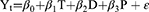Back to Journals » Risk Management and Healthcare Policy » Volume 16
How National Health Insurance Coverage Policy Affected the Use of Trastuzumab and Rituximab in China: A Bicentric Retrospective Study
Authors Shang L, Lin Y , Fang W, Liu Y, Bao Y, Li X , Zhang Y
Received 26 May 2023
Accepted for publication 1 September 2023
Published 4 September 2023 Volume 2023:16 Pages 1739—1753
DOI https://doi.org/10.2147/RMHP.S420899
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jongwha Chang
Linlin Shang,1,* Yingtao Lin,2,* Wenqing Fang,3 Yanyan Liu,4 Yuwen Bao,4 Xin Li,1,2,4 Yuanyuan Zhang5
1Department of Clinical Pharmacy, School of Pharmacy, Nanjing Medical University, Nanjing, People’s Republic of China; 2Center for Global Health, School of Public Health, Nanjing Medical University, Nanjing, People’s Republic of China; 3Department of Medical Ethics Supervision, The First Affiliated Hospital of Soochow University, Suzhou, People’s Republic of China; 4Department of Health Policy, School of Health Policy and Management, Nanjing Medical University, Nanjing, People’s Republic of China; 5Department of Pharmacy, Jiangsu Cancer Hospital, Jiangsu Institute of Cancer Research, The Affiliated Cancer Hospital of Nanjing Medical University, Nanjing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yuanyuan Zhang, Department of Pharmacy, Jiangsu Cancer Hospital, No. 42 Baizi Ting Road, Xuanwu District, Nanjing, Jiangsu, 210009, People’s Republic of China, Email [email protected] Xin Li, Department of Clinical Pharmacy, School of Pharmacy, Nanjing Medical University, No. 101 Longmian Avenue, Jiangning District, Nanjing, Jiangsu, 211166, People’s Republic of China, Email [email protected]
Background: Cancer is a significant health concern and is China’s leading cause of mortality. Targeted therapies, such as trastuzumab and rituximab, have enhanced clinical treatment efficacy. However, their high costs burden patients and healthcare systems considerably. Patient demographic factors further influence the utilization of these expensive drugs. On September 1, 2017, China implemented the National Health Insurance Coverage (NHIC) policy, necessitating additional real-world evidence to assess its impact on patients.
Methods: Data on human epidermal growth factor receptor 2-positive breast cancer and CD20-positive non-Hodgkin B-cell lymphoma patients were gathered in Jiangsu Cancer Hospital and Fujian Cancer Hospital from September 2015 to August 2019, including demographic and clinical information. All eligible patients were divided into two groups. Univariate analysis and multivariable logistic regression were used to investigate the differences between subgroups. An interrupted time-series regression was used to examine the change in trastuzumab and rituximab utilization percentages.
Results: Before and after the NHIC policy, utilization of trastuzumab increased from 61.13% to 75.10%, and the increase was statistically significant. Rituximab therapy increased statistically significantly from 64.79% to 74.88%. The key factor influencing trastuzumab and rituximab use was the NHIC policy. With policy implementation, medical insurance status, occupations, and cancer disease stage affected trastuzumab and rituximab use.
Conclusion: The NHIC policy is essential to the utilization of trastuzumab and rituximab, and the patient’s income level and repayment abilities continue to impact the use of innovative anti-cancer drugs. Appropriate steps, such as reducing the urban-rural gap and broadening medical insurance coverage, would enable more people to access novel anti-cancer drugs.
Keywords: National Health Insurance Coverage, trastuzumab, rituximab, breast cancer, non-Hodgkin lymphoma, utilization
Introduction
Female breast cancer (BC) was the leading cause of cancer incidence (2.3 million cases, 11.7% of total cases) and the fifth leading cause of cancer mortality (685,000 cases) worldwide in 2020.1 According to the World Health Organization (WHO), China will have more new cases and deaths from BC than any other Asian country in 2040, with 491,000 new cases and 176,000 deaths.2 Trastuzumab, is a humanized monoclonal antibody that targets the extracellular domain of the human epidermal growth factor receptor 2 (HER2) protein. It was the first anti-HER2 agent approved by the US Food and Drug Administration (FDA) for clinical use and has become a standard of treatment for patients with HER2-positive breast cancer when combined with chemotherapy.3,4 Non-Hodgkin lymphoma (NHL) caused 544,352 new cases and 259,793 deaths in 2020, according to the GLOBOCAN database.1 A systematic assessment of the burden of lymphoma in China revealed that the burden of NHL in China increased much higher than the global burden.5 NHL caused a large burden of cancer incidence and mortality in men, and it was ranked as one of the top ten most common cancer types in Chinese males.6 Rituximab, a humanized monoclonal antibody against CD20 and the first monoclonal antibody approved for the treatment of lymphoma, has become the standard first-line treatment and maintenance therapy for NHL.7 Although innovative targeted therapies have substantially revolutionized the treatment of certain cancers,8 these targeted anticancer drugs frequently come with high costs,9 thereby escalating the financial burden on patients and healthcare insurance systems.10
To enhance patient access to anticancer drugs, China has introduced a series of policies since 2009, aiming to incorporate these targeted anticancer drugs into the National Reimbursement Drug List (NRDL) to improve their accessibility and affordability for patients.11 In 2017, significant progress was made in China’s national health insurance coverage (NHIC) reform, with drug price negotiations becoming a prerequisite for inclusion in the healthcare catalog.12 By undertaking national price negotiations and incorporating drugs into the NRDL, provinces are mandated to update their Provincial Reimbursement Drug Lists (PRDL) and include all NRDL drugs in the PRDL. Public sector hospitals procure these drugs at the negotiated national prices as their maximum prices. The efficacy of centralized negotiations in controlling drug prices has been evidenced in multiple countries. Some researchers postulate that China’s price negotiation policy is anticipated to reduce the per-unit purchase cost and enhance utilization rates, consequently ameliorating the accessibility and affordability of costly targeted anticancer drugs.13 Nonetheless, concerns persist that national price negotiations may not benefit most patients in need. Despite the considerable price reductions observed for these targeted anticancer drugs, a large proportion of patients might still be unable to afford them.14,15 Most extant studies cannot address these concerns, as there is a dearth of real-world research evidence to substantiate which populations and how many patients benefit from this policy.
