Back to Journals » Open Access Emergency Medicine » Volume 15
How Effective is Angiotensin II in Decreasing Mortality of Vasodilatory Shock? A Systematic Review
Authors Semedi BP , Rehatta NM, Soetjipto S, Nugraha J , Mahyuddin MH, Arnindita JN, Wairooy NAP
Received 25 September 2022
Accepted for publication 20 December 2022
Published 5 January 2023 Volume 2023:15 Pages 1—11
DOI https://doi.org/10.2147/OAEM.S391167
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Hans-Christoph Pape
Bambang Pujo Semedi,1,2 Nancy Margarita Rehatta,2 Soetjipto Soetjipto,3 Jusak Nugraha,4 Muhammad H Mahyuddin,5 Jannatin N Arnindita,5 Nabilah A P Wairooy5
1Doctoral Program of Medical Science, Faculty of Medicine, Universitas Airlangga, Surabaya, East Java, 60132, Indonesia; 2Department of Anesthesiology and Reanimation, Medical Faculty of Medicine, Universitas Airlangga—Dr Soetomo General Hospital, Surabaya, East Java, 60132, Indonesia; 3Department of Medical Biochemistry, Medical Faculty of Universitas Airlangga, Surabaya, East Java, 60132, Indonesia; 4Department of Clinical Pathology, Medical Faculty of Universitas Airlangga, Surabaya, East Java, 60132, Indonesia; 5Medical Faculty Universitas Airlangga, Surabaya, East Java, 60132, Indonesia
Correspondence: Nancy Margarita Rehatta, Email [email protected]
Background: Patients with severe vasodilation accompanied by refractory hypotension despite high doses of vasopressors were associated with a high mortality rate. The Ang-2 for the Treatment of High-Output Shock (ATHOS) 3 trial demonstrated that angiotensin 2 (Ang-2) could effectively increase MAP and blood pressure in vasodilatory shock patients. This systematic review aims to summarize the impact of Ang-2 for the treatment of vasodilatory shock on clinical outcomes, including length of stay, MAP level (before and after), and mortality also Ang-2 dose needed.
Methods: A systematic search in PubMed, Sage, ScienceDirect, Scopus and Gray literature was conducted to obtain studies about the use of Ang-2 in vasodilatory shock patients.
Results: In all of the studies that we obtained, there were different results regarding mortality in patients with vasodilatory shock with Ang-2. Mortality was significantly lower when Ang-2 was administered to patients with elevated renin. The initial dose of Ang-2 can be started at 10– 20 ng/kg/min, but there is no agreement on the maximum dose. Ang-2 may be considered a third-line vasopressor if the targeted MAP has not been achieved after administration of norepinephrine > 200 ng/kg/min for more than 6 hours. Although not statistically significant, the use of Ang-2 can reduce the length of stay in the ICU and in the hospital when compared to patients without Ang-2 therapy, in addition to reducing the dose of vasopressor.
Conclusion: Overall, the use of Ang-2 has potential to be a regimen for patients with vasodilatory shock. Further study is needed to obtain more data.
Keywords: angiotensin II, vasodilatory shock, vasopressor, mortality
Introduction
Vasodilatory shock is the most common type of shock. Patients could fall into a life-threatening condition of circulatory failure that manifests as hypotension (systolic blood pressure less than 90 mmHg or mean arterial pressure (MAP) less than 65 mmHg). When an adequate MAP target of 65 mmHg cannot be achieved, vasopressor therapy should be initiated. Norepinephrine is recommended as the first choice of vasopressor.1 Vasopressin (up to 0.03 U/min) or epinephrine must be titrated in order to increase MAP. However, patients with severe vasodilation who are hypotensive despite taking high doses of vasopressors have a poor prognosis, with a 30-day mortality exceeding 50%.2
Hemodynamically, in any type of vasodilatory shock, cardiac output and heart rate initially increase to compensate for the reduced oxygen supply to the tissues. In addition to that, left ventricular systolic contractions become hyper-dynamic in order to push blood into the tissues. These mechanisms are due to the markedly increased plasma catecholamine concentration and the activation of renin-angiotensin system (RAS). Angiotensin-2 (Ang-2) is capable of stimulating vasoconstriction, aldosterone and vasopressin secretion, sodium and water reabsorption, and cardiac contractility.3
The clinical outcome of the patient with shock is concerning. Compared with patients without shock, patients with shock carry more burden in their clinical outcomes, such as mortality and length of stay. Patients with shock have more mortality rates and longer length of stay. The mortality rate of shock patient is 40–80% at 30 days of treatment.5,6 The findings of mortality from shock differ in each age groups, ranging from 9% in neonates to 63% in the elderly.4 The average length of stay in non-shock patient is 3–7 days comparing with shock patient is 16.5 days. The use of Ang-2 showed the potential to improve these clinical outcomes, by improving the hemodynamic response in shock patients. The Ang-2 for the Treatment of High-Output Shock (ATHOS) 3 trial demonstrated that Ang-2 could effectively increase MAP and blood pressure in vasodilatory shock patients who did not respond to high dose of conventional vasopressors.7,8 Ang-2 is found to be able to normalize blood pressure in 15 patients out of 21 subjects in the previous study.7,8
This systematic review aims to summarize the clinical outcomes of vasodilatory shock patients treated with Ang-2, such as mortality, length of stay, and MAP level (before and after). We also review the optimum Ang-2 dose concentration and decrease in vasopressor dose in Ang-2 treated patients.
