Back to Journals » Cancer Management and Research » Volume 13
How Long is It Safe to Wait for Breast Surgery After Completion of Neoadjuvant Chemotherapy?
Received 16 October 2020
Accepted for publication 18 December 2020
Published 3 February 2021 Volume 2021:13 Pages 989—998
DOI https://doi.org/10.2147/CMAR.S287089
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Bilikere Dwarakanath
Tianyi Ma, Yan Mao, Haibo Wang
Department of Breast Center of the Affiliated Hospital of Qingdao University, Qingdao, Shandong Province, 266000, People’s Republic of China
Correspondence: Haibo Wang; Yan Mao Email [email protected]; [email protected]
Purpose: This study aimed to evaluate the impact of surgical time on postoperative complications and survival outcomes in breast cancer patients after neoadjuvant chemotherapy (NAC).
Patients and Methods: We retrospectively reviewed breast cancer patients treated at Breast Disease Center of the Affiliated Hospital of Qingdao University, from January 2013 to December 2018. The eligibility criteria were female patients with histologically confirmed primary stage II–III breast cancer and initially treated with NAC, who were < 75 years old, and patients for whom medical records were available. The patients with severe comorbidities of other organs, with previous histories of other malignancies or breast cancer, and with distant metastasis or contralateral breast cancer, were excluded. Eligible patients were divided into three groups based on time to surgery (TTS): (A) ≤ 21 days; (B) between 21 and 28 days; and (C) > 28 days. We collected medical records and followed up patients.
Results: Totally 422 patients were enrolled. The median TTS was 26 days. Among these patients, 119 (28.2%) were in Group A, 152 (36.0%) were in Group B, and 151 (35.8%) were in Group C. Eighty-two (19.4%) patients achieved pathologic complete response (pCR). Survival analysis showed that DFS (P=0.012) and OS (P=0.015) were significantly different among three groups. In multivariate analysis, DFS (HR=2.333, P=0.001) and OS (HR=2.783, P=0.030) were significantly worse when TTS > 28 days. Postoperative complications occurred in 96 (22.7%) patients. The incidence of total postoperative complications in the three groups was statistically different (P=0.001) and Group A had worse performance. Multivariate analysis showed that age > 50 years old (P=0.004) and TTS ≤ 21 days (P< 0.001) were independent parameters for total postoperative complications.
Conclusion: Postoperative complications and survival outcomes in breast cancer patients seemed to be influenced by TTS after the NAC. The benefits were remarkable in patients undergoing surgery between 21 and 28 days.
Keywords: breast cancer, neoadjuvant chemotherapy, time to surgery, survival, postoperative complication
Introduction
Neoadjuvant chemotherapy (NAC) has been widely used in breast cancer patients due to its advantages such as reducing tumor stage, increasing the operation opportunity of inoperable patients, improving breast-conserving rate, evaluating drug efficacy and guiding adjuvant treatment strategies.1 Nonetheless, the time interval between surgery and the NAC remains to be determined. Using the National Cancer Database (NCDB) and the Surveillance, Epidemiology, and End Results (SEER) Database, two independent population-based studies were conducted by Bleicher et al.2 It was evidenced from the results that the lower disease-specific survival rate and overall survival rate were closely related to the long-term delay from the diagnosis to the surgery. The timeliness of treatment (including surgery and systemic treatment) could affect the prognosis of patients. Therefore, it can be inferred that prolonged surgery delay affect the prognosis of patients treated with NAC, especially those with high-risk factors. In the past studies, the relationship between the time to surgery (TTS) after NAC and the prognosis of breast cancer patients was controversial. Omarini et al thought that survival would be affected when the TTS was >21 days, while Suleman et al believed that TTS might not affect survival.3,4 Moreover, the incidence of postoperative complications can also affect clinicians’ choice of TTS after NAC. Some studies have suggested that NAC was a risk factor for early postoperative complications, but the specific relationship between TTS and complications was still unknown.5
Therefore, the specific relationship between TTS and patients’ survival was intended to be explored by this study, as well as between TTS and postoperative complications, to obtain the optimal TTS after NAC.