Accordingly, we carried out a retrospective, observational study utilizing real-world data to investigate the changes in patient characteristics and the utilization of trastuzumab and rituximab before and after the implementation of the NHIC policy. Furthermore, we analyzed the factors influencing the utilization of trastuzumab and rituximab, and explored which populations and how many patients might potentially benefit from the NHIC policy.
Materials and Methods
Study Design and Data Sources
This study focused on trastuzumab and rituximab, where trastuzumab is recommended for the treatment of human epidermal growth factor receptor 2 (HER2)-positive invasive breast cancer,16 and rituximab is recommended for the treatment of CD20-positive non-Hodgkin B-cell lymphoma (NHL).7 We collected data from the electronic medical record (EMR) system of Jiangsu Cancer Hospital (the Affiliated Cancer Hospital of Nanjing Medical University) and Fujian Cancer Hospital (Fujian Medical University Cancer Hospital). Jiangsu Cancer Hospital is a 1239-bed tertiary cancer hospital, and Fujian Cancer Hospital is a 1600-bed tertiary cancer hospital. We analyzed patient characteristics and changes in trastuzumab or rituximab use from September 2015 to August 2019, before and after September 1, 2017, which was the time point for the National Health Insurance Coverage (NHIC) policy implementation in Jiangsu and Fujian. Changes in trastuzumab and rituximab before and after the NHIC policy are shown in Table 1.
 |
Table 1 The Differences of Trastuzumab and Rituximab Between the Pre- and Post-Implementation of NHIC Policy in Jiangsu and Fujian Province |
Patients
This study included adult patients diagnosed and treated with either HER2-positive breast cancer or CD20-positive NHL at Jiangsu Cancer Hospital and Fujian Cancer Hospital between September 2015 and August 2019, following the national diagnosis and treatment guidelines.17–19 To mitigate potential selection bias, patients who were diagnosed but not treated at the study hospital, those with contraindications to the study medicines, or those with incomplete data, were excluded. All eligible patients were included in the study, regardless of whether the drugs were administered as first-line therapy or subsequent lines of treatment, and whether they were de novo patients or had already been treated prior to receiving rituximab or trastuzumab. Patients were divided into the investigated drug treatment group and the non-investigated drug treatment group. Specifically, the HER2-positive breast cancer patients were divided into two groups: the trastuzumab group and the non-trastuzumab group. The CD20-positive NHL patients were divided into two groups: the rituximab group and the non-rituximab group.
Data Management and Variable Definition
The collected information from the EMR system includes: (i) demographic data. Gender; age; married or not; occupation (government administrative staff, professionals, businessmen, peasants, retiree, unclear); patient address (urban or rural); method of payment for treatment. In China, payment methods have been divided into 3 types: (1) uninsured or self-paid, implying payment with personal funds. (2) Health insurance, which is divided into two types based on insurance schemes: urban employees’ basic medical insurance (UEBMI) and urban-rural residents’ basic medical insurance (URRBMI). (3) Other types of insurance, such as commercial medical insurance, public expenditure, and so on. (ii) Clinical details. Date of diagnosis, pathological type, pathological stage, and receptor status.
Surveyed Medicines Usage Percentage
Trastuzumab and rituximab utilization percentages were calculated as the proportion of all eligible patients who use trastuzumab or rituximab every month during the study period. The surveyed medicine utilization percentage was analyzed using interrupted time series (ITS) regression analysis during 48 months.
The ITS model is a valuable research design for evaluating the efficacy of public health interventions and allows a more detailed assessment of the longitudinal impact of an intervention (15). The NHIC policy was implemented on September 1, 2017. Hence, there were 24 months before and after the NHIC policy. The formula of the ITS model was as follows:
The monthly utilization percentage at time point t is denoted by Yt. T is a time-dependent continuous variable (T=1, 2, 3,…. 48). D is the dummy variable for the two time periods before and after the NHIC policy (D = 0 indicates before the NHIC policy and D = 1 represents after the NHIC policy). P is the time point after policy intervention (P = 0 indicates before policy intervention and P = 1, 2, 3,…. 24 indicates after policy intervention). β0 is the estimated value of the baseline level of the result (which refers to the outcome when the time is 0), β1 represents the slope of the baseline, β2 represents the level of change following the intervention, and β3 represents the trend change of the outcome caused by the policy intervention, β1 + β3 represents the slope after the intervention, and ε is the error term. The Durbin-Watson test was used in this study to evaluate if the time series had autocorrelation. In addition, the median time point of the preintervention period (September 1, 2016) as a pseudo-start period replaced the true intervention start period to test the robustness of the results.20,21
Statistical Analyses
Chi-square and logistic regression were used to investigate the differences between subgroups. The Student’s t-test or the Mann–Whitney U-test was used to analyze continuous data, which were expressed as the mean standard deviation (SD). Categorical variables were expressed in percentages and were analyzed by chi-square or Fisher’s exact test. The variables impacting the utilization of trastuzumab and rituximab were identified using multivariable logistic regression analysis with backward elimination, and the findings were reported as odds ratios (ORs) and 95% confidence intervals (95% CI). The above analyses were performed using SPSS 23 (IBM Corp., Armonk, NY). P values less than 0.05 were considered statistically significant for all two-tailed statistical tests. ITS regression analyses were conducted using STATA 17 (StataCorp LLC, College Station, TX).
