Back to Journals » Open Access Emergency Medicine » Volume 15
Antioxidant Role in Critically Ill Patients with Vasodilatory Shock: Does Glutathione Peroxidase Correlate to Severity of Tissue Hypoxia and Organ Failure
Authors Semedi BP , Rehatta NM, Nugraha J , Soetjipto
Received 9 February 2023
Accepted for publication 26 April 2023
Published 29 April 2023 Volume 2023:15 Pages 133—143
DOI https://doi.org/10.2147/OAEM.S407958
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Hans-Christoph Pape
Bambang Pujo Semedi,1,2 Nancy Margarita Rehatta,2 Jusak Nugraha,3 Soetjipto4
1Doctoral Program of Medical Science, Faculty of Medicine, Universitas Airlangga, Surabaya, East Java, Indonesia; 2Department of Anesthesiology and Reanimation, Faculty of Medicine, Universitas Airlangga, Dr. Soetomo General Academic Hospital, Surabaya, East Java, 60132, Indonesia; 3Department of Clinical Pathology, Faculty of Medicine, Universitas Airlangga, Dr. Soetomo General Academic Hospital, Surabaya, East Java, 60132, Indonesia; 4Department of Medical Biochemistry, Faculty of Medicine, Universitas Airlangga, Surabaya, East Java, 60132, Indonesia
Correspondence: Nancy Margarita Rehatta, Department of Anesthesiology and Reanimation, Faculty of Medicine, University Airlangga, Dr. Soetomo General Academic Hospital, Surabaya, 60132, East Java, Indonesia, Tel +62315020251, Email [email protected]
Purpose: We aimed to evaluate the antioxidant role in critically ill patients with vasodilatory shock as it relates to severity of tissue hypoxia and organ failure.
Patients and Methods: An observational and prospective study was conducted in critically ill patients with vasodilatory shock. Glutathione peroxidase (GPx) levels as antioxidants were measured based on their levels in the patient’s serum. Tissue hypoxia as micro-hemodynamic status was represented by lactate levels, the macro-hemodynamic status was represented by vasoactive inotropic score (VIS) and mean arterial pressure (MAP), while organ dysfunction severity was represented by the shock index (SI), the sequential organ failure assessment (SOFA) score, and the acute physiology and chronic health evaluation (APACHE) II score.
Results: Thirty-four critically ill patients with vasodilatory shock met the eligibility criteria. The mortality rate was 41.2%. Glutathione peroxidase levels did not show a significant difference between survivors and non-survivors at baseline or after 24 hours. At the initial measurement, there was a correlation between GPx and lactate levels, GPx and SOFA scores. The macrohemodynamic status was represented by VIS and MAP, which were correlated with SI.
Conclusion: Glutathione peroxidase as antioxidant is related to severity of tissue hypoxia and organ failure in critically ill patients with vasodilatory shock.
Keywords: glutathione peroxidase, severity, mortality, vasodilatory shock, critically ill
Introduction
A critically ill patient is one who undergoes life-threatening multisystemic disorders with a high morbidity and mortality rate. Critically ill patients differ in characteristics, depending on the type and severity of organ dysfunction, which influences treatment focus and clinical outcomes.1 Vasodilatory shock is the most common problem in critically ill patients and one of the leading causes of death in the Intensive Care Unit. Septic shock is the most studied vasodilatory shock because it is associated with high mortality. Recent studies have shown that pro-inflammatory mediators and oxidative stress contribute to the development of sepsis and septic shock.2
The role of oxidative stress in the manifestations of critical disorders, including ischemia, reperfusion injury, and systemic inflammatory response. Oxidative stress can occur due to excessive production of oxidant or decreased antioxidant defenses. During septic shock, the balance between reactive oxygen species (ROS) formation and their neutralization by antioxidants is lost, resulting in oxidative stress. Increased oxidative stress further causes tissue damage and leads to the development of multiple organ failure.3 Among endogenous antioxidant defenses in humans, glutathione is quantitatively the most important and has been shown to be depleted during critical illness.2,3 Glutathione peroxidase (GPx) is an antioxidant enzyme that suppresses inflammation and tissue injury due to reactive oxygen species (ROS) produced by phagocytes (neutrophils, monocytes, macrophages, and eosinophils). The