Patients and Methods
Study Population
We retrospectively reviewed patients who were diagnosed with breast cancer at Breast Disease Center of the Affiliated Hospital of Qingdao University, from January 2013 to December 2018. The eligibility criteria were female patients with histologically confirmed primary stage II–III breast cancer and initially treated with NAC, who were <75 years old, and patients for whom information regarding the treatment records was available. The patients with severe comorbidities of other organs, with previous histories of other malignancies or breast cancer, and those with distant metastasis or contralateral breast cancer, were excluded.
In our database, there were 8311 breast cancer patients from January 2013 to December 2018, including 473 patients who received NAC. We extracted the patients’ medical history and treatment information from the database. Then, 422 patients satisfied the criteria and were screened out. And these patients were divided into three groups based on TTS: (A) TTS ≤21 days; (B) TTS between 21 and 28 days; and (C) TTS>28 days.
Before NAC, all patients underwent systemic survey to exclude distant metastasis. With the PET-CT being performed on selected patients, the systemic survey included the brain magnetic resonance imaging (MRI) and bone scans, the chest computed tomography (CT) scans, the ultrasound scans of the liver and the neck, and the serum tumor markers for all the patients. Applying the core-needle biopsy method, all patients’ primary breast cancers were histologically identified. The results of immunohistochemistry were obtained in 365 (86.5%) patients before NAC. Human epidermal growth factor receptor 2 (HER2) positivity was defined as either 3+ on IHC staining or 2+ on IHC with a positive fluorescence in situ hybridization or chromogenic in situ hybridization signal. The molecular subtype of each breast cancer was categorized as follows: ER+ or PR+, and HER2− (luminal); ER+/−, PR+/−, and HER2+ (HER2 enriched); or ER−, PR−, and HER2− (triple-negative). The NAC regimens were determined according to the guidelines. Every two cycles, we evaluated the efficacy of chemotherapy by physical examinations and imaging examinations. Pathologic complete response (pCR) was defined as the absence of histological evidence of malignancy or the presence of only cancer in situ in the primary breast lesions, regardless of axillary lymph node metastasis. Patients with absence of invasive disease in the breast but presence of axillary lymph node metastasis were also defined as pCR patients. The incidence of surgery-related complications and treatment measures were recorded through the patients’ medical records and periodic follow-up. All patients were followed up every six months after surgery. After recurrence or metastasis of tumor, patients were reexamined every two months.
Statistical Analysis
From the initial diagnosis of the primary breast cancer to the tumor recurrence or metastasis, the pertinent time interval was referred to as the disease-free survival (DFS). The overall survival (OS) was considered as the interval between the initial diagnosis of the primary breast cancer and the last follow-up or the death due to any cause. Applying the chi-square test, the rank sum test, or the Fisher’s exact test, the differences in the categorical variables were compared. The Kaplan-Meier method and the Log rank test were used to calculate the actuarial survival rates. In the multivariate analysis, the factors with a p-value<0.05 in the univariate analysis were included. The p<0.05 was considered to be statistically significant in the multivariate analysis, which was performed with the Cox proportional hazards model. The SPSS software (version 17.0.1. SPSS Inc., Chicago, IL) was used in all the statistical analyses.
Ethical Statement
Meeting all the guidelines of the governmental agency, the institutional review committee of the Affiliated Hospital of Qingdao University approved the experimental protocols. All patients provided informed consent, and this study was conducted in accordance with the Declaration of Helsinki.
Results
Cohort Survey
In total, 422 patients were enrolled. Among these patients, 119 (28.2%) patients had surgery within 21 days of their last dose of NAC, 152 (36.0%) patients in 21–28 days, and 151 (35.8%) patients after 28 days. The process of screening and grouping is shown in Figure 1. The median age of all the patients was 49 years old (range, 22–73 years), the median duration of follow-up was 42 months (range, 24 to 93 months), while the median TTS was of 26 days (range, 14 to 63 days) for all the 422 patients. They received anthracycline- or taxane-based NAC, and 82 patients achieved pCR (19.4%). Forty-two (10.0%) patients changed the chemotherapy regimen because of insufficient efficacy, and 20 (4.7%) patients interrupted the scheduled regimen because of the side effects of the drugs.