Results
Patients
As presented in Table 2, a total of 1783 HER-2-positive breast cancer patients were included in this study, including 1112 cases in Fujian Cancer Hospital and 671 cases in Jiangsu Cancer Hospital. Trastuzumab was utilized by 486 of the 795 patients before the policy’s implementation and 742 of the 988 patients after. The use of trastuzumab increased from 61.13% before the implementation of the policy to 75.10% afterward, with a statistically significant increase (P = 0.001). After implementing the NHIC policy, the utilization rate of trastuzumab in Fujian Cancer Hospital increased from 61.38% to 80.32% (P = 0.001), and the utilization rate of trastuzumab in Jiangsu Cancer Hospital increased from 60.73% to 66.30% (P = 0.135).
 |
Table 2 Univariate Analysis of the Factors Associated with the Use of Trastuzumab |
Meanwhile, this study included 1445 patients, who were all diagnosed with CD20-positive NHL, and the baseline information on these patients was provided in Table 3. Among them, rituximab was utilized by 379 of the 585 patients before the policy implementation and by 644 of the 860 patients after. The average proportion of patients who started rituximab therapy increased from 64.79% before the NHIC policy to 74.88% subsequently, and the increase was statistically significant (P = 0.020). Following the implementation of the policy, the utilization rate of rituximab increased from 76.85% to 83.53% in Fujian Cancer Hospital (P = 0.013) and increased from 49.81% to 62.29% in Jiangsu Cancer Hospital (P = 0.002).
 |
Table 3 Univariate Analysis of the Factors Associated with the Use of Rituximab |
Univariate Analysis of the Factors Associated with the Use of Surveyed Medicine
According to univariate analysis, patients in the surveyed drug treatment group and the non-surveyed drug treatment group had different demographic characteristics in the two hospitals. The results are displayed in Table 2 and Table 3.
In the study of trastuzumab, patients living in rural areas, clerks, patients with stage II cancer, and after the policy implementation were more likely to choose trastuzumab for therapy in Fujian Cancer Hospital (P < 0.05). In Jiangsu Cancer Hospital there were significant differences between urban and rural distribution, location of residence, medical insurance, occupational type, and cancer stage (P <0.05). Combining data from two hospitals revealed that the proportion of medical insurance patients, clerks, patients with stage II cancer and after the NHIC policy using trastuzumab increased significantly (P < 0.05).
Furthermore, in the rituximab study, at Fujian Cancer Hospital, there were significant variations in the proportion of patients using rituximab in the subgroups of gender, types of insurance scheme, and the implementation of the policy (P < 0.05). In Jiangsu Cancer Hospital, the distribution of patients using rituximab in subgroups such as urban and rural distribution, location of residence, usage of medical insurance, occupational type, cancer stage, and the implementation of the policy revealed statistical differences (P < 0.05). Combined with the statistics of the two hospitals, it was found that the proportion of patients using rituximab increased in most subgroups, and the increase was significantly different, such as local patients, medical insurance, UEBMI, clerk, advanced cancer, after the policy implemented, and so on (P < 0.05).
Multivariate Logistic Regression Analysis
Multivariable logistic regression analysis was performed to identify further the factors associated with trastuzumab or rituximab. All the factors were included in the multivariable logistic regression analysis to compare the factors’ impact. The details of the multivariable analysis are shown in Tables 4 and Table 7, and the multivariate logistic analysis of Fujian Cancer Hospital is shown in Tables 5 and Table 8. The results of Jiangsu Cancer Hospital are shown in Table 6 and Table 9.
 |
Table 4 Factors Related to the Use of Trastuzumab in the Multivariate Logistic Regression Model |
 |
Table 5 Factors Related to the Use of Trastuzumab in Fujian Cancer Hospital in the Multivariate Logistic Regression Model |
 |
Table 6 Factors Related to the Use of Trastuzumab in Jiangsu Cancer Hospital in the Multivariate Logistic Regression Model |
 |
Table 7 Factors Related to the Use of Rituximab in the Multivariate Logistic Regression Model |
 |
Table 8 Factors Related to the Use of Rituximab in Fujian Cancer Hospital in the Multivariate Logistic Regression Model |
 |
Table 9 Factors Related to the Use of Rituximab in Jiangsu Cancer Hospital in the Multivariate Logistic Regression Model |
In the trastuzumab study, we observed that after the NHIC policy implementation, the patients were statistically considerably more likely to obtain trastuzumab therapy than before (OR=0.610, 95% CI: 0.489–0.761, P = 0.001). Patients with medical insurance were more likely to receive trastuzumab therapies than patients paying for their care (OR=0.804, 95% CI: 0.648 to 0.998, P = 0.048). In addition, patients with non-distant metastatic cancer were more likely to receive trastuzumab than those with distant metastatic cancer (stage IV) (stage I: OR=1.406, 95% CI: 1.073–1.843, P = 0.014; stage II: OR=3.137, 95% CI: 2.26–4.354, P = 0.001; stage III: OR=2.627, 95% CI: 1.969–3.504, P = 0.001). In addition, government administrative staff (OR=1.744, 95% CI: 1.037–2.935, P = 0.036) and clerks (OR=2.3, 95% CI: 1.659–3.188, P = 0.001) were much more likely to receive trastuzumab than patients with other occupational types.