Materials and Methods
A systematic search of primary literature was performed based on recommendations by the Cochrane Collaboration.
Information Sources and Eligibility Criteria
Plain text and medical subject heading terms were used to search studies discussing the use of Ang-2 in vasodilatory shock patients from PubMed, Sage, ScienceDirect, and Scopus. Reference lists of gray literature (Clinical Trial.gov, Preprints, Medxriv, and Google) were also manually screened for potentially relevant articles that may have eluded the initial search.
Eligible studies were retrospective studies and controlled trials with vasodilatory shock. Vasodilatory shock was defined as shock with difficulty in maintaining mean arterial pressure (MAP) above 65 mmHg treated with vasopressor dose ranging from above 200 ng/kg/min to 500 ng/kg/min norepinephrine equivalents or more and with a cardiac index (CI) of at least 2.2 L/min/m2.9 In this review, we compared the use of Ang-2 for patients with vasodilatory shock. Primary outcomes of interest to be collected included, mortality rate, age, Ang-2 dose, length of stay, other vasopressor dose, and MAP before and after the administration of Ang-2.
Search Strategy
We used text words and medical subject heading (MeSH) term. Search terms included (“Angiotensin II” OR “Angiotensin 2”) AND (“Vasodilatory Shock” OR “Refractory Shock”) OR (“Mortality” AND ‘Mortality Rate’). All studies were restricted to English language-publications in the last 5 years (2017–2022) (Figure 1). We only included the last five years studies because the first study of Ang-2 for the treatment of vasodilatory shock was published in 2017. Further search by reviewing gray literature was performed manually.
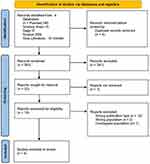 |
Figure 1 Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram of studies identified, excluded and included. |
Study Selection and Data Extraction
Three authors (M.H.M, J.N.A, and N.A.P.W) reviewed each study candidate from the literature search by title and abstract, and if necessary full text, to determine its eligibility for inclusion in the quality evidence synthesis. Disagreements were settled with discussion by all of the authors. The data from eligible studies were extracted into a summary table (Tables 1 and 2).
 |
Table 1 Characteristics of Studies |
 |
Table 2 Summary of Studies on Angiotensin 2 in Vasodilatory Shock Patients |
Risk of Bias Assessment
We followed recommendations from Cochrane Handbook for Systematic Reviews of Interventions to assess risk of bias, including Newcastle–Ottawa Scale (NOS)10 for observational studies and Risk of Bias Judgements. In Non-Randomized Studies of Interventions (ROBINS-I)11 for clinical trial studies to assess the quality of the body of evidence. The NOS was utilized for each item with the value “0” (in the case the item was not contemplated) or “1” (if the item was contemplated); a maximum score of 2 could be given for the item “comparability.”10 Studies are rated from 0 to 9, with those studies rating 0–2 (poor quality), 3–5 (fair quality), 6–9 (good/high quality).10 The response options for each domain-level in ROBINS-I are (1) Low risk of bias (the study is comparable to a well-performed randomized trial with regard to this domain), (2) Moderate risk of bias (the study is sound for a non-randomized study with regard to this domain but cannot be considered comparable to a well-performed randomized trial) (3) Serious risk of bias (the study has some important problems in this domain), (4) Critical risk of bias (the study is too problematic in this domain to provide any useful evidence on the effects of intervention); and (5) No information on which to base a judgement about risk of bias for this domain.11
Results from the risk of bias assessment are presented in Tables 3 and 4. All of the studies were low or unclear risk of bias.
 |
Table 3 Risk of Bias Assessment (Newcastle–Ottawa Scale) |
 |
Table 4 Risk of Bias Assessment (Risk of Bias Judgements in Non-Randomized Studies of Interventions) |
Result
Overall, the initial search yielded 555 total results from databases and 10 results from gray literature. Four duplicate studies were removed. Among 561 studies, screening was done based on inclusion and exclusion criteria, and therefore 20 studies were remained. One study was removed because the full text was not retrieved. Two journals were excluded because of the wrong population, and twelve studies were excluded because of the wrong type of publication (Figure 1). From the screening process, we obtained four studies that were ultimately eligible for inclusion in the qualitative evidence synthesis. Results were summarized for each study and presented in detail in Table 1.