 |
Figure 1 The procedure of screening and grouping patients. Abbreviations: NAC, neoadjuvant chemotherapy; TTS, time to surgery. |
The main pathological type of primary breast cancer is non-specific invasive ductal carcinoma (401/422, 95.0%), in addition to some invasive lobular carcinoma, mucinous carcinoma, medullary carcinoma and so on. In terms of the molecular subtype, 41.2% (174/422), 45.7% (193/422) and 13.0% (55/422) of the patients were luminal, HER2-enriched and triple-negative, respectively. The pCR rates of patients with molecular subtype luminal, HER2-enriched and triple-negative were 7.5% (13/174), 28.0% (54/193) and 27.3% (15/55), respectively. Among these 193 HER2-enriched patients, 72.0% (139/193) patients were treated with trastuzumab in neoadjuvant therapy, and 91.2% (176/193) patients were treated with trastuzumab in adjuvant therapy. Of all 422 patients, 147 (33.3%) patients had tumor recurrence or metastasis during follow-up and 52 (12.3%) patients died. The median DFS was 36 months (range, 4 to 93 months), and the 3-year OS rate was 94.1% (397/422). When tumor progression was first detected, 30 patients had local recurrence, 12 patients were observed to have had distant metastasis and local recurrence both, while 79 patients showed distant metastasis. The lung, followed by the bone, was indicated to be the most common metastatic organ.
Survival Analysis of Patients in the Different TTS Groups
Regarding the different TTS, 442 patients were divided into three groups. Table 1 shows the comparison of patients’ characteristics in three groups. There was no significant difference in age, menstrual state, family history relative to breast cancer, tumor T stage before NAC, axillary lymph nodes involvement before NAC, histological grade, HR status, HER2 status, Ki-67 value, molecular subtype, drugs of NAC, clinical response to NAC, breast surgery type, pCR or non-pCR, pN stage after surgery, adjuvant chemotherapy, adjuvant radiotherapy, and targeted therapy.
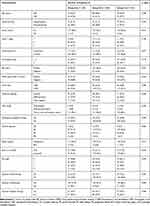 |
Table 1 The Comparison of Patients’ Characteristics in Three Groups |
The median DFS of the Group A was 43 months (range, 9 to 87 months), the median DFS of the Group B was 35.5 months (range, 4 to 81 months), and the median DFS of the Group C was 33 months (range, 6 to 93 months). In the three groups, the median OS was found to be 46 months (range, 25 to 87 months), 41 months (range, 24 to 81 months) and 39 months (range, 25 to 93 months) respectively. The 3-year OS rate of the three groups was 95.8% (114/119), 96.7% (147/152) and 90.1% (136/151), respectively.
Figure 2 indicates the results of the Kaplan-Meier survival curves using the Log rank test for the DFS and the OS. Survival analysis showed that DFS (P=0.012) and OS (P=0.015) were significantly different among three groups. Between Groups A and B, no significant difference in the DFS (P=0.595) and the OS (P=0.716) was observed. Nonetheless, between Groups A and C, the survival analysis indicated that DFS (P=0.006) and the OS (P=0.014) were found to be significantly different. At the same time, the DFS (P=0.024) and OS (P=0.027) of Group B were significantly different from that of Group C. This indicated that TTS >28 days did affect the survival outcome of these patients.
 |
Figure 2 Kaplan–Meier curves for survival according to different time to surgery. (A) Disease-free survival (P=0.012). (B) Overall survival (P=0.015). |
Univariate and Multivariate Analyses for DFS and OS
The univariate analysis of DFS showed that pCR, pN stage, adjuvant chemotherapy and TTS were significant factors (P<0.001, P<0.001, P=0.001 and P=0.003, respectively). For OS, the univariate analysis showed that pN stage, adjuvant chemotherapy and TTS were significant factors (P=0.014, P=0.001 and P=0.032, respectively). In the multivariate analyses, DFS and OS were significantly worse when TTS >28 days with a hazard ratio of 2.333 (95% CI, 1.444–3.770, P=0.001) and 2.783 (95% CI, 1.007–7.687, P=0.030) respectively. Patients with pCR, pN2-3 stage and adjuvant chemotherapy were independent parameters for DFS (P=0.042, P<0.001 and P=0.001, respectively), while pN2-3 stage and adjuvant chemotherapy were also independent parameters for OS (P=0.024 and P=0.010, respectively). The relevant statistics are shown in Table 2.