In the rituximab study, the probability of using rituximab after the policy implementation was more than before (OR=0.721, 95% CI: 0.562–0.926, P = 0.010), and the probability of using rituximab in patients with stage IV tumor was greater than in patients with stage I (OR=0.640, 95% CI: 0.459–0.893, P = 0.009). The patients with medical insurance were more likely to use rituximab than self-pay patients (OR=0.713, 95% CI: 0.552–0.919, P = 0.009). Peasants (OR=0.401, 95% CI: 0.293–0.551, P = 0.001) and Retiree (OR=0.511, 95% CI: 0.327–0.798, P = 0.003) were less likely to use rituximab than other occupational types, and clerk (OR=1.712, 95% CI: 1.15–2.55, P = 0.008) were more likely to use rituximab than patients with other occupational types.
The Impact of NHIC Policy on the Use of Trastuzumab and Rituximab
Table 10 and Figure 1 illustrate scatter plots of trastuzumab and rituximab monthly usage and trends from September 2015 to August 2019. September 2017 was regarded as the time point of the implementation of NHIC policy, which divided the time series into two sections, ie, before and after the national insurance coverage inclusion of trastuzumab and rituximab. According to the results of the segmented linear regression model, we plotted the two lines before and after September 2017. From September 2015 to August 2017, the average monthly usage rate of trastuzumab and rituximab increased by 0.102% (P = 0.289) and 0.331% (P < 0.001), respectively. Following the implementation of the NHIC policy in September 2017, both trastuzumab and rituximab experienced a sudden surge in monthly usage rate, with increases of 5.797% (P = 0.003) and 6.328% (P < 0.001), respectively. Between September 2017 and August 2019, the growth in monthly usage rate of trastuzumab and rituximab accelerated compared to the period before NHIC policy implementation, with average monthly increases of 0.842% (P < 0.001) and 0.850% (P = 0.090), respectively. Specifically, at Fujian Cancer Hospital, the average monthly usage rate of trastuzumab and rituximab increased by 0.235% (P = 0.614) and 0.148% (P = 0.051) per month, respectively, between September 2015 and August 2017. After September 2017, trastuzumab and rituximab experienced a sudden surge in monthly usage rate, with increases of 3.207% (P = 0.004) and 6.058% (P = 0.001), respectively. Between September 2017 and August 2019, the growth in monthly usage rate of trastuzumab and rituximab accelerated compared to the period prior to the NHIC policy implementation, yielding average monthly increases of 0.849% (P = 0.002) and 0.556% (P = 0.156), respectively. At Jiangsu Cancer Hospital, the average monthly usage rate of trastuzumab decreased by 0.113% (P = 0.004) between September 2015 and August 2017, while that of rituximab increased by 0.161% (P = 0.002) per month. After September 2017, both trastuzumab and rituximab experienced a sudden surge in their monthly usage rate, with increases of 9.599% (P < 0.001) and 7.073% (P = 0.006), respectively. From September 2017 to August 2019, the growth in monthly usage rate of trastuzumab and rituximab accelerated relative to the period before the NHIC policy implementation, registering average monthly increases of 0.268% (P = 0.021) and 0.956% (P = 0.007), respectively. In addition, we used the median time point of the preintervention period (September 1, 2016) as a pseudo-start period to test the robustness of the results. The results showed that after applying the pseudo-start period (September 1, 2016) for ITS regression analysis, the instantaneous and long-term effects of policy implementation disappeared, indicating the stability of the true intervention start period (Table 11).
 |
Table 10 The Interrupted Time-Series (ITS) Regression Analysis of Monthly Trastuzumab and Rituximab Usage Proportions |
 |
Table 11 The ITS Regression Analysis of Monthly Usage Ratio of Trastuzumab and Rituximab Using Pseudo-Start Period |
 |
Figure 1 Regression analysis of monthly utilization percentage of Trastuzumab and Rituximab before and after policy implementation. |
Discussion
The implementation of the NHIC policy has enhanced the accessibility of trastuzumab and rituximab for patients with cancer in China. After the policy’s implementation, the utilization rate of trastuzumab increased from 61.13% to 75.10%, while the utilization rate of rituximab rose from 64.79% to 74.88%. These findings suggest that a larger number of patients are benefitting from the NHIC policy and can afford these targeted therapies. The multivariate logistic regression analysis further supports this perspective. In the ITS model, comparable observations were made, with the average monthly utilization rates of trastuzumab and rituximab increasing by 20.06% and 24.14%, respectively, in comparison to the period before the policy’s implementation. The introduction of policies typically enhances patients’ access to medications in the short term.13,22 In 2013, the local Jiangsu government established a negotiation mechanism to address patients’ financial burden by selecting clinically essential but expensive medications as special drugs within the medical insurance reimbursement scope for the treatment of major (rare) diseases. The costs of these medications were shared among medical insurance beneficiaries. Trastuzumab was among the first special drugs to be incorporated into the medical insurance reimbursement scheme, leading to an increase in its utilization rate from 16.21% prior to the special drug policy implementation to 44.59% thereafter.23 Notably, the current NHIC policy has not only improved the short-term utilization rates of trastuzumab and rituximab but also demonstrated potential long-term benefits for patients, as indicated by the long-term trend simulation in the ITS model. This outcome aligns with a previous study conducted at a provincial medical center in Fujian Province, where the utilization rate of trastuzumab escalated from 37.4% before the NHIC policy implementation to 69.2% afterward.24 This evidence further underscores that the NHIC policy has increased the accessibility of innovative targeted anticancer drugs and exerted a positive influence on patients utilizing these novel targeted therapies.