Mortality
The study by Wieruszewski et al12 used severity-adjusted multivariate analysis. It showed that 30-day mortality rate was related to hemodynamic responsiveness (HR 0.50, 95% CI 0.35–0.71, p < 0.001), surgery (HR 0.71, 95% CI 0.50–1.00, p = 0.048), and low lactate levels (HR 0.94, 95% CI 0.91–0.96, p < 0.001). Higher vasopressor dose at initiation of Ang-2 also contributed to 30-day mortality (HR 1.61, 95% CI 1.03–2.51, p = 0.037).
In contrast, the study by Quan et al3 compared groups receiving Ang-2 and other vasopressors as third-line agents, and found that patients in the Ang-2 group had a higher mortality rate (91.1% vs 78%; p = 0.04). After propensity score weighting, a trend of higher mortality was found in the Ang-2 group but not statistically significant (86% vs 71%; p = 0.16). Intriguingly, patients with elevated renin treated with Ang-2 showed a 51.1% mortality compared with 69.9% in patients treated with placebo (p = 0.01). Increased renin was independently associated with an increased risk of death (HR 2.15; 95% CI 1.35–3.42) and Ang-2 therapy in patients with elevated renin showed a reduced risk of death (HR 0.62; 95% CI, 0.39–0.98). Another significant predictor of mortality was Sequential Organ Failure Assessment (SOFA) score (OR 1.25, 95% CI 1.05–1.49, p = 0.01). SOFA score reflects the extent of organ failure in a patient, comprised of 6 systems: respiratory, coagulation, cardiovascular, central nervous system, and renal. Score 0 means normal function, while score 4 is the most abnormal.
Different results were presented by Khanna et al13 in which there was no significant difference in mortality at day 7 (HR 0.78; 95% CI, 0.53 to 1.16; P = 0.22) and day 28 (HR 0.78; 95% CI, 0.57 to 1.07; P = 0.12) between group receiving Ang-2 and placebo.
Age
A study from Quan et al3 showed that the mean age was 59.5 ± 15.7 years and the study of Wieruszewski et al12 was 60±15 years. Meanwhile the studies of Smith et al14 and Khanna et al13 used median with results 63 years old (IQR 51–71 years) and 64 years (IQR 52–75 years).
Mean Arterial Pressure (MAP)
A study from Wieruszewski et al12 found that mean MAP before treatment was 65±11 mmHg. Three hours after Ang-2 administration, the responders had a significant greater increase in MAP + 10.3 mmHg compared with non-responders MAP + 1.6 mmHg (p < 0.001). A similar study was shown by Quan, et al3 who explained that there was an improvement in MAP in responder subjects (Mean 74.5 ± 2.8 mm Hg) compared to non-responders (Mean 56.5 ± 2.6 mm Hg). Smith et al14 explained that the Median MAP before Ang-2 administration was 62 (IQR 57–70) mmHg. Then, three hours post Ang-2 administration the median MAP was 73 (IQR 65–79) mmHg (p < 0.001). Improvements in MAP were also found in the study of Khanna et al13 who explained that before administration of Ang-2 the median MAP was 66 (IQR 65.8–66.5) mmHg and after administration of Ang-2 the median MAP was 77 (IQR 76.5–77.7) mmHg (P < 0.001; OR, 7.95; 95%).
Length of Stay (LOS)
In Quan, et al3 there was a decrease in ICU length of stay (LOS) in patients with Ang-2 therapy (Mean 6.9 ± 6.7 days) compared to those without Ang-2 therapy (Mean 9.8 ±12.8 days). Similarly, there was a decrease in hospital LOS in patients with Ang-2 therapy compared to those who are not. However, there was no statistically significant difference between ICU LOS (p = 0.08) and hospital LOS (p = 0.56) in patients treated with Ang-2 and patients not treated with Ang-2. Wieruszewski et al12 found that the median ICU length of stay in responders was 9 days (IQR 6–16 days) compared with 11 days (IQR 8–24 days) in Ang-2 nonresponders (p = 0.26). Wieruszewski et al12 also found that neither there was a difference in LOS according to hemodynamic response (24 (IQR 13–36) v. 30 (IQR 17–36)) days for patients on Ang-2 and without Ang-2 therapy (p = 0.43). Smith et al14 found that median ICU LOS was 7 (IQR 2–17) days and median hospital LOS was 10 (IQR 4–26) days.