 |
Table 2 Univariate and Multivariate Analysis for DFS and OS |
Relationship Between TTS and Postoperative Complications
Postoperative complications occurred in 96 (22.7%) patients, including poor healing of incision, infection, hematoma or hemorrhage, subcutaneous effusion and skin flap necrosis. The incidence of postoperative complications is shown in Table 3 and the classification of severity is shown in Table 4. Most of the patients were treated in the outpatient department; only 4.2% (4/96) of the patients returned to the operating room and received general anesthesia operations. The incidence of total postoperative complications in the three groups was statistically different (P=0.001). Compared with Group B (P=0.003) and Group C (P<0.001), Group A had worse performance in total postoperative complications. The total complication rates of Group B and Group C were similar (P=0.566). This suggested that TTS ≤21 days was significantly associated with a higher incidence of total postoperative complications. And among the five kinds of complications, the incidence of poor incision healing (P=0.024) and infection (P=0.031) was significantly different among the three groups. The univariate analysis indicated that age and TTS were the significant factors for total postoperative complications (P=0.009 and P<0.001, respectively). In the multivariate analysis, age >50 years old and TTS ≤21 days were independent parameters for total postoperative complications (P=0.004 and P<0.001, respectively). The statistical details are shown in Table 5.
 |
Table 3 Relationship Between TTS and Postoperative Complications |
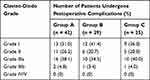 |
Table 4 Classification of the Severity of Postoperative Complications |
 |
Table 5 Univariate and Multivariate Analysis for Postoperative Complications |
Causes of Surgery Delay
The causes of surgery delay in Group C were analyzed. There were six main reasons: neutropenia caused by chemotherapy drugs (47.0%), liver dysfunction caused by chemotherapy drugs (33.1%), heart disease or other comorbidities (14.6%), waiting for breast MRI appointment (9.3%), discussion time of treatment strategy (7.9%) and medical insurance or hospitalization appointment (7.3%). The most common causes were due to the side effects of chemotherapy drugs, such as hematological toxicity and liver toxicity.
Discussion
The effectiveness and safety of NAC in the treatment of breast cancer had been confirmed from the numerous clinical trials.6,7 Besides not presenting any specific interval from the completion of the NAC to the surgery, none of the trials could evaluate the relationship between NAC and postoperative complications. At present, clinicians mainly infer the interval from NAC to surgery based on the interval data of adjuvant chemotherapy, but its applicability to the NAC is unclear.8 Therefore, we conducted this retrospective study to analyze the relationship between TTS and patient survival as well as the relationship between TTS and postoperative complications, trying to find the appropriate TTS interval.
In this study, we retrospectively collected data from 422 patients and analyzed the TTS and survival. Through analysis, it was observed that in the case of the patients with TTS >28 days, the OS and the DFS were evidently different when compared with those from the other two groups. The TTS >28 days also being an independent prognostic factor capable of influencing the survival outcome was simultaneously indicated by the multivariate analysis. This suggested that prolonged TTS did have an adverse effect on the survival of patients, and TTS ≤28 days may be the most favorable surgery time for the prognosis of patients. In addition, we found that pCR, pN2-3 stage and adjuvant chemotherapy were factors affecting DFS; pN2-3 stage and adjuvant chemotherapy were factors affecting OS while pCR did not have an impact on OS. This suggested that pCR after neoadjuvant chemotherapy may not translate into long-term survival benefits. The incidence of tumor recurrence or metastasis in 3 years in patients with adjuvant chemotherapy was significantly higher than that in patients without adjuvant chemotherapy. This may be because most of the patients receiving adjuvant chemotherapy after surgery were patients with worse NAC curative effect, and their tumors may have worse biological characteristics.