The patients’ medical insurance status could pose significant impacts on the utilization of innovative anti-cancer drugs. In China, the UEBMI and URBMI scheme have comprehensive healthcare service coverage and financial protection. It means that cancer patients who use innovative anti-cancer drugs under the UEBMI and URBMI can get adequate reimbursement. However, uninsured cancer patients had to pay out-of-pocket for expensive anti-cancer drugs. Lack of adequate financial protection leads to the underuse of trastuzumab and rituximab for self-paying patients. Medical insurance coverage has consistently played a crucial role in enhancing the accessibility of expensive cancer treatments, as extensively investigated by numerous scholars.25–27 Doctors typically recommend the use of trastuzumab for HER2+ breast cancer beyond stage IA, while the recommendation for rituximab spans across all stages. Therefore, theoretically, the frequency of trastuzumab use is higher in stages 2, 3, and 4 compared to stage 1, while rituximab use is similar across all stages. However, the high cost of medication needs to be taken into consideration, as many patients may not be able to afford the treatment and choose not to follow the doctor’s recommendations. Similarly, this study demonstrates the essential role of medical insurance in the utilization of trastuzumab and rituximab. The results of multivariate logistic regression analysis reveal that the likelihood of out-of-pocket patients using trastuzumab and rituximab is 0.804 and 0.713, respectively, in comparison to patients with medical insurance. Thus, it can be inferred that patients with medical insurance are more inclined to receive these treatments than out-of-pocket patients. The broadening of medical insurance coverage has been empirically demonstrated as a vital element in enhancing the accessibility of targeted therapeutics, such as trastuzumab and rituximab. More comprehensive coverage not only facilitates access but also eases financial burdens, potentially amplifying the utilization of these drugs. Physicians, assured that expenses are likely to be covered by insurance, may be more inclined to recommend these therapeutic regimens. Such policies do more than merely escalate drug availability; they may positively impact patient survival rates and overall quality of life. Although the NHIC policy manifests similarities in its approach to reducing costs and augmenting availability of anti-cancer drugs, our research delineates unique benefits attributed to the NHIC policy for patients. The NHIC policy diverges from previous strategies that solely aimed to promote drug accessibility through medical insurance. The NHIC policy not only benefits insured patients but also directly reduces the prices of these innovative targeted anticancer drugs. This approach allows all patients to access these medications at more affordable prices. As a result, out-of-pocket patients can obtain clinically effective treatments at costs approximately equivalent to those incurred by patients with medical insurance. Currently, medical insurance reimbursement policies in various regions of China are not without flaws. Patients with severe diseases, such as cancer, are more inclined to seek treatment in tertiary hospitals.28,29 However, a considerable number of patients from other provinces are unable to utilize their medical insurance for reimbursement at out-of-town facilities.30 These patients may benefit significantly from the implementation of the National Health Insurance Coverage (NHIC) policy. Additionally, in this study, many patients in the target population for trastuzumab and rituximab treatments are from regions outside Jiangsu and Fujian provinces. These out-of-town patients are likely to return to their medical insurance locations to purchase trastuzumab and rituximab after receiving initial treatment at the hospital.31 Consequently, the actual utilization rate of trastuzumab and rituximab may be higher than the results reported in this study.