antioxidant system’s ability to respond to increased production of oxidants and oxidative damage is an indicator for oxidative stress.4 During critical illness, a similar decrease in the reduced glutathione (GSH) concentration is seen, but in addition, a change in the redox status indicative of an elevated oxidized glutathione (GSSG) level occurs. Furthermore, correlations exist between the concentrations of glutamine as well as glutamate and GSH in these patients. From the available evidence accumulated, glutathione plays a pivotal role in the maintenance of the intracellular redox status, the antioxidant vitamin levels, and the antioxidant enzyme functions under various metabolic conditions. The effectiveness of glutathione protection in the individual tissues depends on the tissue concentration of glutathione as well as the capacity of the tissue to import GSH and export GSSG.5
Physiological signs of worsening hemodynamics in critically ill patients who develop shock commonly occur in its early phases. However, numerous evidence points out that these signs are often overlooked. This late response leads to prolonged hypoxia and correlates with poor outcome, due to resulting refractory vasodilatory shock, among other factors.6 In critically ill patients, the severity of organ failure can be assessed using scoring systems, such as the sequential organ failure assessment (SOFA) score as well as the acute physiology and chronic health evaluation (APACHE) score.7 Other studies recommended the use of shock index (SI).8 The hemodynamic status can be represented by vasoactive inotropic score (VIS), mean arterial pressure (MAP), and lactate levels.9,10
To date, the role of GPx as an early biomarker for tissue hypoxia, hemodynamic status and organ failure severity in critically ill patients undergoing vasodilatory shock, as measured with the APACHE II, SOFA, and SI scoring systems, has not been widely studied. Therefore, we conducted a study to analyze the role of GPx as an early biomarker for tissue hypoxia, hemodynamic status and organ failure severity in critically ill patients experiencing vasodilatory shock.
Patient and Methods
Study Design
The design of the study was a cohort prospective study, which enrolled critically ill patients with vasodilatory shock hospitalized at the Intensive Care Unit (ICU) and emergency department of Dr. Soetomo General Hospital, a teaching hospital of Universitas Airlangga. The study was conducted from December 1, 2021 to June 30, 2022. Inclusion criteria included patients aged >18 years, a clinical diagnosis of vasodilatory shock, and the signing of an informed consent by family or relatives. The comorbidity of congenital heart disease (CHD) and end-stage renal disease (ESRD) were excluded from the study.
Criteria for Vasodilatory Shock
Shock that is unresponsive to fluid administration of at least 1000 mL and using a vasopressor of >0.1 µg/kgBW/minute.
Hemodynamic Status and Organ Severity
Macro hemodynamic indicators were VIS and MAP. Lactate levels are a biomarker of tissue hypoxia as micro hemodynamic status. Lactate levels were measured from a vein blood sample using IStat tools. The APACHE II score, the SOFA score and the Shock Index were used as scoring indicators. The Shock Index (SI) was also calculated by dividing HR over SBP. SOFA Score and APACHE II Score were calculated within 24 hours of emergency department admission and at the Intensive Care Unit (ICU).
Macro hemodynamic indicator was represented by a vasoactive inotropic score calculated with the formula as dopamine dose (μg kg−1 min−1) + dobutamine dose (μg kg−1 min−1) + 100 × epinephrine dose (μg kg−1 min−1) + 100 × norepinephrine dose (μg kg−1 min−1) + 10,000 × vasopressin dose (U kg−1 min−1) + 10 × milrinone dose (μg kg−1 min−1).10
Glutathione Peroxidase
After getting permission, blood samples were taken to get GPx and lactate serum at the same time. As much as 2 mL of blood was withdrawn from the cubital vein and stored in a venoject tube containing EDTA anticoagulant. The tube was then centrifuged at 3000 rpm for 15 minutes to separate the serum from the precipitate or pellet within 30 minutes after sample collection. The serum obtained was stored at −20°C if not immediately analyzed. An ELISA test was subsequently performed to determine the GPx levels using the Human Glutathione Peroxidase ELISA Kit Catalog No. E3696Hu. Quantification was performed using ELISA Reader (iMark Microplate Absorbance Reader).