The results of Omarini et al indicated that prolonged TTS was not conducive to the survival of patients. They studied 319 patients and found that TTS was an independent prognostic factor for OS (P=0.03) and recurrence-free survival (RFS) (P=0.01), even in the pCR subgroup. And they believed that the best time for surgery was TTS ≤21 days.3 However, some clinicians believed that TTS might have little relationship with the prognosis of patients. Sanford et al followed up 1011 patients and found that although there were differences in 5-year OS among patients with TTS ≤4 weeks, 4–6, and >6 weeks (P=0.03), there was no difference in 5-year RFS (P=0.28) and local recurrence-free survival (LRFS) (P=0.31) among the three groups. In multivariate analysis, OS, LRFS and RFS of patients with TTS ≤4 weeks, 4–6 weeks and >6 weeks were comparable; sensitivity analysis showed that patients who underwent surgery at >8 weeks had worse OS (P=0.02), but there was no significant difference between RFS and LRFS.9 Suleman et al found that TTS had no effect on DFS (P=0.3) and OS (P=0.5), but the pCR rate of ER +/HER2 + patients decreased when TTS ≥8 weeks (12.9% versus 25.3%).4 Although the results of these retrospective studies were suggestive, they were controversial. Large-sample and multi-center clinical cases are needed for further analyses.
By inhibiting the cell division, the protein synthesis, the RNA, or DNA production, most of the antineoplastic drugs were observed to exert their cytotoxic impact in the NAC of breast cancer. Previous animal experimental data showed that wound healing was affected within 28 days after the application of cytotoxic drugs.10 Previous clinical studies have shown that immunosuppression after chemotherapy is a known factor leading to postoperative complications.11,12 The association of TTS in cases of postoperative complications was revealed from this study. In patients with shorter TTS, the incidence of complications such as poor incision healing and infection had an obvious increasing trend. In the multivariate analysis, age <50 years old and TTS ≤21 days were independent parameters for total postoperative complications. The decrease of neutrophils, the increase of vascular fragility and tissue edema may be the reasons for the increased incidence of these complications. However, we also observed that most of the postoperative complications were mild and could be treated in the outpatient department. Similarly, defining the time interval to be 28 days, the higher incidence of postoperative complications was found to have a shorter TTS by a study of Sutton et al.13 It was also observed that with age as the dominant predictor, compared to TTS greater than 28 days (P <0.05), the TTS of 28 days or less was associated with almost 70% increased chances of a wound complication. Considering the influence of chemotherapy and radiotherapy, only a few patients choose immediate reconstruction surgery after NAC, so we failed to evaluate the impact of TTS on postoperative complications of patients with immediate breast reconstruction. In previous studies, in the case of perioperative complications in patients with immediate breast reconstruction, NAC was not observed to be a risk factor.14 As an increase in the number of patients with immediate breast reconstruction in our center recently, we will further do some research in this area.
In clinical practice, the surgery delay after NAC was affected by multiple factors. One of the most common causes of delayed surgery is the time required to recover from the side effects of short-term chemotherapy (mainly hematological toxicity and liver toxicity). Therefore, clinicians should well control the side effects of chemotherapy, such as regular blood examination and timely use of granulocyte colony stimulating factor (G-CSF) preparation, etc.15 It was worth noting that 14 patients delayed the TTS due to the appointment of preoperative breast MRI in Group C. Zhang et al also reported that preoperative breast MRI prolonged the operation waiting time by 11 days.16 This result reminded clinicians to optimize the patient’s examination process and minimize the operation delay due to waiting for examination.
Nonetheless, this study is not free of limitations. First, as is typical of retrospective studies, there could have been potential biases. The TTS of patients were individually selected based upon the condition of the patients, rather than being assigned randomly. The interpretation of the survival analysis was thus limited by the inevitable biases in the selection of the treatment. Second, the follow-up time was short. Especially for the patients who achieved pCR after NAC, we observed fewer death events, so we could not make subgroup analysis on these patients. Third, due to the retrospective study, patients may have memory loss or mistakes in the occurrence of postoperative complications, which affects our analysis. Last but not least, the study represented a single institutional experience and therefore the results may be hard to extrapolate to other institutions or countries with different patient populations and different practice patterns. Nevertheless, this study can provide some ideas and guidance for clinicians’ decisions. At the same time, it also provides the direction for further research.