The implementation of the NHIC policy or expansion of medical insurance coverage could potentially lead to an increase in the use of high-cost treatments such as trastuzumab and rituximab. However, clinical misuse may follow, possibly driving an overall rise in healthcare spending and placing strains on both the supply chain and existing medical infrastructure. Therefore, safeguarding the sensible and sustainable use of medical resources is essential. Moreover, it should be understood that neither the NHIC policy nor expanded medical insurance coverage can indefinitely reduce the costs associated with trastuzumab and rituximab. In China’s diverse landscape, policy impacts may vary across different regions and income levels. Even with increased coverage, there may remain areas where policy implementation is less effective, leaving some population segments with reduced benefits from these measures. Drug usage disparities between patients in various demographic subgroups are minimized. In univariate analysis, we discovered that in the trastuzumab and rituximab studies, the proportion of patients in various demographic groupings grew, and the differences were reduced. In China, urban-rural inequality in life expectancy, maternal mortality, infant mortality, and health resources has aroused attention among policymakers in recent years. Interestingly, results from our study showed that there were no significant differences in accessing trastuzumab and rituximab between patients living in urban areas and patients living in rural areas. The most likely reason is that Jiangsu and Fujian are located in the eastern coastal economic-developed region, which reduced the effects of urban-rural differences. However, we argue that the specific challenges faced by rural populations in accessing trastuzumab and rituximab. For instance, inadequate supply of health service resources and inconvenient transportation in rural areas could lead to urban-rural inequality in the utilization of innovative anti-cancer drug therapy. It implies that transportation infrastructure and medical service capacity play critical roles in accessing trastuzumab and rituximab for patients living in remote rural districts. Currently, the key strategies to bridge this divide and ensure equitable access across urban and rural geographical areas are to promote the equality of health services utilization among the rural population. The policy recommendation would be that the government should facilitate the two-way referral system between urban tertiary hospitals and remote rural community hospitals to reduce the urban-rural gap in health service utilization. The utilization rate of trastuzumab in rural regions was higher than it was in urban regions but not statistically significant. In multivariate analysis, the utilization rate of trastuzumab and rituximab among rural and urban regions also showed that it was not statistically significant. Therefore, it was estimated that these surveyed drugs’ inclusion in NHIC policy could promote medical equity for regions.32,33
In this study, there were significant impacts of socioeconomic status on the selection of trastuzumab and rituximab in cancer patients. This implies that income level, medical insurance, occupation, and repayment abilities play an important role in the selection of innovative anti-cancer drug therapy for cancer patients. For instance, the cancer patients’ occupation type not only links to income levels but also poses an impact on employment status. If the patients’ jobs involved heavy physical effort, they had to change their jobs when they are diagnosed with cancer. Some pieces of evidence demonstrated that manual labor was negatively correlated with a return to work.34 The Chinese peasants still have to deal with heavy manual labor and are more likely to lose their jobs due to serious diseases. As opposed to this, the government administrative staff have mental labor and can take sick leave during treatment. Unemployment means a dramatic change in economic position, which may result in lower acceptability of innovative anti-cancer drugs in cancer patients. However, among the occupations, the univariate and multivariate analysis also indicated that peasants had fewer benefits from the policy than other occupations. The utilization rate of peasants was the last rank in the study, only 0.922 times that of other occupations in the trastuzumab study and 0.401 in the rituximab study, respectively. This might be because peasants have lower incomes and the capacity to pay, making them less willing to receive trastuzumab or rituximab.35–37 Even though trastuzumab and rituximab are already covered by health insurance, the illness burden remains high. In China, different occupations represent different social classes and income levels. Inequality of income in different social classes has gradually become a major concern in China. Recently, a previous study showed that businessmen have been regarded as one of the richest social classes in China.38 Moreover, it was demonstrated that the highest-income populations in China were government administrative staff, whereas the lowest incomes were earned by peasants.39 Therefore, huge differences in the occupations of Chinese cancer patients may lead to inequality in individual earnings and household incomes. The major cause of inequality is the patients’ different salaries and work stability.40 Due to low-income levels or poverty, the possibility of obtaining innovative anti-cancer drug therapy decreased significantly, especially when it was high-priced and paid for privately. In certain parts of China, a huge number of low-income peasants exist. Patients do not have financial capacity to afford trastuzumab or rituximab therapy. Although the implementation of NHIC, financial burden is still a source of strain for rural patients during cancer therapy.41 The government should increase its efforts to lower the illness burden among rural patients.42 Given the economic disparities between cancer patients with different socioeconomic statuses, it is crucial to improve the multi-level medical insurance coverage for economically disadvantaged patients. The multi-level medical security systems include but are not limited to, basic medical insurance, medical assistance, serious illness insurance, and commercial insurance jointly established by the government, enterprises, the market, and individuals.
Our study has several strengths, including examining the impact of China’s NHIC policy on targeted cancer therapies’ utilization, specifically trastuzumab and rituximab. Results show a statistically significant increase in usage after policy implementation, with the NHIC policy being a key determinant. However, factors like patients’ income levels, repayment abilities, medical insurance status, occupations, and cancer disease stages still affect utilization. Limitations include being a two-center study, potentially causing selection bias and limited representativeness. Data sources may be influenced since the study was conducted in urban medical centers. Some patients had insufficient illness information and lacked economic indicators, possibly affecting the utilization of new anti-cancer medications. The research may not accurately represent the benefits of national policy implementation due to regional economic differences. Further investigation into the effects of expanding medical insurance coverage on the utilization of trastuzumab and rituximab remains an area for future in-depth research. Our study provides an initial understanding of the potential long-term benefits of the NHIC policy in enabling access to trastuzumab and rituximab in China over a 4-year period. However, we cannot yet confirm whether the initial growth in utilization rates is sustainable over time or if the policy leads to a lasting impact on patient access to these targeted therapies. Further comprehensive investigations, enhanced data collection, and careful analyses will be necessary to elucidate these aspects. The retrospective study design without a simultaneous control group makes it difficult to attribute results solely to the NHIC policy, necessitating further research with larger sample sizes and more rigorous designs.
Conclusion
This study compared the utilization of trastuzumab in patients with HER2-positive breast cancer and rituximab in patients with NHL. According to the findings, the NHIC policy positively impacted the utilization of trastuzumab and rituximab. Following the NHIC policy, medical insurance status, occupations, and cancer disease stage affected trastuzumab and rituximab use, indicating that the patient’s income level and repayment ability remain to influence the novel anti-cancer drug usage. As a result, appropriate measures, such as reducing the occupations of urban-rural divide and broadening medical insurance coverage, would allow more individuals to access novel anti-cancer medicines.
