 were confirmed COVID-19 and 11 were confirmed bacterial pneumonia. The median (interquartile range) age in COVID-19 and patients with bacterial pneumonia were 53 years (40– 64) vs 57 years (53– 71), respectively. Compared with patients with bacterial pneumonia, HJBLIs were significantly higher in COVID-19 patients [21/22 (80.8%) vs 5/11 (45.5%), p 0.001].
Conclusion: Howell-Jolly body-like inclusions could be a potential feature to help differentiate between COVID-19 and bacterial pneumonia.
Keywords: Howell-Jolly body-like inclusions, COVID-19, bacterial pneumonia, peripheral blood smear
Introduction
During COVID-19 pandemic many patients with acute febrile illness were hospitalized. It was difficult to distinguish between patients with SARS-CoV-2 and those who have bacterial pneumonia. Diagnostic laboratory confirmation such as polymerase-chain reaction for COVID-19 and bacterial culture need time and specific resources that were not available in every health facility. Especially in severe patients, we need to early differentiate between COVID-19 and bacterial pneumonia patients to determine the appropriate treatment.
The peripheral blood smear is a simple evaluation that can potentially differentiate between bacterial and viral infection.1 One of the abnormalities that could be detected in peripheral blood smear is Howell-Jolly bodies, nuclear remnants found in red blood cells under various pathological states. They are most commonly present in patients with absent or impaired function of the spleen2 Others causes of Howell-Jolly bodies are post-splenectomy, sepsis, post-high-dose corticosteroid treatment, autoimmune and neoplastic disorders.3,4
In peripheral blood smears, the nuclear fragments appear as small but strongly basophilic (purple) dots inside the eosinophilic (pink) red blood cells.5 Howell-Jolly body-like inclusions (HJBLIs) were detached nuclear fragments resembling Howell-Jolly bodies within neutrophils from Human Immunodeficiency Virus (HIV)-infected patients, organ-transplant recipients, and patients on immunosuppressive drugs.6 In our previous case report, interestingly we found HJBLIs in our COVID-19 patient.7
In this study, we sought to determine whether the presence of HJBLIs could be used to help differentiating between patients with confirmed COVID-19 and bacterial pneumonia.
Methods
We performed cross-sectional study using secondary data from COVID-19 database and re-evaluated peripheral blood smears to identify HJBLIs. We included confirmed COVID-19 adults age >18 years who were hospitalized in Dr. Hasan Sadikin General Hospital, Bandung, Indonesia from March 1st 2020–May 31st 2020. We also examined peripheral blood smears in patients with suspected COVID-19 but had negative SARS-CoV-2 RT-PCR and confirmed bacterial pneumonia in that period as a control group. From this database, we excluded the patients who had not complete data of hematological examination and peripheral blood smears. Clinical characteristics including disease severity, CURB-65 scoring, comorbidity, and the present of HJBLIs in peripheral blood smears were compared on the hospital admission. Disease severity was determined using WHO interim guidelines of novel coronavirus (2019-nCoV) (WHO 2020).8
We retrieved complete blood cell counts (CBCs) data that were performed using Sysmex XN-1000. Peripheral blood smears were re-evaluated manually by two independent observers, a board-certified haematologist and a hematopathologist. The present of HJBLIs, morphological characteristics in red blood cells, white blood cells (WBC), and platelets were qualitatively and quantitatively documented. The morphology grading system was reported according to the International Council for Standardization in Hematology (ICSH), and the examiners were blinded to the COVID-19 test result during the smear review.
Qualitative variables of peripheral blood smear were analysed using the Chi-square or Fisher’s Exact test, while the Mann–Whitney test was used to conduct quantitative analysis. All statistical analyses were conducted using SPSS software (SPSS Inc. 24).
Results
A total of 33 patients which consist of 22 COVID-19 infected cases and 11 bacteria pneumonia cases were included in the analysis. More than half of patients were male. Median (IQR) age of COVID-19 and bacterial pneumonia patients were 53 years (40–64) vs 57 years (53–71) years, respectively. The most common comorbidity was hypertension in patient with COVID-19 (40.9%), and chronic kidney disease in patients with bacterial pneumonia (36.4%). Severe disease was found in approximately half of COVID-19 and two-third of bacterial pneumonia patients. CURB-65 score 3 (severe pneumonia) was significantly higher in bacterial pneumonia compared to COVID-19 patients [3/11 (27.3%) vs 0/22 (0%), p = 0.03]. The majority of our COVID-19 and bacterial pneumonia patients were discharged. Death was found in approximately 14% COVID-19 patients and one-fourth of those with bacterial pneumonia (Table 1).
 |
Table 1 Characteristics of Patients with COVID-19 and Bacterial Pneumonia |
Haemoglobin level was higher in COVID-19 patients compared to those with bacterial pneumonia [12.5 (6.4–12.8) vs 9.5 (7.2–12.6) g/dL, p 0.004]. There were no significant differences in white blood cells count and morphology in peripheral blood between COVID-19 and bacterial pneumonia patients, except for HJBLIs. Proportion of COVID-19 patients that had HJBLIs was significantly higher than those with bacterial pneumonia [21/22 (80.8%) vs 5/11 (45.5%), p 0.002]. Atypical lymphocytes were commonly found in COVID-19 patients [9/22 (40.9%)]. There was no COVID-19 patient with thrombocytopenia compared to half of those with bacterial pneumonia patients (p = 0.001) (Table 2).
 |
Table 2 Red Blood Cells, White Blood Cells and Platelets in Patient with COVID-19 and Bacterial Pneumonia |
Howell-Jolly body-like inclusions are depicted in Figure 1.
