Back to Journals » Clinical Ophthalmology » Volume 16
How to Stop People from Going Blind from Glaucoma Using Early Cataract Surgery/Refractive Lensectomy and Microinvasive Glaucoma Surgery
Received 17 December 2021
Accepted for publication 14 February 2022
Published 15 March 2022 Volume 2022:16 Pages 815—821
DOI https://doi.org/10.2147/OPTH.S354338
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Daniel Laroche,1,2 Melanie Scheive3
1Department of Ophthalmology, New York Eye and Ear Infirmary, Icahn School of Medicine of Mount Sinai, New York, NY, USA; 2Advanced Eyecare of New York, New York, NY, USA; 3Department of Ophthalmology, Indiana University School of Medicine, Indianapolis, IN, USA
Correspondence: Daniel Laroche, Department of Ophthalmology, New York Eye and Ear Infirmary and Advanced Eyecare of New York, 49 West 127th Street, New York, NY, 10027, USA, Tel +1 212663-0473, Email [email protected]
Abstract: Glaucoma continues to be a leading cause of blindness worldwide for the same reasons as in the past several decades, including the lack of early detection, improper treatment, and non-adherence to therapy. Medical therapy continues to be the first-line therapy even as surgical techniques are improving in their safety and efficacy. To turn the tide in preventing blindness from glaucoma, attention must be focused on targeted patient education, screening, effective treatment, and addressing health disparities. To achieve this, early safer cataract surgery and microinvasive glaucoma surgery must be considered as a first-line therapy in addition to medical therapy to best lower both intraocular pressure and the medication burden.
Keywords: glaucoma, prevention, cataract surgery, lensectomy, MIGS
Introduction
Glaucoma is the leading cause of irreversible blindness affecting 3–5% of the worldwide population over 40 years old.1,2 The most recent estimate of bilateral glaucoma blindness from 2020 is 5.9 million people.3 The number affected is expected to continue to grow to a projected more than 111 million people by 2040 disproportionately in Africa, which has the highest primary open angle glaucoma (POAG) prevalence, and Asia, which has the highest primary acute angle glaucoma prevalence.4
The answer to the question as to why people go blind from glaucoma continues to be the same as in 1982 when Grant et al posed this question.5,6 One third of patients are undiagnosed, one third are not treated for glaucoma appropriately, and one third are non-adherent to glaucoma therapy.5 According to the Preferred Practice Patterns (PPP) of the American Academy of Ophthalmology (AAO), medical therapy continues to be the first-line therapy for POAG.7 We must revisit this.
Glaucoma has both economic and quality of life impacts based on the extent of visual field loss and disease severity.8 As a result, attention to prevention of glaucoma progression through education, screening, effective treatment, and addressing health disparities is essential. This can be best accomplished through a change in focus from early medical therapy to earlier safer surgical interventions based on glaucoma severity. Here are five recommendations to bend the curve to stop people from going blind from glaucoma.
We Need to be More Aggressive About Educating and Screening for Undiagnosed Glaucoma in High-Risk Populations Using Targeted Outreach and Multimodal Testing Methods in Order to Detect and Intervene Earlier in the Disease Course.
Large population-based studies indicate that the majority of glaucoma cases, specifically in the United States (US), are undiagnosed.9 The prevalence of glaucoma has been shown to increase with advancing age to include 2.9% of adults at least 40 years old and 6.6% of adults at least 70 years old in the US.10 In other parts of the world such as South Africa, the proportion of undiagnosed is even higher, including 87% of cases in the population of black residents of at least 40 years old in the city of Temba.10
Glaucoma disproportionately impacts Black, Hispanic, and elderly populations, so education and screening efforts should focus on these high-risk populations.9,11,12 One contributing reason why glaucoma is underdiagnosed and presents late is that patients underestimate and are not knowledgeable about their risk of developing glaucoma.13 As a result, education through media sources and accessible mediums, especially mobile devices, being used now is essential. Mobile-based education can improve both public and patient awareness about glaucoma given knowledge gaps in these populations, especially high-risk groups including the elderly and Black and Afro-Latino communities in the US.14,15 Targeted educational funding of health campaigns supported by the National Institutes of Health and as a part of other government research funding can involve targeted media of television, local press, local radio, and social media to reach elderly and Black and Afro-Latino communities which can be further facilitated through Black and Afro-Latino-owned media.