Angiotensin II Dose
Khanna et al13 and Quan et al studies used the same initial Ang-2 dose of 20 ng/kg/min, while the average initial dose used in the Wieruszewski et al12 study revealed an average initial dose of 20 ± 12 ng/ kg/min (range 1–80 ng/kg/min). Meanwhile, Smith et al14 started Ang-2 therapy at a median dose of 10 ng/kg/minute (IQR 10–20 ng/kg/minute). The maximum dose in the studies of Quan et al3 was 40 ng/kg/minute, but the dose could be increased to 80 ng/kg/min with the approval of attending physician. Quan et al3 also stated that the titration used were 5 ng/kg/min every 5 minutes as needed. Smith et al14 used the median maximum dose, which was 40 (IQR 29–80) ng/kg/min. In the study of Khanna et al13 it was stated that in the first 3 hours to reach the target MAP, the dose of Ang-2 could be increased by 200 ng/kg/min. However, after 3 hours and 15 minutes, the maximum dose to maintain the target MAP was 40 ng/kg/min. In the study of Wieruszewski et al12 the average maximum dose was 52 ± 24 ng/kg/min (IQR 10–82 ng/kg/min).
Decrease in Vasopressor Dose
In the study conducted by Wieruszewski et al12 Ang-2 was generally used as a third-line (N = 104, 40%) or fourth-line (N = 127, 47%) vasopressor, starting at a median of 11 hours from the initiation of the first vasopressor. Simultaneous initiation of Ang-2 was accompanied by escalation of other vasopressors, with a mean increase of 50 ± 140 ng/kg/min cumulative dose of norepinephrine equivalent (NEE) within 1 hour prior to initiation of Ang-2 initiation. The mean vasopressor dose at the time of initiation of Ang-2 was 580 ± 330 ng/kg/min NEE. Compared to non-responders, there was a significant reduction in the vasopressor NEE dose (−200 versus +40 ng/kg/min, p < 0.001) at 3 hours. Similar results were demonstrated by the study of Khanna et al13 which the mean dose of the main vasopressor in the first 48 hours of the Ang-2 group was consistently lower than the placebo group. Likewise, Smith et al14 revealed that at 3 hours after Ang-2 administration, the median dose of NEE decreased to 390 ng/kg/min (IQR 0.16–0.64) and the mean change in NEE dose was 160 ng/kg/min (95% CI 0.10–0.22, p < 0.001). Quan et al3 stated that there was no significant difference in the total dose of NEE vasopressor between the Ang-2 and non-Ang-2 groups and accordingly Ang-2 should only be administered after administration of norepinephrine and vasopressin equivalent to a dose of NEE > 200 ng/kg/min).
Discussion
The pathophysiology of vasodilatory shock is shown in Figure 2. In the RAAS system, angiotensinogen with renin and angiotensin converting enzyme (ACE) will form Ang-2 bioactive and its receptors, namely AT-1 and AT-2.15 Under physiological condition, Ang-2 will bind to the AT-1 receptor followed by events such as an increase in expression of pro-inflammatory mediators, rise in vascular permeability by inducing vascular endothelial growth factor (VEGF), and stimulate the expression of endothelial adhesion molecules (P-selectin and E-selectin), intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1). On the other hand, binding of Ang-2 to the AT-II receptor results in vasodilation and a decrease in systemic vascular resistance, although this is not the dominant effect of Ang-2.15,16
Angiotensin 2 receptor type II (AT-II) and Mas receptor (MasR) belong to the non-classical G protein-coupled receptor family and mediate functions such as diuresis, natriuresis, vasorelaxation, and reduction of blood pressure in various animal models.17 AT-II and MasR also show signaling overlap in terms of nitric oxide (NO) formation and inhibition of Na+, K+-ATPase activity in the proximal tubule of the kidney.18 There is evidence that suggest AT-II and MasR form heterodimers with AT-I, and this heterodimerization provides a mechanism in which AT-II and MasR attenuate AT-I-induced signaling and cellular function.17 Due to the interaction between AT-II and MasR, several studies have suggested that these receptors may be functionally interdependent. Some study using AT-II antagonist namely PD123319 reduces the vasodepressor effect of endogenous MasR agonists Ang-(1–7).19,20 Similarly, Ang-(1–7)–mediates cerebral arterial vasodilatation21 and the aortic ring22 AT-II antagonist PD123319 and MasR antagonist A-779.