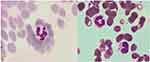 |
Figure 1 Howell-Jolly body-like inclusions found in neutrophil (arrow sign). |
Discussion
This study demonstrated significant morphologic changes between patients with and without COVID-19 infection. Our most interesting findings are the significant HJBLIs found in these patients. COVID-19 patient also showed normal platelet, and a slight increase in atypical lymphocytes.
The findings of HJBLIs in neutrophils were previously reported in COVID-19 patient.7 And observed in majority of our patients. Bain coined the inclusion in 1989, a detached nuclear fragment in the neutrophils caused by dysplastic granulopoiesis.9 It was occasionally identified in monocytes, lymphocytes, eosinophils, and neutrophils.10 The occurrence of the inclusions could hypothetically be a response to a specific viral infection. However, the pathogenic and prognostic consequences of HJBLIs in COVID-19 are questions that demand further studies. We also found HJBLIs in 5/11 (45.5%) patients with bacterial infected group due to possibility previous or concomitant other viral infection in these patients.
Interestingly and different from other common viral infections, about 90% of the COVID-19 patients had normal platelet count. This finding is also consistent with the previous study by Pozdnyakova, which reported normal mean platelet count in Non-ICU and ICU COVID-19 patients.11 Infected patients often have mild thrombocytopenia and increased platelet consumption, together with a corresponding increase in platelet production.12 On the contrary, we found approximately half of bacterial pneumonia patients had thrombocytopenia. Elmaraghy et al revealed the association of thrombocytopenia with high number of mortality in patient with pneumonia.13 Compared to patients with severe pneumonia but without COVID-19, those with COVID-19 disease actually had higher platelet counts, according to Yin et al.14
In our study, atypical lymphocytes were found in 9/22 (40.9%) COVID-19 patients compared to 2/11 (18.2%) in those with bacterial pneumonia. This finding was lower than previously study by Weinberg et al who reported atypical lymphocytes in 93.3% COVID-19 cases in Chicago, USA.15 This variance could be caused by the difference in clinical characteristics of the patients. Most of the patients (9/15) in previous study were admitted in intensive care with severe disease compared to half of our patients with severe COVID-19 disease. These atypical lymphocytes are likely reactive to severe SARS-CoV-2 infection.
We found that haemoglobin level was significantly higher in COVID 19 patients compare to bacterial infected cases [12.5 (6.4–12.8) vs 9.5 (7.2–12.6) g/dl, p = 0.004]. Previous study reported that patients with severe COVID-19 had significantly lower hemoglobin levels than those with moderate or mild COVID-19. The optimal hemoglobin cut-off value for prediction of disease severity was 11.6 g/dL.16 These different could be caused by different severity between COVID-19 and bacterial pneumonia patients in our study. On the other hand, there was no significant difference in WBC and platelet count between the groups. However, Kamel et al revealed COVID-19 cases showed a significant decrease in mean WBC count, as well as basophil, neutrophil, lymphocyte, and eosinophil counts compared with the controls.17 This dissimilarity could be caused by different characteristics of bacterial pneumonia patients in our study.
In regard of clinical characteristics, the most common comorbidity in COVID-19 patient was hypertension followed by diabetes. These results were consistent with a meta-analysis study on COVID-19 comorbidities, in total of 1786 patients that stated the most common comorbidities identified in these patients were hypertension (15.8%), cardiovascular and cerebrovascular conditions (11.7%), and diabetes (9.4%).18 In bacterial pneumonia patients, the most common comorbidity was chronic kidney disease followed by hypertension. HJBLIs previously reported in HIV-infected patients, organ-transplant recipients, and patients on immunosuppressive drugs.6 So, we assumed that the comorbidities in our patients did not affect the present of HJBLIs.
The majority of our COVID-19 patients were discharged and approximately 14% ended in death. These results were consistent with previous study by Chen et al that found discharged and death of COVID-19 patients were 68% and 15%, respectively.19
The limitations of this study are the small number of subjects; hence, analysis cannot be conducted according to the severity of the disease, and the possibility of operator-dependent morphologic changes in blood smear. We also could not retrieve list of drugs used in our patients, so we could not rule out the potential bias caused by drugs especially steroid use that could have influence on the present of HJBLIs. Post high-dose steroid treatment had been reported as one of the causes of Howell-Jolly bodies in red blood cells.3,4 It is unknown whether steroid used could influence HJBLIs. In addition, due to cross-sectional method, we could not evaluate the relation between these new findings in the peripheral blood smear with the course of the patients. In conclusion, blood smear analysis could be considered as an additional important examination in patients with suspected COVID-19 to help differentiate those from bacterial pneumonia. In addition, attention should be focused on HJBLIs in neutrophils. Further studies are needed to evaluate the pathogenic and prognostic consequences of HJBLIs in COVID-19 patients with analysis of additional variables among the groups and stratification by disease severity.