To expand glaucoma screenings in high-risk populations, there should be a focus on the expansion of quality medical facilities in Black and Afro-Latino communities that are staffed by a representatively diverse workforce of community members who can provide excellent culturally competent care. The evidence for the efficacy of student-run free eye clinics is not well studied, but one recent national survey indicated that glaucoma is among the most common eye condition encountered in 23% of the 13 clinics surveyed.16 One screening student-run free eye clinic in an underserved urban area of Indianapolis that serves a large Black and Afro-Latino population had glaucoma as one of the most common reasons for referral to the local county hospital.17
To improve the sensitivity of glaucoma screening, access to a combination of structural and functional tests and imaging technologies for high-risk populations is essential based on recommendations from the Consensus of the World Glaucoma Association on Glaucoma Screening and Glaucoma Diagnosis.18 The glaucoma screening threshold should be relatively low given that the mean normal intraocular pressure (IOP) is 15 mmHg and the mean IOP in patients with untreated glaucoma is 18 mmHg. Patients with an IOP of 18 mmHg or higher should undergo gonioscopy, optic nerve assessment, and optical coherence tomography (OCT) assessment. Incorporation of OCT into glaucoma screening programs can facilitate both detection with sensitivity between 70% and 80% and diagnosis with specificity of 80%. OCT has the potential to reduce the prevalence undiagnosed glaucoma from 75% to 38%.19
Stopping Pigment Liberation and Lower IOP Targets Based on Glaucoma Severity Can be Achieved Through Early Surgical Intervention of Cataract Surgery and Micro Invasive Glaucoma Surgery (MIGS) with Adjuvant Medical Therapy
First-line medical therapy to lower IOP in patients with glaucoma has been shown to be inadequate in fully preventing disease progression. For instance, the average IOP decrease of 25% or 5.1 mmHg in the Early Manifest Glaucoma Trial (EMGT) was insufficient to prevent visual field loss in the majority of patients over the four-year study. Despite substantial advances in the field showing that surgical intervention, including earlier cataract surgery and MIGS for mild to moderate glaucoma, can eliminate the risk of angle closure and significantly lower IOP, this has not traditionally been offered as initial or early glaucoma treatment.20,21
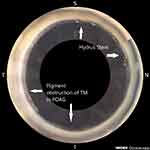
Early surgical treatment should be considered given that POAG is often caused by the lens enlarging with aging. The enlarging lens compresses Schlemm’s canal and liberates pigment through contact with the posterior iris which becomes trapped in the trabecular meshwork. Both the Schlemm’s canal and trabecular meshwork are required for aqueous drainage, so the enlarged lens decreases aqueous outflow leading to elevated IOP. There are multiple mechanisms by which surgical interventions for glaucoma are proposed to counter these effects. Clear lensectomy results in enhanced patency of Schlemm’s canal and trabecular meshwork expansion. Concurrently, MIGS bypasses the partially obstructed trabecular meshwork and expands aqueous access to Schlemm’s canal and collector channels. Figure 1 demonstrates the placement of the Hydrus form of MIGS to treat POAG by bypassing the trabecular meshwork obstructed by visible pigment. The Hydrus is preferred over the iStent because this device has been found to more likely be burrowed in the trabeculum rather than be placed appropriately in the Schlemm canal.22
For primary angle-closure glaucoma (PACG), clear lensectomy is better at lowering IOP than first-line therapy of laser peripheral iridotomy (LPI) and appears to produce similar outcomes alone and in conjunction with other therapies. Clear lensectomy, goniosynechialysis, and the Hydrus stent have been reported to be successful in angle closure glaucoma at reducing mean IOP.23 The Effectiveness in Angle-Closure Glaucoma of Lens Extraction (EAGLE) study showed that clear lensectomy was better than LPI at lowering IOP.20 Other studies similarly showed that phacoemulsification results in a lower IOP increase over time and fewer complications compared to LPI over two years.24
The benefits of early uncomplicated phacoemulsification are beginning to outweigh the risk and costs of medical therapy over time. Technological advances have led to the prevalence of severe postoperative adverse events being increasingly rare now at 0.5%.25 Although the evidence is mixed, one large cohort study of US veterans showed that older age is associated with greater odds of postsurgical complications, making the benefit of cataract surgery greater at a younger age.26 Uncomplicated phacoemulsification may also become safer in one group at higher risk of postoperative complications, that is ophthalmology resident surgeries, particularly the first 50 to 80 procedures, with the incorporation of surgical simulator training into the curriculum.27,28
Trabeculectomy is associated with a higher postoperative complication rate, including cataract formation, with similar IOP reduction compared to phacoemulsification, so this procedure should be reserved for severe cases.29,30 According to the Collaborative Initial Glaucoma Treatment Study (CIGTS), the average IOP reduction was shown to reach the target level and be lower in the trabeculectomy group ranging from 35% to 48% compared to medical therapy. Surgical intervention in the Advanced Glaucoma Intervention Study (AGIS) resulted similarly in reductions in IOP preventing glaucoma progression over nine years, including a mean IOP of 12.3 mmHg and intervisit IOP measurements below 18 mmHg. Given its side effect profile and IOP reduction effects, trabeculectomy should be considered as an initial approach in advanced glaucoma patients.