Data Collections
Data collected included patient name, age, gender, vital signs, and primary diagnosis. The degree of severity and lactate levels were evaluated on the first day of hospitalization.
All subjects were given early intervention, which include appropriate antibiotics, hemodynamic resuscitation, and organ support therapy using mechanical ventilation in accordance with the patient’s diagnosis. GPx levels were evaluated on day-0 and day-1 after resuscitation.
Data Analysis
Data were analyzed using SPSS for Windows 20.0 (IBM, Armonk, NY, USA). Normality for numerical data was tested using Shapiro–Wilk, in which data with normal distribution were presented as mean and standard deviation (SD) and data with non-normal distribution were presented as median and minimum and maximum range values. Nominal data were analyzed using Chi-square test. Numerical data with normal distribution were analyzed using t-test, while Mann–Whitney test was used for data with non-normal distribution. Bivariate analysis was conducted to identify the correlation between variables: data with normal distribution were analyzed using Pearson test, and data with non-normal distribution were analyzed using Spearman test. A receiver operating curve (ROC) analysis was performed to determine the cut-off for severity level score in relation to mortality outcome. The sensitivity and specificity of the severity level score were calculated to predict the cut-off for mortality outcome.
Results
A total of 44 patients who fulfilled the study criteria were included in this study. Of these 44 patients, only 34 patients received examinations for all study variables, of whom 14 patients died and 20 patients survived. No subject dropped out during the study period (Figure 1). Over half of the patients with vasodilatory shock were men (61.8%). The prevalence rate for in-hospital mortality was 41.2%. The mean MAP was 69.46 ± 14.17 mmHg. The MAP was lower in the non-survivor than in the survivor group (63.31 ± 16.67 mmHg vs 73.77 ± 10.56 mmHg, p = 0.032). The mean shock index was 1.28 ± 0.48. The shock index was higher in the non-survivor than in the survivor group (1.54 ± 0.52 vs 1.10 ± 0.35, p = 0.005). The mean SOFA score was 9.26 ± 3.95. The SOFA score was higher in non-survivor group than in the survivor group (10.86 ± 3.69 vs 8.15 ± 3.82, p = 0.047) (Table 1). The area under the receiver operating curve of the APACHE II score, SOFA score, and shock index to predict in-hospital mortality was 0.7 (p=0.050), 0.71 (p=0.041), and 0.764 (p=0.010), respectively (Figure 2). Based on the Youden index, the mortality cut-off score for each scoring method was as follows: 16.5 for APACHE II score, 10.5 for SOFA score, and 1.225 for the shock index (Figure 2). The sensitivity and specificity were as follows: 64.3% and 65% for the APACHE II score, 64.3% and 70% for the SOFA score, and 71.4% and 70% for the Shock Index (Figure 2).
 |
Table 1 Baseline Characteristics of Study Participants |
 |
Figure 1 Flow chart of the study population. |
There was no statistically different GPx at day 1 between the survivor and non-survivor groups. A correlation analysis was carried out to evaluate the correlation of severity scores, hemodynamic indicators, and GPx. The lactate levels were significantly negatively correlated with the GPx levels at baseline (r = −0.36, p=0.040) (Table 2; Figure 3). The SOFA score was significantly negatively correlated with the GPx levels at baseline (r = −0.43, p =0.011) (Table 3; Figure 4). The lactate levels were significantly positively correlated with the SOFA score (r = 0.5, p =0.003) (Table 4; Figure 5). The VIS was significantly positively correlated with the SI (r = 0.35, p=0.040) (Table 4). The MAP was significantly negatively correlated with the SI (r = -0.72, p=0.000) (Table 4).