Conclusion
In conclusion, TTS after NAC seemed to influence survival outcomes and postoperative complications. Breast cancer patients who underwent surgery between 21 and 28 days benefited the most. Clinicians should make a comprehensive evaluation of patients, balance the impact of TTS on short-term complications and long-term survival rate, and select the appropriate operation time.
Disclosure
There are no relevant conflicts of interests to be disclosed by the authors.
References
1. Apuri S. Neoadjuvant and adjuvant therapies for breast cancer. South Med J. 2017;110(10):638–642. doi:10.14423/SMJ.0000000000000703
2. Bleicher RJ. Timing and delays in breast cancer evaluation and treatment. Ann Surg Oncol. 2018;25(10):2829–2838. doi:10.1245/s10434-018-6615-2
3. Omarini C, Guaitoli G, Noventa S, et al. Impact of time to surgery after neoadjuvant chemotherapy in operable breast cancer patients. Eur J Surg Oncol. 2017;43(4):613–618. doi:10.1016/j.ejso.2016.09.020
4. Suleman K, Almalik O, Haque E, et al. Does the timing of surgery after neoadjuvant therapy in breast cancer patients affect the outcome? Oncology. 2020;98(3):168–173. doi:10.1159/000504964
5. Radovanovic Z, Ranisavljevic M, Radovanovic D, et al. Nipple-sparing mastectomy with primary implant reconstruction: surgical and oncological outcome of 435 breast cancer patients. Breast Care. 2018;13(5):373–378. doi:10.1159/000489317
6. Wolmark N, Wang J, Mamounas E, et al. Preoperative chemotherapy in patients with operable breast cancer: nine-year results from national surgical adjuvant breast and bowel project B-18. J Natl Cancer Inst Monogr. 2001;30(30):96–102. doi:10.1093/oxfordjournals.jncimonographs.a003469
7. Bear HD, Anderson S, Brown A, et al. The effect on tumor response of adding sequential preoperative docetaxel to preoperative doxorubicin and cyclophosphamide: preliminary results from national surgical adjuvant breast and bowel project protocol B-27. J Clin Oncol. 2003;21(22):4165–4174. doi:10.1200/JCO.2003.12.005
8. Gagliato D, Gonzalez-Angulo AM, Lei X, et al. Clinical impact of delaying initiation of adjuvant chemotherapy in patients with breast cancer. J Clin Oncol. 2014;32(8):735–744. doi:10.1200/JCO.2013.49.7693
9. Sanford RA, Lei X, Barcenas CH, et al. Impact of time from completion of neoadjuvant chemotherapy to surgery on survival outcomes in breast cancer patients. Ann Surg Oncol. 2016;23(5):1515–1521. doi:10.1245/s10434-015-5020-3
10. Falcone RE, Nappi JF. Chemotherapy and wound healing. Surg Clin. 1984;64(4):779–794. doi:10.1016/S0039-6109(16)43394-3
11. Trojan TP, Trojan J, Bechstein W, Woeste G. Impact of neoadjuvant chemotherapy on postoperative morbidity after gastrectomy for gastric cancer. Dig Surg. 2015;32(4):229–237. doi:10.1159/000381884
12. Berkel AE, Woutersen DP, van der Palen J, et al. Prognostic factors for postoperative morbidity and tumour response after neoadjuvant chemoradiation followed by resection for rectal cancer. J Gastrointest Surg. 2014;18(9):1648–1657. doi:10.1007/s11605-014-2559-4
13. Sutton TL, Johnson N, Schlitt A, et al. Surgical timing following neoadjuvant chemotherapy for breast cancer affects postoperative complication rates. Am J Surg. 2020;219(5):741–745.
14. Song J, Zhang X, Liu Q, et al. Impact of neoadjuvant chemotherapy on immediate breast reconstruction: a meta-analysis. PLoS One. 2014;9(5):e98225. doi:10.1371/journal.pone.0098225
15. Müller C, Juhasz Böss I, Schmidt G, et al. Factors influencing the time to surgery after neoadjuvant chemotherapy in breast cancer patients. Arch Gynecol Obstet. 2020;301(4):1055–1059. doi:10.1007/s00404-020-05494-6
16. Zhang M, Sun S, Mesurolle B. The impact of pre-operative breast MRI on surgical waiting time. PLoS One. 2017;12:1–10.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