Early surgery not only lowers IOP but also reduces the amount of medication needed to reach target IOP. This will reduce the number of missed doses that is associated with visual field progression in glaucoma. In Black and Afro-Latino populations, cataract surgery and MIGS, specifically the Hydrus stent, lowered IOP. Nearly 80% of patients no longer needed eye medications. The earlier the glaucoma surgical intervention was performed, the less medication was required.31 Once glaucoma is detected, earlier cataract surgery/lensectomy and MIGS should be considered to prevent glaucoma progression, especially when there are challenges with follow-up. A retrospective study of private, community-based practices of ophthalmologists found that patients with unstable or uncontrolled glaucoma were the least likely group of patients to have follow-up scheduled within the recommended time intervals of the AAO’s PPP guidelines.32
General targets for IOP should vary by glaucoma severity, including 15 mmHg for mild glaucoma, 12 mmHg for moderate to advanced glaucoma, and 8 to 12 mmHg for advanced glaucoma involving field progression in patients with normal corneal thickness. These IOP targets can often be better achieved with early surgical intervention to reduce the medication burden and enhance adherence to therapy. MIGS using the Hydrus stent has longitudinal evidence supporting clinical benefits over the course of five years showing reduction in medication use and likelihood of requiring subsequent invasive glaucoma surgery with a similar safety profile.33 Cataract extraction/refractive lensectomy and MIGS should be considered for mild to moderate glaucoma and initial trabeculectomy for more advanced cases.
Diurnal IOP Peaks Can be Lowered Through Early Surgical Interventions for Glaucoma
IOP peak measurement is a significant factor in glaucoma progression, potentially more than mean IOP.23,40 This diurnal measurement is often difficult to detect given time restraints of testing and capturing peaks during office hours given that 50% of IOP peak measurements occur outside of these hours.34–36 One etiology comes from the additive effect of episcleral venous pressure increasing at night while sleeping in the supine position, a measurement of retrograde aqueous drainage, on IOP in glaucoma.37
Diurnal IOP peak has been shown to be lowered significantly using MIGS for glaucoma. This can also reduce the diurnal nighttime IOP in patients with hypotension. Post-hoc analysis of the GEMINI study showed that the difference between the highest and lowest daily IOP measurements was shown to reduce from 2.8 mmHg on average preoperatively to 1.8 mmHg on average 12 months postoperatively with greater proportions of lower IOPs compared to preoperative levels. Diurnal IOP peaks were similarly affected in the prospective study of two iStent implants which showed mean IOP reduction by 46% at four years postoperatively without use of glaucoma medication.38 Medications for glaucoma do not typically affect diurnal IOP peaks with the exception of prostaglandin analogs. As a result, early MIGS intervention is advantageous by reducing diurnal IOP peaks to slow glaucoma progression.