 |
Table 2 Correlation Between Macro- and Microhemodynamic Status and Glutathione Peroxidase Levels |
 |
Table 3 Correlation Between Severity Score and Glutathione Peroxidase Levels |
 |
Table 4 Correlation Analysis Between Hemodynamic Indicators and Severity Scores |
 |
Figure 3 Significant negative correlation between the glutathione peroxidase levels at baseline and lactate levels. |
 |
Figure 4 Significant negative correlation the glutathione peroxidase levels at baseline and SOFA score. |
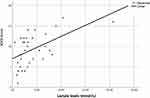 |
Figure 5 Significant positive correlation between SOFA score and lactate levels. |
Discussion
Patients hospitalized in the emergency department or intensive care unit with a diagnosis of vasodilatory shock over the period of the trial were eligible for participation. It was found that the mortality rate was 41.2%. We discovered that the group that did not survive had much higher shock index and SOFA scores, but significantly lower MAP. The area under the curve (AUC) was calculated to determine the cut-off, sensitivity, and sensitivity. In this study, the median GPx levels on day-0 was 32.38 ng/mL, with a minimum value of 17.52 ng/mL and a maximum value of 273.95 ng/mL. On day-1, The median GPx levels were 31.98 ng/mL, with a minimum value of 11.74 ng/mL and a maximum value of 266.03 ng/mL. The GPx levels appeared to be higher both on day-0 and day-1 in the patients who survived compared to those who passed away, although this was not statistically significant. Identifying antioxidant activity on the first day in the ICU is necessary due to its correlation with length of stay, prognosis, and treatment course. Antioxidant levels are reduced in patients with severe conditions, and medical treatment often further reduce antioxidant levels.11 GPx is an antioxidant enzyme that suppresses inflammation and tissue injury due to ROS released by phagocytes (neutrophils, monocytes, macrophages, and eosinophils).12
The SOFA and SI scores showed significant differences in the mortality of critically ill patients with vasodilatory shock. The SI value for the non-survivor group was significantly higher. The cut-off to predict in-hospital mortality was 1.225 (95% CI 0.93–0.99, p=0.050) with a sensitivity of 71.4% and a specificity of 70%, while the AUC was 0.76. In the interim, a range of values from 0.5 to 0.7 is deemed appropriate for SI.13 According to the results of several studies, a higher SI is associated with a higher mortality rate in the emergency department.14–16 In addition, it may be used as a triage tool to identify the patient’s severity without waiting for test findings. Indeed, our data support the notion that patients with vasodilatory shock had greater SI levels associated with death.
The SOFA score was found to be related to mortality in patients with vasodilatory shock. Previous studies have shown that the SOFA score is an accurate predictor of mortality in the ICU.17,18 An increase in the SOFA score reflects high risk of mortality. Furthermore, it has been shown that the SOFA score is a valid indication of long-term mortality in persons who have recently had an acute myocardial infarction.19 A link exists between a lower SOFA score and a decreased mortality rate.20 In addition, the SOFA score is used to evaluate the course of disease and organ failure over time.21 Then, it was expanded to include the association with patients’ outcomes.22–25 We assessed that, to predict mortality, a cut-off of 10.5 had a sensitivity of 64.3% and a specificity of 70% in patients with vasodilatory shock. Among other scores, the SOFA score had the highest AUC [0.71 (95% CI 0.81–0.94, p=0.709)]. The AUC result suggested that the SOFA score was effective in predicting the outcome of patients with vasodilatory shock.