There is an increased activity of renin, angiotensin 1 (Ang-1), and angiotensin 2 (Ang-2) in shock. High levels of plasma Ang-2 which binds to AT-I will cause oxidative stress and endothelial dysfunction that will induce pro-inflammatory mediators (interleukin (IL)-1β, tumor necrosis factor (TNF)-α, interferon (IFN) γ and nitric oxide (NO).16 Endothelial dysfunction and oxidative stress disrupt the vasopressor ability of Ang-2 thus vasoconstriction does not occur.23
This condition will cause pathological vasodilation, usually due to the effect of inducible nitric oxide synthase (iNOS) which produces excessive amounts of nitric oxide (NO).24 Nitric oxide increases cAMP (cyclic adenosine monophosphate) and cGMP (cyclic guanosine monophosphate) to trigger vasodilation.25 Activation of adenosine triphosphate (ATP)-sensitive potassium channels in vascular smooth muscle cells prevents the entry of calcium, which is necessary for vasoconstriction, leading to metabolic disturbances (tissue hypoxia and acidosis) and inflammation (including NO production) with vasoplegia.23,26–28
Patients could fall into vasodilatory shock influenced by several factors, one of them is age. There was a significant increase in the incidence of sepsis along with increasing age. Elderly patients are at high risk due to their susceptibility and comorbidities.29 Patients with preexisting chronic comorbidities, such as pulmonary or renal disease, may be associated with increased susceptibility to sepsis.30 Sepsis patients aged 65 years who had at least one comorbid are twice more prevalent than younger patients (<65 years).31 Changes in functional status, namely disuse atrophy due to inactivity, sarcopenia due to decreased muscle mass, changes in response to hormones (growth hormones, androgens, and estrogens), neurological changes, changes in cytokine regulation, changes in protein metabolism, and changes in food intake to pre-admission status are also important because they affect the patient’s condition.32,33
Patients in this age group also receive a lot of Angiotensin 2 therapy for management of shock. Angiotensin 2 acts as an endogenous vasoconstrictor via the AT-1 receptor.34 Previous research said that there is a decrease in plasma renin activity with age.35 This results in a decrease in circulating Angiotensin 2 in the population compared to the younger population. The body will respond to this reduced substrate by increasing the sensitivity of AT-1. This is an important regulatory factor in the hemodynamics of the elderly population.34
In 2017, the Food and Drug Administration (FDA) approved Ang-2 as a vasopressor to increase blood pressure in cases of septic shock or distributive shock in adult patients.3 In ATHOS-3, the patients started receiving Ang-2 after a dose of norepinephrine equivalent dose (NED) 200 ng/kg/minute for more than six hours and was used as a third line vasopressor after norepinephrine and vasopressin.13 In the latest Surviving Sepsis Campaign guidelines, it is stated that Ang-2 is not the first line but can act as an additional vasopressor therapy.36
However, in the studies, we collected, it appears that the maximum dose administered is different. Wong et al noted that the dosing of Ang-2 was often not in accordance with the recommendations in the protocol, especially with regard to the maximum dose.37 They stated that although the institution had determined the Ang-2 administration protocol, in practice this was up to the attending physician to determine the timing of initiation and dose modification. They also added that the doctors are reluctant to use Ang-2 in the treatment of shock due to lack of familiarity with this new vasopressor and uncertainty about its availability and adjustment for use.37
In this systematic review study, we found that Ang-2 was able to increase MAP above 65mmHg.3,12–14 Ang-2 binds to AT-I causing vasoconstriction in both afferent and efferent glomerular arterioles. The effect is greatest on the efferent side, causing a decrease in renal blood flow and an increase in glomerular filtration pressure.16 In shock patients, Ang-2 is produced endogenously in response to decreased renal afferent arterial perfusion, through the production of renin which triggers the conversion of angiotensinogen into Ang-1, which then converted by the ACE enzyme into Ang-2.38 This reaction will directly trigger the sympathetic nervous system while also triggering the release of calcium (Ca2+) from the sarcoplasmic reticulum of smooth muscle cells, causing vasoconstriction.39 This interaction prompts the hemodynamic to improve which can then reduce the mortality rate in shock patients. This is in line with the results of the study by Wieruszewski et al which states that the level of hemodynamic responsiveness of patients has a significant effect on the 30-day mortality rate of patients in shock.12 Furthermore, Khanna et al explained that the use of Ang-2 was able to improve the cardiovascular SOFA score within 48 hours from mean 4.00 become mean 2.25 (SD 1.771) and mean 2.72 (SD 1.654) (−1.75 versus −1.28, p = 0.01).13
Fluid therapy and administration of vasopressors are the mainstay of treatment for shock used to treat hypotension in patients.40,41 Patients with refractory shock usually receive catecholamine vasopressor therapy as first-line therapy in shock and are given high doses.36 Catecholamine vasopressors may be associated with excessive vasoconstriction which may result in an impairment in tissue perfusion, even when perfusion pressure is restored. However, non-adrenergic vasopressors are also associated with some adverse effects, and switching from adrenergic to non-adrenergic vasopressors is not always optimal.42 Administration of non-catecholamine vasopressors in the treatment of refractory shock can reduce catecholamine doses and can reduce the potential of toxicity associated with high-dose catecholamine therapy through vasoconstriction with different mechanisms.39 Chawla et al showed that the number of catecholamine use in patients who had been given Ang-2 was lower than those who received placebo.7 Using a sparing effect on a vasopressor patient will reduce the patient’s morbidity and length of stay (LOS).43 Our study showed that administration of Ang-2 could be a sparing effect, thus reducing the dose of vasopressors which could minimize the adverse effect of using them.