Early Cataract Surgery with MIGS Lowers the Medication Burden and IOP for Glaucoma While Preserving Visual Field
Medication adherence is a significant concern for long-term multimodal glaucoma medical management. The frequency of dosing during the day proportionately decreases medication adherence based on the results of systemic review evidence.39 There are multifaceted barriers to medication adherence, including forgetfulness, other life priorities, and lack of education.40 For adherence to glaucoma medical therapy, specifically, factors that decrease adherence based on the results of the Glaucoma Adherence and Persistency Study include receiving all knowledge about glaucoma from the physician, lack of understanding of the effect medication non-adherence has on vision outcomes, difficulty paying for medication or managing medication when away from home, and not acknowledging side effects of stinging and burning.41
Early surgical intervention through cataract surgery/clear lensectomy with MIGS can reduce the long-term medication therapy requirements of glaucoma treatment through its reduction in IOP, including affecting diurnal levels. Future long-term study of medication therapy requirements is needed to elucidate the extent of reduction in medication burden and subsequent extent of medical therapy adherence. The HORIZON study has shown that 73% patients on one glaucoma medication at baseline that underwent Hydrus microstent placement remained medication-free after five years compared to the control group that only underwent cataract surgery at 48%. Involving over 3000 data points, eyes treated with the Hydrus microstent showed a 47% lower rate of mean deviation visual field loss relative to the control group. Additionally, there was a 20% to 30% increase in the medication free rate in the Hydrus group at all timepoints up to five years compared with the control group.33 Similar effects have been seen in other studies, including a retrospective review showing similar improvements in medication free levels and visual field test stabilization.31 Clear lensectomy and the Hydrus stent has been reported to lower IOP and preserve visual field in patients with open angle glaucoma at a younger age at an average of 68 years old compared to the national average of 73 years old.42
Investing in Training a Diverse Workforce to Become Experts in Early Cataract Surgery Will Reduce Health Disparities in Glaucoma Care
Among racial groups in the US, glaucoma disproportionately affects Blacks compared to Whites with a prevalence 4 to 5 times higher.9 Wealth disparities between White and Black households at an average of $171,000 for Whites and $17,000 for Blacks indicate that economic access is more challenging for Blacks who have a greater disease burden. Therefore, universal health insurance access will be important in equalizing equal access to healthcare as the short-term cost of surgical intervention is much greater than medical therapy.
The disparities are also compounded by limited representation of Black physicians in the US who make up only 5% of physicians, an increase of 4% over the course of 120 years solely from an increase in Black female physicians, which influences Black patient willingness to seek preventative healthcare and to share health concerns.43,44 There are over 40 million Blacks in the US and only 400 Black ophthalmologists. Additionally, about 13.5% of US physicians are from underrepresented minoritized groups even though these groups make up 31% of the US population.45 Many communities in the United States are segregated by race still today. As such, many of the healthcare institutions are not located in those segregated communities. There are also few Black physicians in these communities. This must change. Disproportionately low representation of Blacks extends to POAG clinical trials as there are Black participants at much lower rates compared to Whites despite the higher disease burden.46
More diversity in medical training, especially underrepresented Black and Afro-Latino surgeons, is essential to address community needs in areas with higher prevalence of glaucoma. Better training of glaucoma surgeons across the world, especially where glaucoma is most prevalent in Africa, should be provided by improving expertise in cataract surgery and affordable MIGS with a straight cystotome or Sinskey hook.47,48
Many studies show that Black physicians are more likely to work in underserved Black communities. Many studies have also shown that Black patients prefer to seek care from culturally concordant physicians and have greater trust in them. This is due to the unfortunate history of institutionalized medical experimentation on Black patients, including most notably from the infamous Tuskegee study.49–51
There needs to be increased diversity in the leadership of all academic ophthalmology institutions, hospital executive boards, research teams, ophthalmic companies, and NIH funding tied to ensuring diversity and inclusion in ophthalmic glaucoma research to further enhance education and learning and develop future advances for everyone.52–54 Only 2.5% of practicing ophthalmologists are Black and there is no significant difference in residency program representation.45 This issue needs to be carefully monitored, invested in and improved with annual metrics of success evaluated and enhanced.
Conclusion
Future studies focused on earlier cataract surgery/clear lensectomy and MIGS as initial surgery are warranted to stop blindness from glaucoma. Increasing the surgical workforce, diversity, and public health education to higher risk communities with these new advances in safer early surgical treatments will be essential to stop blindness from glaucoma. Secondary glaucomas, such as uveitis, neovascular, or congenital, that may not be lens related will need a more individualized approach.
Disclosure
Daniel Laroche is a speaker/consultant for Aerie, Sight Sciences, and Nidek.The authors report no other conflicts of interest in this work.