In our study, we found significant negative correlation between GPx levels and tissue hypoxia represented by lactate levels (p < 0.05); and no significant correlation between GPx levels and hemodynamic status, as represented by MAP and VIS. Glutathione peroxidase at an early phase in severely septic patients makes it possible to predict the degree of severity of tissue hypoxia. Wiecek et al in 2018 conducted a study in healthy adult patients evaluating the difference between superoxide dismutase, catalase (CAT), and GPx enzymatic antioxidant defense at early phase after anaerobic exercise and within 24 hours after exercise. Anaerobic exercise would induce a significant increase of lactate levels. At the early phase of exercise, the antioxidant enzyme activity decreased; 24 hours after anaerobic exercise the GPx activity in the blood plasma of men and women was higher compared to before exercise. Increased activity of GPx will in turn increase the rate of GSH oxidation into GSSG in the presence of H2O2, which caused a reduction in GSH concentration, an increase in GSSG concentration, and a reduction in GSH/GSSG ratio (Figure 6).26
In addition, we found a negative correlation between GPx levels and SOFA score (r = −0.43; p = 0.011). The mean organ failure severity levels in this study were 9.26 ± 3.95 and 16.35 ± 8.22, as measured using SOFA and APACHE II scores. Based on classification reported in the study by Raith et al, the range of SOFA score in this study is relatively high with mortality prediction of more than 30%,27 while based on the APACHE II score, the mortality prediction in the study subjects was more than 20%.28
A study conducted by Chuang et al in 73 patients with severe sepsis suggested that the level of antioxidant capacity represented the clinical severity of the sepsis.29 There was a significant correlation between APACHE II score and serum total antioxidant capacity, as measured through serum uric acid. The SOFA score was negatively correlated with the GPx levels at baseline. Indeed, the SOFA score represents organ dysfunction during an ICU stay. In the past, the SOFA score was given to patients in hospitals or critical care units to classify the degree of organ dysfunction.17,30,31 Organ dysfunction results from the imbalance of oxidative stress, which includes the reduced GPx levels, since it causes inflammation, endothelial cell malfunction, and increased vascular permeability.32,33 As a result, the SOFA score, a simple, affordable, and rapid approach, may be used to evaluate early organ dysfunction. Moreover, an early onset of organ failure may occur during a patient’s stay in the ICU.34 Previous investigations have shown that there is a negative correlation between the GPx and the SOFA score.35
In addition, our study also assessed the shock severity level using SI. The mean SI value was 1.28 + 0.38. Several studies suggested that the normal range of SI value was between 0.5 and 0.7, although some evidence also stated that SI value up to 0.9 was still acceptable.36–39 SI value near 1.0 indicates worsening hemodynamic status or shock.40 Based on the SI characteristics, the majority of the current study population can be considered severe cases.
There was a correlation between the SOFA score and lactate concentration. This is consistent with previously conducted study.41–43 Blood lactate levels are a sensitive and universal sign of metabolic stress and tissue hypoxia.44 There is an increase in tension when there is a shortage of oxygen, and this may result in a number of critical illness.45 According to the results of our investigation, there was a negative correlation between the lactate levels and the GPx levels upon admission. In addition, our study demonstrates that individuals who did not survive sepsis had much greater lactate concentrations than those who survived. A previous study presented an association between higher levels of lactate and increased mortality.46,47 High lactate levels are believed to indicate organ malfunction and the need for immediate medical care.48
In our study, the VIS was significantly positively correlated with the SI (r = 0.35, P =0.040). In Setyaningtyas’ study, a significant negative correlation was found between VIS and ANP levels on day-2. ANP is a marker for congestive heart failure (r −0.475, p<0.05). This explained the role of VIS as the strongest predictor of mortality in in children with septic shock.49
Our study is constrained in many significant aspects. The design of the study, which was done in a single location, included an inherent bias that could not be eliminated. Not infrequently, the findings of a research only apply to the patients who were formally examined as part of the study. Moreover, our ICU often accepts severely ill patients as our ICUs are located at tertiary academic hospital. Our results support the evidence that the role of oxidative stress in vasodilatory shock is complex and requires further research. In addition, this study emphasizes the dynamic and balanced nature of oxidative stress rather than the static nature of measuring oxidative stress in critically ill patients, which can affect hemodynamic disturbances in the microcirculation that correlate with severe organ dysfunction.
Study Limitations
Our study could not determine the appropriate biomarker with an exact cut-off value to assess antioxidant capacity, which influenced mortality in critically ill patients with vasodilatory shock. Further research is needed to evaluate the factors that influence mortality in critically ill patients with vasodilatory shock.
Conclusion
Glutathione peroxidase as antioxidant is related to lactate levels as a marker of severity of tissue hypoxia and the SOFA score as a marker of organ failure but not with mortality in critically ill patients with vasodilatory shock.
Abbreviations
APACHE, Acute Physiology and Chronic Health Evaluation; AUC, area under curve; GPx, glutathione peroxidase; GR, glutathione reductase; GSH, reduced glutathione; GSSG, oxidized glutathione; ICU, Intensive Care Unit; MAP, mean arterial pressure; NADPH, reduced nicotinamide adenine dinucleotide phosphate; NADP+, nicotinamide adenine dinucleotide phosphate; ROS, reactive oxygen species; SI, Shock Index; SOFA, Sequential Organ Failure Assessment; SVR, systemic vascular resistance; VIS, vvasoactive inotropic score.