Length of stay (LOS) is an indicator to determine the efficiency of therapy and hospital care management.44 A decrease in LOS indicates a good outcome of the given therapy. Reducing LOS is also able to reduce the risk of infection, side effects of treatment, and improve the quality of treatment.45 Administration of Ang-2 to patients is able to improve the patient’s condition, however the result is not statistically significant.12 This is probably due to differences in the patient’s clinical background including severity, comorbidities, and patient age.
Although treating patients in shock with Ang-2 makes sense pathophysiologically, the study by Quan et al and Bellomo et al showed that patients in shock treated with Ang-2 had higher mortality rate.3,46 However, in the same study, the mortality rate was significantly lower when Ang-2 is given to patients with elevated renin.3,46 In vasodilatory shock, renin production is triggered to increase Ang-2 and induce vasoconstriction, but in these patients the mechanism failed.3 The study by Bellomo et al showed that there was an increase in the levels of Ang-1 and Ang-1/Ang-2 ratio in patients with vasodilatory shock compared to controls, while the levels of Ang-2 in both groups tended to remain.46 The increase in renin is caused by the ACE, single nucleotide polymorphism (SNP) gene, and endothelial lesions, which is directly associated with 28-day mortality.47,48 An increase in renin activity also coincides with a decrease in aldosterone levels, indicating dysfunction of the RAAS pathway.49,50 Thus, increased renin may be a predictor of RAAS dysfunction and a suitable candidate for Ang-2 therapy.
Conclusion
Overall, the administration of Ang-2 in treating vasodilatory shock had potential to improve the patient’s outcome which can be seen from the increased MAP, decreased vasopressors dose, mortality rate, and length of stay. However, the results of increasing MAP, decreasing the dose of vasopressors, mortality and length of stay were inconsistent. Until now there has been no guideline for the administration of angiotensin 2 in shock patients. Further study on this matter shall be continued to obtain more data.
Acknowledgments
We thanked the Doctoral Program in Medical Sciences, Department of Anesthesiology and Reanimation, Department of Medical Biochemistry, Department of Clinical Pathology, Faculty of Medicine, Airlangga University, Indonesia for their support.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Vincent JL, De Backer D. Circulatory Shock. N Engl J Med. 2013;369(18):1726–1734. doi:10.1056/NEJMra1208943
2. Brown SM, Lanspa MJ, Jones JP, et al. Survival after shock requiring high-dose vasopressor therapy. Chest. 2013;143(3):664–671. doi:10.1378/chest.12-1106
3. Quan M, Cho N, Bushell T, et al. Effectiveness of angiotensin II for catecholamine refractory septic or distributive shock on mortality: a propensity score weighted analysis of real-world experience in the medical ICU. Crit Care Explor. 2022;4(1):e0623. doi:10.1097/CCE.0000000000000623
4. Health Issues India. The death toll of sepsis in India; 2022. Available from: https://healthissuesindia.com/2018/09/13/the-death-toll-of-sepsis-in-india/.
5. Divatia JV, Amin PR, Ramakrishnan N, et al. Intensive care in India: the Indian intensive care case mix and practice patterns study. Indian J Crit Care Med Peer Rev off Publ Indian Soc Crit Care Med. 2016;20(4):216–225.
6. Martin GS. Sepsis, severe sepsis and septic shock: changes in incidence, pathogens and outcomes. Expert Rev Anti Infect Ther. 2012;10(6):701–706. doi:10.1586/eri.12.50
7. Chawla LS, Russell JA, Bagshaw SM, et al. Angiotensin II for the treatment of high-output shock 3 (ATHOS-3): protocol for a Phase III, double-blind, randomised controlled trial. Crit Care Resusc. 2017;19(1):43–49.
8. Chawla LS, Busse L, Brasha-Mitchell E, et al. Intravenous angiotensin II for the treatment of high-output shock (ATHOS trial): a pilot study. Crit Care. 2014;18(5):534. doi:10.1186/s13054-014-0534-9
9. Ortoleva JP, Cobey FC. A systematic approach to the treatment of vasoplegia based on recent advances in pharmacotherapy. J Cardiothorac Vasc Anesth. 2019;33:1310–1314. doi:10.1053/j.jvca.2018.11.025
10. Wells GA, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. Newcastle-Ottawa quality assessment scale. Ottawa Hosp Res Inst. 2014;2014(3):2–4.