References
1. Kingman S. Glaucoma is second leading cause of blindness globally. Bull World Health Organ. 2004;82:887–888.
2. Jonas JB, Aung T, Bourne RR, Bron AM, Ritch R, Panda-Jonas S. Glaucoma. Lancet. 2017;390(10108):2183–2193. doi:10.1016/S0140-6736(17)31469-1
3. Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br j Ophthalmol. 2006;90:262–267. doi:10.1136/bjo.2005.081224
4. Tham Y-C, Li X, Wong TY, Quigley HA, Aung T, Cheng C-Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121:2081–2090. doi:10.1016/j.ophtha.2014.05.013
5. Susanna R, De Moraes CG, Cioffi GA, Ritch R. Why Do People (Still) Go Blind from Glaucoma? Transl Vis Sci Technol. 2015;4:1. doi:10.1167/tvst.4.2.1
6. Grant WM, Burke JF. Why Do Some People Go Blind from Glaucoma? Ophthalmology. 1982;89:991–998. doi:10.1016/S0161-6420(82)34675-8
7. Gedde SJ, Vinod K, Wright MM, et al. Primary Open-Angle Glaucoma Preferred Practice Pattern®. Ophthalmology. 2021;128:P71–P150. doi:10.1016/j.ophtha.2020.10.022
8. Wang Y, Alnwisi S, Ke M, Hoch JS, Ducruet T, Chaillet N. The impact of mild, moderate, and severe visual field loss in glaucoma on patients’ quality of life measured via the Glaucoma Quality of Life-15 Questionnaire: a meta-analysis. Medicine. 2017;15:96. doi:10.1186/s12916-017-0859-8
9. Tielsch JM, Sommer A, Katz J, Royall RM, Quigley HA, Javitt J. Racial variations in the prevalence of primary open-angle glaucoma: the Baltimore Eye Survey. JAMA. 1991;266:369–374. doi:10.1001/jama.1991.03470030069026
10. Shaikh Y, Yu F, Coleman AL. Burden of undetected and untreated glaucoma in the United States. Am J Ophthalmol. 2014;158:1121–1129. e1121. doi:10.1016/j.ajo.2014.08.023
11. Nathan N, Joos KM. Glaucoma disparities in the Hispanic population. Semin Ophthalmol. 2016;31(4):394–399. doi:10.3109/08820538.2016.1154165
12. Queen JH, Beaver HA. Glaucoma in the Elderly. Geriatric Ophthalmology. Springer; 2019:27–38.
13. Hou C-H, Lee J-S, Lin -K-K, Liu L, Lee Y-S, Pu C. Accuracy of perceived glaucoma risk by patients in a clinical setting. PLoS One. 2021;16:e0257453. doi:10.1371/journal.pone.0257453
14. Peralta E, Muir KW, Rosdahl JA. Systematic Review of Knowledge Assessments for Glaucoma Patients. Semin Ophthalmol. 2018;33:377–388. doi:10.1080/08820538.2016.1247180
15. Li J, Huang W, Gao J, Li D, Xu L, Huang J. Impact of Mobile-Based Health Education on the Awareness and Knowledge of Glaucoma in Chinese Patients. Telemed J E Health. 2019;25:455–461. doi:10.1089/tmj.2018.0123
16. Okaka Y, Meah YS, Fallar R, Chadha N. Ophthalmology services at student-run free clinics: a national survey. J Natl Med Assoc. 2021;113(4):431–435. doi:10.1016/j.jnma.2021.02.004
17. Rowe LW, Scheive M, Tso HL, et al. A seven-year analysis of the role and impact of a free community eye clinic. BMC Med Educ. 2021;21:1–8. doi:10.1186/s12909-021-03026-7
18. Weinreb RN, Garway-Heath D, Leung C, Medeiros F, Liebmann J. Diagnosis of Primary Open Angle Glaucoma: WGA Consensus Series-10. Kugler Publications; 2017.