Ethical Consideration
This study was conducted in compliance with the Declaration of Helsinki. We obtained ethical approval from the Ethical Committee of the Faculty of Medicine, Universitas Airlangga - Dr. Soetomo General Academic Hospital (ethical clearance number 0348/KEPK/XII/2021).
Acknowledgments
We thank all teachers, the resident, nursing staff, laboratory staff in Soetomo General Academic Hospital, Faculty of Medicine, Universitas Airlangga; and the staff of Institute of Tropical Disease, Universitas Airlangga who are involved in patient management and data collection. We also thank Reza Affandi for helping us with the editing assistance.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The authors report no funding source.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Vincent JL, Creteur J. The critically ill patient. In: Critical Care Nephrology.
2. Flaring UB, Rooyackers OB, Wernerman J, Hammarqvist F. Temporal changes in muscle glutathione in ICU patients. Intensive Care Med. 2003;29:2193–2198. doi:10.1007/s00134-003-2031-5
3. Hammarqvist F, Luo J-L, Andersson K, Wernerman J, Wernerman J. Skeletal muscle glutathione is depleted in critically ill patients. Crit Care Med. 1997;25:78–84. doi:10.1097/00003246-199701000-00016
4. Kandar R. The ratio of oxidized and reduced forms of selected antioxidants as a possible marker of oxidative stress in humans. Biomed Chromatogr. 2016;30:13–28. doi:10.1002/bmc.3529
5. Wemerman J, Luo JL, Hammarqvist F. Glutathione status in critically ill patients: possibility of modulation by antioxidants. Proc Nutr Soc. 1999;58:677–680.
6. Robertson LC, Al-Haddad M. Recognizing the critically ill patient. Anaesth Intensive Care Med. 2013;14(1):11–14. doi:10.1016/j.mpaic.2012.11.010
7. Ho KM, Lee KY, Williams T, Finn J, Knuiman M, Webb SAR. Comparison of Acute Physiology And Chronic Health Evaluation (APACHE) II score with organ failure scores to predict hospital mortality. Anaesthesia. 2007;62(5):466–473. doi:10.1111/j.1365-2044.2007.04999.x
8. Berger T, Green J, Horeczko T, et al. Shock index and early recognition of sepsis in the Emergency Department: pilot study. Western J Emerg Med. 2013;14(2:):168–174. doi:10.5811/westjem.2012.8.11546
9. Jeong H, Park I, Lee JH, et al. Feasibility study using longitudinal bioelectrical impedance analysis to evaluate body water status during fluid resuscitation in a swine sepsis mode. Intensive Care Med Exp. 2022;10:51. doi:10.1186/s40635-022-00480-5
10. Baysal PK, Güzelmeriç F, Kahraman E, Gürcü ME, Erkılınç A, Orki T. Is vasoactive-inotropic score a predictor for mortality and morbidity in patients undergoing coronary artery bypass surgery? Braz J Cardiovasc Surg. 2021;36(6):802–806. doi:10.21470/1678-9741-2020-0219
11. Gajardo A, Von Dessaur B, Molina V, Vera S, Libuy M, Rodrigo R. Plasma antioxidant potential at admission is associated with length of ICU stay in child with sepsis: a pilot study. Fetal Pediatr Pathol. 2018;37(5):348. doi:10.1080/15513815.2018.1517845
12. Mittal M, Siddiqui MR, Tran K, et al. Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal. 2014;20:7(7):1126–1167. doi:10.1089/ars.2012.5149
13. Balhara KS, Hsieh YH, Hamade B, Circh R, Kelen GD, Bayram JD. Clinical metrics in emergency medicine: the shock index and the probability of hospital admission and inpatient mortality. Emerg Med J. 2017;34(2):89–94. doi:10.1136/emermed-2015-205532
14. Pandit V, Rhee P, Hashmi A, et al. Shock index predicts mortality in geriatric trauma patients: an analysis of the National Trauma Data Bank. J Trauma Acute Care Surg. 2014;76(4):1111–1115. doi:10.1097/TA.0000000000000160