11. Sterne JACC, Hernán MA, Reeves BC, et al. Cochrane; ROBINS-I_detailed_guidance. BMJ. 2016;366:1–53.
12. Wieruszewski PM, Wittwer ED, Kashani KB, et al. Angiotensin II infusion for shock: a multicenter study of postmarketing use. Chest. 2021;159(2):596–605. doi:10.1016/j.chest.2020.08.2074
13. Khanna A, English SW, Wang XS, et al. Angiotensin II for the treatment of vasodilatory shock. N Engl J Med. 2017;377(5):419–430. doi:10.1056/NEJMoa1704154
14. Smith SE, Newsome AS, Guo Y, et al. A multicenter observational cohort study of angiotensin II in shock. J Intensive Care Med. 2020;37(1):75–82. doi:10.1177/0885066620972943
15. Khalique SC, Ferguson N. Angiotensin II (Giapreza): a distinct mechanism for the treatment of vasodilatory shock. Cardiol Rev. 2019;27(3):167–169. doi:10.1097/CRD.0000000000000247
16. Corrêa TD, Takala J, Jakob SM. Angiotensin II in septic shock. Crit Care. 2015;19(1):1–6. doi:10.1186/s13054-015-0802-3
17. Patel SN, Ali Q, Samuel P, Steckelings UM, Hussain T. Angiotensin II type 2 receptor and receptor mas are colocalized and functionally interdependent in obese Zucker rat kidney. Hypertens. 2017;70(4):831–838. doi:10.1161/HYPERTENSIONAHA.117.09679
18. Gwathmey TM, Westwood BM, Pirro NT, et al. Nuclear angiotensin-(1–7) receptor is functionally coupled to the formation of nitric oxide. Am J Physiol Renal Physiol. 2010;299(5):F983–90. doi:10.1152/ajprenal.00371.2010
19. Walters PE, Gaspari TA, Widdop RE. Angiotensin-(1–7) acts as a vasodepressor agent via angiotensin II type 2 receptors in conscious rats. Hypertens. 2005;45(5):960–966. doi:10.1161/01.HYP.0000160325.59323.b8
20. Bosnyak S, Widdop RE, Denton KM, Jones ES. Differential mechanisms of ang (1–7)-mediated vasodepressor effect in adult and aged candesartan-treated rats. Int J Hypertens. 2012;2012:192567. doi:10.1155/2012/192567
21. Durand MJ, Raffai G, Weinberg BD, Lombard JH. Angiotensin-(1–7) and low-dose angiotensin II infusion reverse salt-induced endothelial dysfunction via different mechanisms in rat middle cerebral arteries. Am J Physiol Heart Circ Physiol. 2010;299(4):H1024–33. doi:10.1152/ajpheart.00328.2010
22. Roks AJM, Nijholt J, van Buiten A, van Gilst WH, de Zeeuw D, Henning RH. Low sodium diet inhibits the local counter-regulator effect of angiotensin-(1–7) on angiotensin II. J Hypertens. 2004;22(12):2355–2361. doi:10.1097/00004872-200412000-00018
23. Senatore F, Balakumar P, Jagadeesh G. Dysregulation of the renin-angiotensin system in septic shock: mechanistic insights and application of angiotensin II in clinical management. Pharmacol Res. 2021;174:1. doi:10.1016/j.phrs.2021.105916
24. Ahmad A, Dempsey SK, Daneva Z, et al. Role of nitric oxide in the cardiovascular and renal systems. Int J Mol Sci. 2018;19(9):2605. doi:10.3390/ijms19092605
25. Lambden S. Bench to bedside review: therapeutic modulation of nitric oxide in sepsis—an update. Intensive Care Med Exp. 2019;7(1). doi:10.1186/s40635-019-0274-x
26. Tinker A, Aziz Q, Thomas A. The role of ATP-sensitive potassium channels in cellular function and protection in the cardiovascular system. Br J Pharmacol. 2014;171(1):12–23. doi:10.1111/bph.12407
27. Buckley JF, Singer M, Clapp LH. Role of KATP channels in sepsis. Cardiovasc Res. 2006;72(2):220–230. doi:10.1016/j.cardiores.2006.07.011
28. Levy B, Collin S, Sennoun N, et al. Vascular hyporesponsiveness to vasopressors in septic shock: from bench to bedside. Intensive Care Med. 2010;36(12):2019–2029. doi:10.1007/s00134-010-2045-8
29. Martín S, Pérez A, Aldecoa C. Sepsis and immunosenescence in the elderly patient: a review. Front Med. 2017;4:20. doi:10.3389/fmed.2017.00020
30. Juneja D. Severe sepsis and septic shock in the elderly: an overview. World J Crit Care Med. 2012;1(1):23. doi:10.5492/wjccm.v1.i1.23
31. Martin GS, Mannino DM, Moss M. The effect of age on the development and outcome of adult sepsis*. Crit Care Med. 2006;34(1):15–21. doi:10.1097/01.CCM.0000194535.82812.BA
32. Rusinova K, Guidet B. Are you sure it’s about “age”? Intensive Care Med. 2014;40(1):114–116. doi:10.1007/s00134-013-3147-x