19. Blumberg DM, Vaswani R, Nong E, Al-Aswad L, Cioffi GA. A comparative effectiveness analysis of visual field outcomes after projected glaucoma screening using SD-OCT in African American communities. Invest Ophthalmol Vis Sci. 2014;55:3491–3500. doi:10.1167/iovs.14-14014
20. Azuara-Blanco A, Burr J, Ramsay C, et al. Effectiveness of early lens extraction for the treatment of primary angle-closure glaucoma (EAGLE): a randomised controlled trial. Lancet. 2016;388:1389–1397. doi:10.1016/S0140-6736(16)30956-4
21. Tanner L, Gazzard G, Nolan WP, Foster PJ. Has the EAGLE landed for the use of clear lens extraction in angle-closure glaucoma? And how should primary angle-closure suspects be treated? Eye. 2020;34:40–50. doi:10.1038/s41433-019-0634-5
22. Gillmann K, Bravetti GE, Mermoud A, Mansouri K. A Prospective Analysis of iStent Inject Microstent Positioning: schlemm Canal Dilatation and Intraocular Pressure Correlations. J Glaucoma. 2019;28:613–621. doi:10.1097/IJG.0000000000001273
23. Laroche D, Brown A, Ng C. Clear Lensectomy, Goniosynechiolysis and Hydrus Microstent in a Patient with Mixed Mechanism Glaucoma. J Natl Med Assoc. 2020;112:339–343. doi:10.1016/j.jnma.2020.04.003
24. Wright C, Tawfik MA, Waisbourd M, Katz LJ. Primary angle-closure glaucoma: an update. Acta Ophthalmol. 2016;94:217–225. doi:10.1111/aos.12784
25. Stein JD, Grossman DS, Mundy KM, Sugar A, Sloan FA. Severe Adverse Events after Cataract Surgery Among Medicare Beneficiaries. Ophthalmology. 2011;118:1716–1723. doi:10.1016/j.ophtha.2011.02.024
26. Greenberg PB, Tseng VL, Wu WC, et al. Prevalence and predictors of ocular complications associated with cataract surgery in United States veterans. Ophthalmology. 2011;118:507–514. doi:10.1016/j.ophtha.2010.07.023
27. Staropoli PC, Gregori NZ, Junk AK, et al. Surgical Simulation Training Reduces Intraoperative Cataract Surgery Complications Among Residents. Simul Healthc. 2018;13:11–15. doi:10.1097/SIH.0000000000000255
28. Stein JD. Serious adverse events after cataract surgery. Curr Opin Ophthalmol. 2012;23:219–225. doi:10.1097/ICU.0b013e3283524068
29. Li X, Liu Y, Li Y, Wang M. Effects of modified trabeculectomy combined with phacoemulsification and intraocular lens implantation on intraocular pressure and complications in patients with primary open angle glaucoma. Int J Clin Exp Med. 2019;12:1778–1784.
30. Tham CC, Kwong YY, Baig N, Leung DY, Li FC, Lam DS. Phacoemulsification versus trabeculectomy in medically uncontrolled chronic angle-closure glaucoma without cataract. Ophthalmology. 2013;120:62–67. doi:10.1016/j.ophtha.2012.07.021
31. Laroche D, Nkrumah G, Ng C. Real-world efficacy of the Hydrus microstent in Black and Afro-Latinx patients with glaucoma: a retrospective study. Therapeutic Adv Ophthalmol. 2020;12:2515841420964311. doi:10.1177/2515841420964311
32. Hertzog LH, Albrecht KG, LaBree L, Lee PP. Glaucoma care and conformance with preferred practice patterns. Examination of the private, community-based ophthalmologist. Ophthalmology. 1996;103:1009–1013. doi:10.1016/S0161-6420(96)30573-3