15. King RW, Plewa MC, Buderer NMF, Knotts FB. Shock index as a marker for significant injury in trauma patients. Acad Emerg Medi. 1996;3(11):1041–1045. doi:10.1111/j.1553-2712.1996.tb03351.x
16. McNab A, Burns B, Bhullar I, Chesire D, Kerwin A. A prehospital shock index for trauma correlates with measures of hospital resource use and mortality. Surgery. 2012;152(3):473–476. doi:10.1016/j.surg.2012.07.010
17. Lopes Ferreira F, Peres Bota D, Bross A, Mélot C, Vincent JL. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA. 2001;286(14):1754–1758. doi:10.1001/jama.286.14.1754
18. Fuchs PA, Czech IJ, Krzych ŁJ. Mortality prediction using SOFA score in critically ill surgical and non-surgical patients: which parameter is the most valuable? Medicina. 2020;56(6):1–8. doi:10.3390/medicina56060273
19. Huang SS, Chen YH, Lu TM, Chen LC, Chen JW, Lin SJ. Application of the Sequential Organ Failure Assessment score for predicting mortality in patients with acute myocardial infarction. Resuscitation. 2012;83(5):591–595. doi:10.1016/j.resuscitation.2011.12.014
20. Goldhill DR, Sumner A. Outcome of intensive care patients in a group of British intensive care units. Crit Care Med. 1998;26:1337–1345. doi:10.1097/00003246-199808000-00017
21. Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22(7):707–710. doi:10.1007/BF01709751
22. Regel G, Grotz M, Weltner T, Sturm JA, Tscherne H. Pattern of organ failure following severe trauma. World J Surg. 1996;20:422–429. doi:10.1007/s002689900067
23. Vincent JL, de Mendonca A, Cantraine F, et al. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on “sepsis-related problems” of the European Society of Intensive Care Medicine. Crit Care Med. 1998;26(11):1793–1800. doi:10.1097/00003246-199811000-00016
24. Antonelli M, Moreno R, Vincent JL, et al. Application of SOFA score to trauma patients. Intensive Care Med. 1999;25(4):389–394. doi:10.1007/s001340050863
25. Moreno R, Vincent JL, Matos R, et al. The use of maximum SOFA score to quantify organ dysfunction/failure in intensive care. Results of a prospective, multicentre study. Intensive Care Med. 1999;25(7):686–696. doi:10.1007/s001340050931
26. Kantorowicz M, Szygula Z. Anaerobic exercise-induced activation of antioxidant enzymes in the blood of women and men. Front Physiol. 2018;27(9):1006. doi:10.3389/fphys.2018.01006
27. Raith EP, Udy AA, Bailey M, et al. Prognostic accuracy of the SOFA score, SIRS criteria, and qSOFA score for in-hospital mortality among adults with suspected infection admitted to the intensive care unit. JAMA. 2017;317:3::290–300. doi:10.1001/jama.2016.20328
28. Costa JI, Gomes JL, Munechika M, Juliano Y, Filho JGB. Severity and prognosis in intensive care: prospective application of the APACHE II index. São Paulo Med J. 1999;117:5::205–214. doi:10.1590/S1516-31801999000500005
29. Chuang CC, Shiesh SC, Chi CH, et al. Serum total antioxidant capacity reflects severity of illness in patients with severe sepsis. Crit Care. 2006;10:R36. doi:10.1186/cc4826
30. Hynninen M, Valtonen M, Markkanen H, et al. Interleukin 1 receptor antagonist and E-selectin concentrations: a comparison in patients with severe acute pancreatitis and severe sepsis. J Crit Care. 1999;14:63–68. doi:10.1016/S0883-9441(99)90015-1
31. Di Filippo A, De Gaudio AR, Novelli A, et al. Continuous infusion of vancomycin in methicillin-resistant staphylococcus infection. Chemotherapy. 1998;44:63–68. doi:10.1159/000007092
32. Boisramé-Helms J, Kremer H, Schini-Kerth V, Meziani F. Endothelial dysfunction in sepsis. Curr Vasc Pharmacol. 2013;11(2):150–160.