33. Martin GS. Optimal fluid management in sepsis. Qatar Med J. 2019;2019(2):1.
34. Wray DW, Nishiyama SK, Harris RA, Richardson RS. Angiotensin II in the elderly: impact of angiotensin II type 1 receptor sensitivity on peripheral hemodynamics. Hypertension. 2008;51(6):1611–1616. doi:10.1161/HYPERTENSIONAHA.108.111294
35. Nanba K, Vaidya A, Rainey WE. Aging and Adrenal aldosterone production. Hypertens. 2018;71(2):218–223. doi:10.1161/HYPERTENSIONAHA.117.10391
36. Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Crit Care Med. 2021;49(11):e1063–143. doi:10.1097/CCM.0000000000005337
37. Wong A, Alkazemi A, Eche IM, Petri CR, Sarge T, Cocchi MN. A retrospective review of angiotensin II use in adult patients with refractory distributive shock. J Intensive Care Med. 2020;35(12):1490–1496. doi:10.1177/0885066619872720
38. Silva ACS, Lanza K, Palmeira VA, Costa LB, Flynn JT. update on the renin–angiotensin–aldosterone system in pediatric kidney disease and its interactions with coronavirus. Pediatr Nephrol. 2020;2021(36):1407–1426.
39. Heavner MS, McCurdy MT, Mazzeffi MA, Galvagno SMJ, Tanaka KA, Chow JH. Angiotensin II and vasopressin for vasodilatory shock: a critical appraisal of catecholamine-sparing strategies. J Intensive Care Med. 2021;36(6):635–645. doi:10.1177/0885066620911601
40. Chawla LS, Ostermann M, Forni L, Tidmarsh GF. Broad spectrum vasopressors: a new approach to the initial management of septic shock? Crit Care. 2019;23(1):1–3. doi:10.1186/s13054-019-2420-y
41. Malbrain MLNG, Langer T, Annane D, et al. Intravenous fluid therapy in the perioperative and critical care setting: executive summary of the International Fluid Academy (IFA). Ann Intensive Care. 2020;10(1). doi:10.1186/s13613-020-00679-3
42. De Backer D, Foulon P. Minimizing catecholamines and optimizing perfusion. Crit Care. 2019;23(Suppl 1):1–7. doi:10.1186/s13054-019-2433-6
43. Guinot PG, Martin A, Berthoud V, et al. Vasopressor-sparing strategies in patients with shock: a scoping-review and an evidence-based strategy proposition. J Clin Med. 2021;10(14):3164. doi:10.3390/jcm10143164
44. Siddique SM, Tipton K, Leas B, et al. Interventions to reduce hospital length of stay in high-risk populations: a systematic review. JAMA Netw Open. 2021;4(9):e2125846. doi:10.1001/jamanetworkopen.2021.25846
45. Baek H, Cho M, Kim S, Hwang H, Song M, Yoo S. Analysis of length of hospital stay using electronic health records: a statistical and data mining approach. PLoS One. 2018;13(4):e0195901. doi:10.1371/journal.pone.0195901
46. Bellomo R, Wunderink RG, Szerlip H, et al. Angiotensin i and angiotensin II concentrations and their ratio in catecholamine-resistant vasodilatory shock. Crit Care. 2020;24(1):1–8. doi:10.1186/s13054-020-2733-x
47. Hannula-Jouppi K, Massinen S, Siljander T, et al. Genetic susceptibility to non-necrotizing erysipelas/cellulitis. PLoS One. 2013;8(2):e56225. doi:10.1371/journal.pone.0056225
48. Dou XM, Cheng HJ, Meng L, et al. Correlations between ACE single nucleotide polymorphisms and prognosis of patients with septic shock. Biosci Rep. 2017;37(2). doi:10.1042/BSR20170145
49. Davenport MW, Zipser RD. Association of hypotension with hyperreninemic hypoaldosteronism in the critically ill patient. Arch Intern Med. 1983;143(4):735–737. doi:10.1001/archinte.1983.00350040125016
50. du Cheyron D, Lesage A, Daubin C, Ramakers M, Charbonneau P. Hyperreninemic hypoaldosteronism: a possible etiological factor of septic shock-induced acute renal failure. Intensive Care Med. 2003;29(10):1703–1709. doi:10.1007/s00134-003-1986-6
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.