Ethical Statement
This study was approved by the Ethics Committee of Nanjing Medical University (No.: 2020103). Government administrative staff permission was granted by Nanjing Medical University to access and use the datasets/medical records described in this study. This study does not involve human participants or animals. The manuscript does not contain any personally identifiable data in any form. According to Article 39 of the Regulation of Ethical Review of Human Biomedical Research, issued by the National Health and Family Planning Commission of the People’s Republic of China in 2016, when the data used for the study cannot be traced back to the patients, and the study is not for commercial purposes, patients’ informed consent to participate can be waived upon approval from the Ethics Committee. All procedures were in compliance with the Declaration of Helsinki.
Acknowledgments
The authors are grateful for the support of the department of Information Technology of Jiangsu Cancer Hospital and Fujian Cancer Hospital. The authors also appreciate all participants who contributed to the data collection and analysis in this study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the grants of the National Natural Science Foundation of China, (Grant Number:72074123) and the General Project of Philosophy and Social Science Research Project of Jiangsu Universities (No: 2021SJA0306); The funder was not involved in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Disclosure
The authors declare that this work has no conflicts of interest.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Yacong Z, Zhangyan L, Fangfang S, Kexin C. Trends of incidence and mortality of breast cancer worldwide and in China. J Multidiscip Cancer Manag. 2021;7(2):14–20.
3. Escriva-de-Romani S, Arumi M, Bellet M, Saura C. HER2-positive breast cancer: current and new therapeutic strategies. Breast. 2018;39:80–88. doi:10.1016/j.breast.2018.03.006
4. Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344(11):783–792. doi:10.1056/NEJM200103153441101
5. Liu W, Liu J, Song Y, et al. Burden of lymphoma in China, 2006–2016: an analysis of the global burden of disease study 2016. J Hematol Oncol. 2019;12(1):115. doi:10.1186/s13045-019-0785-7
6. Cao W, Chen HD, Yu YW, Li N, Chen WQ. Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics 2020. Chin Med J. 2021;134(7):783–791. doi:10.1097/CM9.0000000000001474
7. Yu-Wen H, Mei-Bian Z, Xiang X, Xiao-Hua X, Quan Z, Le J. Socioeconomic inequality in the use of rituximab therapy among non-Hodgkin lymphoma patients in Chinese public hospitals. Asia Pac J Public Health. 2014;26(2):203–214. doi:10.1177/1010539512464648
8. Sledge GW. What is targeted therapy? J Clin Oncol. 2005;23(8):1614–1615. doi:10.1200/JCO.2005.01.016
9. Shih YT, Xu Y, Liu L, Smieliauskas F. Rising prices of targeted oral anticancer medications and associated financial burden on medicare beneficiaries. J Clin Oncol. 2017;35(22):2482–2489. doi:10.1200/JCO.2017.72.3742
10. Gyawali B, Sullivan R. Economics of cancer medicines: for whose benefit? New Bioeth. 2017;23(1):95–104. doi:10.1080/20502877.2017.1314885
11. Si L, Xu L, Chen M, Jan S. Using strategic price negotiations to contain costs and expand access to medicines in China. BMJ Glob Health. 2020;5(1):e002256. doi:10.1136/bmjgh-2019-002256
12. Tang M, Song P, He J. Progress on drug pricing negotiations in China. Biosci Trends. 2020;13(6):464–468. doi:10.5582/bst.2019.01339
13. Guan X, Wushouer H, Yang M, et al. Influence of government price regulation and deregulation on the price of antineoplastic medications in China: a controlled interrupted time series study. BMJ Open. 2019;9(11):e031658. doi:10.1136/bmjopen-2019-031658
14. Moye-Holz D, van Dijk JP, Reijneveld SA, Hogerzeil HV. Policy approaches to improve availability and affordability of medicines in Mexico - an example of a middle income country. Global Health. 2017;13(1):53. doi:10.1186/s12992-017-0281-1
15. Limwattananon C, Waleekhachonloet O. Access to and price trends of antidiabetic, antihypertensive, and antilipidemic drugs in outpatient settings of the universal coverage scheme in Thailand. PLoS One. 2019;14(2):e0211759. doi:10.1371/journal.pone.0211759
16. Lammers P, Criscitiello C, Curigliano G, Jacobs I. Barriers to the use of trastuzumab for HER2+ breast cancer and the potential impact of biosimilars: a physician survey in the united states and emerging markets. Pharmaceuticals. 2014;7(9):943–953. doi:10.3390/ph7090943
17. NHC. Chinese guidelines for diagnosis and treatment of breast cancer; 2018. Available from: http://www.nhc.gov.cn/cms-search/xxgk/getManuscriptXxgk.htm?id=b21802b199814ab7b1219b87de0cae51.
18. NHC. Guidelines for the clinical application of new anti-tumour drugs; 2018. Available from: http://www.nhc.gov.cn/cms-search/xxgk/getManuscriptXxgk.htm?id=0ea15475f58a4f36b675cfa4716fa1e4.
19. Kaiyuan S, Yan S, T L. China standard for diagnosis and treatment of malignant lymphoma. Chin J Cancer. 2015;37(2):148–158.