33. Babior BM. Phagocytes and oxidative stress. Am J Med. 2000;109(1):33–44. doi:10.1016/S0002-9343(00)00481-2
34. Cryer HG, Leong K, McArthur DL, et al. Multiple organ failure: by the time you predict it, it’s already there. J Trauma. 1999;46(4):597–606. doi:10.1097/00005373-199904000-00007
35. Rodas PC, Rooyackers O, Hebert C, Norberg A, Wernerman J. Glutamine and glutathione at ICU admission in relation to outcome. Clin Sci. 2012;122(12):591–597. doi:10.1042/CS20110520
36. Rousseaux J, Grandbastien B, Dorkenoo A, Lampin ME, Leteurtre S, Leclerc F. Prognostic value of shock index in children with septic shock. Pediatr Emerg Care. 2013;29:10. doi:10.1097/PEC.0b013e3182a5c99c
37. Acker SN, Ross JT, Partrick DA, Tong S, Bensard DD. Pediatric specific shock index accurately identifies severely injured children. J Pediatr Surg. 2015;50:2:331–334. doi:10.1016/j.jpedsurg.2014.08.009
38. Linnaus ME, Notrica DM, Langlais CS, et al. Prospective validation of the shock index pediatric-adjusted (SIPA) in blunt liver and spleen trauma. J Pediatr Surg. 2017;52:2:340–344. doi:10.1016/j.jpedsurg.2016.09.060
39. Vandewalle RJ, Peceny JK, Dolejs SC, Raymond JL, Rouse TM. Trends in pediatric adjusted shock index predict morbidity and mortality in children with severe blunt injuries. J Pediatr Surg. 2017;53(2):362–366. doi:10.1016/j.jpedsurg.2017.10.045
40. Allgöwer M, Burri C. Shock Index. Deutsch Med Wochenschr. 1967;92(43):1947–1950. doi:10.1055/s-0028-1106070
41. Shapiro NI, Howell MD, Talmor D, et al. Serum lactate as a predictor of mortality in emergency department patients with infection. AnN Emerg Med. 2005;455:524–528. doi:10.1016/j.annemergmed.2004.12.006
42. Trzeciak S, Dellinger RP, Chansky ME, et al. Serum lactate as a predictor of mortality in patients with infection. Intensive Care Med. 2007;33(6):970–977. doi:10.1007/s00134-007-0563-9
43. Jansen TC, Van Bommel J, Woodward R, Mulder PGH, Bakker J. Association between blood lactate levels, Sequential Organ Failure Assessment subscores, and 28-day mortality during early and late intensive care unit stay: a retrospective observational study. Crit Care Med. 2009;37(8):2369–2374. doi:10.1097/CCM.0b013e3181a0f919
44. Kraut JA, Madias NE, Ingelfinger JR. Lactic acidosis. N Engl J Med. 2014;371(24):2309–2319. doi:10.1056/NEJMra1309483
45. Gomez H, Kellum JA. Lactate in sepsis. JAMA. 2015;313(2):194–195. doi:10.1001/jama.2014.13811
46. Singer AJ, Taylor M, Domingo A, et al. Diagnostic characteristics of a clinical screening tool in combination with measuring bedside lactate level in emergency department patients with suspected sepsis. Acad Emerg Medi. 2014;21(8):853–857. doi:10.1111/acem.12444
47. Nichol AD, Egi M, Pettila V, et al. Relative hyperlactatemia and hospital mortality in critically ill patients: a retrospective multi-centre study. Crit Care. 2010;14(1):1–9. doi:10.1186/cc8888
48. Jones AE, Shapiro NI, Trzeciak S, et al. Lactate clearance vs central venous oxygen saturation as goals of early sepsis therapy: a randomized clinical trial. JAMA. 2010;303(8):739–746. doi:10.1001/jama.2010.158
49. Setyaningtyas A, Soetjipto S, Endaryanto A, Pudjiadi AH. The correlations of human atrial natriuretic peptide on cardiac function and hemodynamics in pediatric septic shock. Open Access Emerg Med. 2022;14:525–534. doi:10.2147/OAEM.S379543
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