33. Ahmed IK. 5 Year Follow Up from the HORIZON Trial. Am Glaucoma Soc Virtual Annu Meeting. 2021;1:736.
34. Bengtsson B, Leske MC, Hyman L, Heijl A. Fluctuation of intraocular pressure and glaucoma progression in the early manifest glaucoma trial. Ophthalmology. 2007;114:205–209. doi:10.1016/j.ophtha.2006.07.060
35. Konstas AG, Quaranta L, Mikropoulos DG, et al. Peak intraocular pressure and glaucomatous progression in primary open-angle glaucoma. J Ocular Pharmacol Therapeutics. 2012;28:26–32. doi:10.1089/jop.2011.0081
36. Barkana Y, Anis S, Liebmann J, Tello C, Ritch R. Clinical utility of intraocular pressure monitoring outside of normal office hours in patients with glaucoma. Arch Ophthalmol. 2006;124:793–797. doi:10.1001/archopht.124.6.793
37. Selbach JM, Posielek K, Steuhl K-P, Kremmer S. Episcleral venous pressure in untreated primary open-angle and normal-tension glaucoma. Ophthalmologica. 2005;219:357–361. doi:10.1159/000088378
38. Lindstrom R, Sarkisian SR, Lewis R, Hovanesian J, Voskanyan L. Four-Year Outcomes of Two Second-Generation Trabecular Micro-Bypass Stents in Patients with Open-Angle Glaucoma on One Medication. Clin Ophthalmol. 2020;14:71–80. doi:10.2147/OPTH.S235293
39. Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin Ther. 2001;23:1296–1310. doi:10.1016/S0149-2918(01)80109-0
40. Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–497. doi:10.1056/NEJMra050100
41. Friedman DS, Hahn SR, Gelb L, et al. Doctor–patient communication, health-related beliefs, and adherence in glaucoma: results from the glaucoma adherence and persistency study. Ophthalmology. 2008;115:1320–1327. e1323. doi:10.1016/j.ophtha.2007.11.023
42. Laroche D, Nkrumah G, Ng C. Clear lensectomy and the Hydrus stent lower IOP and medication use in Black and Afro-Latino patients with glaucoma. Am Soc Cataract Refractive Surg. 2021.
43. Ly DP. Historical Trends in the Representativeness and Incomes of Black Physicians, 1900–2018. J Gen Intern Med. 2021;19:1–3.
44. Alsan M, Garrick O, Graziani G. Does diversity matter for health? Experimental evidence from Oakland. Am Economic Rev. 2019;109:4071–4111. doi:10.1257/aer.20181446
45. Xierali IM, Nivet MA, Wilson MR. Current and Future Status of Diversity in Ophthalmologist Workforce. JAMA Ophthalmol. 2016;134:1016–1023. doi:10.1001/jamaophthalmol.2016.2257
46. Allison K, Patel DG, Greene L. Racial and Ethnic Disparities in Primary Open-Angle Glaucoma Clinical Trials: a Systematic Review and Meta-analysis. JAMA network open. 2021;4:e218348–e218348. doi:10.1001/jamanetworkopen.2021.8348
47. Laroche D, Okaka Y, Ng C. A Novel Low Cost Effective Technique in Using a 23 Gauge Straight Cystotome to Perform Goniotomy: making Micro-invasive Glaucoma Surgery (MIGS) Accessible to the Africans and the Diaspora. J Natl Med Assoc. 2019;111:193–197. doi:10.1016/j.jnma.2018.09.006
48. Laroche D. Affordable Sinskey hook goniotomy for glaucoma Daniel Laroche MD. 2021.
49. Marrast LM, Zallman L, Woolhandler S, Bor DH, McCormick D. Minority Physicians’ Role in the Care of Underserved Patients: diversifying the Physician Workforce May Be Key in Addressing Health Disparities. JAMA Intern Med. 2014;174:289–291. doi:10.1001/jamainternmed.2013.12756
50. Bajaj SS, Stanford FC. Beyond Tuskegee—Vaccine distrust and everyday racism. N Engl J Med. 2021;384:e12. doi:10.1056/NEJMpv2035827
51. Ma A, Sanchez A, Ma M. The impact of patient-provider race/ethnicity concordance on provider visits: updated evidence from the Medical Expenditure Panel Survey. J Racial Ethnic Health Disparities. 2019;6:1011–1020. doi:10.1007/s40615-019-00602-y
52. Yashadhana A, Clarke NA, Zhang JH, et al. Gender and ethnic diversity in global ophthalmology and optometry association leadership: a time for change. Ophthalmic Physiol Optics. 2021;41:623–629. doi:10.1111/opo.12793
53. Yashadhana A, Zhang JH, Yasmin S, et al. Action needed to improve equity and diversity in global eye health leadership. Eye. 2020;34:1051–1054. doi:10.1038/s41433-020-0843-y
54. Scott AW, Elam AR, Nwanyanwu K. Addressing Disparities in Eye Care—The Time Is Now. JAMA Ophthalmol. 2021;139(9):935. doi:10.1001/jamaophthalmol.2021.2053
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.