20. Imbens GW, Lemieux T. Regression discontinuity designs: a guide to practice. J Econometrics. 2008;142(2):615–635. doi:10.1016/j.jeconom.2007.05.001
21. Linden A. Conducting interrupted time-series analysis for single- and multiple-group comparisons. Stata J. 2015;15(2):480–500. doi:10.1177/1536867X1501500208
22. Berkemeier F, Whaley C, Robinson JC. Increasing divergence in drug prices between the United States and Germany after implementation of comparative effectiveness analysis and collective price negotiations. J Manag Care Spec Pharm. 2019;25(12):1310–1317. doi:10.18553/jmcp.2019.25.12.1310
23. Xia Y, Zheng M, Zhan X, et al. The use of trastuzumab affected by health insurance policy in Jiangsu Province of China. Transl Cancer Res. 2021;10(1):509–519. doi:10.21037/tcr-20-3329
24. Diao Y, Lin M, Xu K, et al. How government health insurance coverage of novel anti-cancer medicines benefited patients in China - a retrospective analysis of hospital clinical data. Bmc Health Serv Res. 2021;21(1):856. doi:10.1186/s12913-021-06840-3
25. Diao Y, Qian J, Liu Y, et al. How government insurance coverage changed the utilization and affordability of expensive targeted anti-cancer medicines in China: an interrupted time-series study. J Glob Health. 2019;9(2):020702. doi:10.7189/jogh.09.020702
26. Cho DY, Park J, Kim DS. The impact of expanding health insurance coverage for anti-cancer drugs on cancer survival in Korea. Cancer Med. 2021;10(13):4555–4563. doi:10.1002/cam4.3979
27. Meng Q, Fang H, Liu X, Yuan B, Xu J. Consolidating the social health insurance schemes in China: towards an equitable and efficient health system. Lancet. 2015;386(10002):1484–1492. doi:10.1016/S0140-6736(15)00342-6
28. Liu M, Hu L, Xu Y, Wang Y, Liu Y. Patient healthcare experiences of cancer hospitals in China: a multilevel modeling analysis based on a national survey. Front Public Health. 2023;11:1059878. doi:10.3389/fpubh.2023.1059878
29. Chen C, Feng Z, Ding Y, et al. What factors hindered the access to essential anticancer medicine in public hospitals for the local population in Hubei Province, China. Front Pharmacol. 2021;12:734637. doi:10.3389/fphar.2021.734637
30. Zhu Y, Wang Y, Sun X, Availability LX. Price and affordability of anticancer medicines: evidence from two cross-sectional surveys in the Jiangsu Province, China. Int J Environ Res Public Health. 2019;16(19):3728. doi:10.3390/ijerph16193728
31. Zhang A, Nikoloski Z, Mossialos E. Does health insurance reduce out-of-pocket expenditure? Heterogeneity among China’s middle-aged and elderly. Soc Sci Med. 2017;190:11–19. doi:10.1016/j.socscimed.2017.08.005
32. Semprini JT, Biddell CB, Eberth JM, et al. Measuring and addressing health equity: an assessment of cancer center designation requirements. Cancer Causes Control. 2023. doi:10.1007/s10552-023-01680-4
33. Baeten SA, Baltussen RM, Uyl-de Groot CA, Bridges J, Niessen LW. Incorporating equity-efficiency interactions in cost-effectiveness analysis-three approaches applied to breast cancer control. Value Health. 2010;13(5):573–579. doi:10.1111/j.1524-4733.2010.00718.x
34. Taskila-Brandt T, Martikainen R, Virtanen SV, Pukkala E, Hietanen P, Lindbohm ML. The impact of education and occupation on the employment status of cancer survivors. Eur J Cancer. 2004;40(16):2488–2493. doi:10.1016/j.ejca.2004.06.031
35. Chiu SYR, Yang Z. Influence of family income and medical insurance coverage on health-related quality of life and optimism in cancer patients at a Hong Kong private hospital: a cross-sectional study. Psychooncology. 2019;28(10):1971–1977. doi:10.1002/pon.5175
36. Jiang H, Mou W, Lyu J, et al. Assessment of self-reported financial toxicity among patients with nasopharyngeal carcinoma undergoing radiotherapy: a cross-sectional study in western China. Front Oncol. 2022;12:1011052. doi:10.3389/fonc.2022.1011052
37. Fu W, Shi J, Zhang X, et al. Effects of cancer treatment on household impoverishment: a multicentre cross-sectional study in China. BMJ Open. 2021;11(6):e044322. doi:10.1136/bmjopen-2020-044322
38. Yule Z. An analysis on the inequality of income in China. J Econ Water Resour. 2006;24(6):12–14.
39. Qiang L. A primary investigation on the high-income population in China. Data. 2009;2009(1):54–56.
40. Yoon TH, Lee SY, Kim CW, Kim SY, Jeong BG, Park HK. Inequalities in medical care utilization by South Korean cancer patients according to income: a retrospective cohort study. Int J Health Serv. 2011;41(1):51–66. doi:10.2190/HS.41.1.d
41. Sun CY, Shi JF, Fu WQ, et al. Catastrophic health expenditure and its determinants among households with breast cancer patients in china: a multicentre, cross-sectional survey. Front Public Health. 2021;9:704700. doi:10.3389/fpubh.2021.704700
42. Zhang X, Liu S, Liu Y, et al. Economic burden for lung cancer survivors in urban China. Int J Environ Res Public Health. 2017;14(3):1.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.