Ethical Approval
Ethical approval was obtained from Ethics Committee of Health Research Dr Hasan Sadikin Hospital, (instituition name: RSUP Dr Hasan Sadikin) with following number: LB.02.01/X.6.5/100/2020. We confirm that informed consent was obtained from the study participants. We confirm that the guidelines outlined in the Declaration of Helsinki were followed.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Prokocimer M, Potasman I. The added value of peripheral blood cell morphology in the diagnosis and management of infectious diseases—part 1: basic concepts. Postgrad Med J. 2008;84(997):579–585. doi:10.1136/pgmj.2008.069609
2. Sears DA, Udden MM. Howell-Jolly bodies: a brief historical review. Am J Med Sci. 2012;343(5):407–409. doi:10.1097/MAJ.0b013e31823020d1
3. William BM, Corazza GR. Hyposplenism: a comprehensive review. Part I: basic concepts and causes. Hematology. 2007;12(1):1–13.
4. Ong SY, Ng HJ. Howell–Jolly bodies in systemic amyloidosis. Int J Hematol. 2018;108(2):119–120. doi:10.1007/s12185-018-2473-8
5. Mathew H, Dittus C, Malek A, Negroiu A. Howell-Jolly bodies on peripheral smear leading to the diagnosis of congenital hyposplenism in a patient with septic shock. Clin Case Rep. 2015;3(8):714. doi:10.1002/ccr3.323
6. Chang B, Huang RSP, Dasgupta A, Nguyen N, Wahed A. Prevalence of Howell-Jolly body-like inclusions in HIV patients and their correlation with CD4 counts and HIV RNA viral load. Ann Clin Lab Sci. 2015;45(1):23–26.
7. Alisjahbana B, Huang I, Oehadian A. The detection of Howell-Jolly body-like inclusions in a case of coronavirus disease-2019 (COVID-19). Blood Res. 2020;55(4):191. doi:10.5045/br.2020.2020186
8. Koçyiğit B, Sayin S, Şişman FO, Kizilkaya S, Ayrik MY. Coronavirus disease-19 (Covid-19): the disease that changed the world. Turkish Med Student J. 2020;7(2):96–105. doi:10.4274/tmsj.galenos.2020.07.02.07
9. Bain BJ. Blood Cells: A Practical Guide. Philadelphia: Lippincott; 1989.
10. Andre E, Chevalier C, Scheiff JM. Howell‐Jolly‐like bodies in leucocytes: first description in leucocytes other than neutrophils. Eur J Haematol. 2011;86(2):182–183. doi:10.1111/j.1600-0609.2010.01543.x
11. Pozdnyakova O, Connell NT, Battinelli EM, Connors JM, Fell G, Kim AS. Clinical significance of CBC and WBC morphology in the diagnosis and clinical course of COVID-19 infection. Am J Clin Pathol. 2021;155(3):364–375. doi:10.1093/ajcp/aqaa231
12. Güçlü E, Kocayiğit H, Okan HD, et al. Effect of COVID-19 on platelet count and its indices. Revista da Associação Médica Brasileira. 2020;66:1122–1127. doi:10.1590/1806-9282.66.8.1122
13. ElMaraghy AA, AbdelFattah EB, Ahmed MS. Platelet count: is it a possible marker for severity and outcome of community acquired pneumonia? Egypt J Chest Dis Tuberc. 2016;65(2):499–504. doi:10.1016/j.ejcdt.2015.09.001
14. Yin S, Huang M, Li D, Tang N. Difference of coagulation features between severe pneumonia induced by SARS-CoV2 and non-SARS-CoV2. J Thromb Thrombolysis. 2021;51(4):1107–1110. doi:10.1007/s11239-020-02105-8
15. Weinberg SE, Behdad A, Ji P. Atypical lymphocytes in peripheral blood of patients with COVID‐19. Br J Haematol. 2020;190:24–39. doi:10.1111/bjh.16848
16. Fouad SH, Allam MF, Taha SI, et al. Comparison of hemoglobin level and neutrophil to lymphocyte ratio as prognostic markers in patients with COVID-19. J Int Med Res. 2021 Jul;49(7):03000605211030124
17. Kamel FO, Magadmi RM, Alqutub ST, et al. Clinical and hematologic presentations of adults with COVID-19 patients in Jeddah: a case control study. J Infect Public Health. 2021;14(6):709–716. doi:10.1016/j.jiph.2021.03.007
18. Paudel SS. A meta-analysis of 2019 novel Corona virus patient clinical characteristics and comorbidities; 2020.
19. Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. bmj. 2020;368
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
