Back to Journals » Clinical Interventions in Aging » Volume 18
Homocysteine Combined with Apolipoprotein B as Serum Biomarkers for Predicting Carotid Atherosclerosis in the Oldest-Old
Authors Liu Z, Li Y, Cheng F, Zhou Y, Chen M, Ning C, Zhang B, Zhao Y
Received 4 July 2023
Accepted for publication 7 November 2023
Published 24 November 2023 Volume 2023:18 Pages 1961—1972
DOI https://doi.org/10.2147/CIA.S428776
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Maddalena Illario
Zhaoyu Liu,1,* Yan Li,2,* Fei Cheng,1 Yue Zhou,1 Miao Chen,1 Chaoxue Ning,3 Bingqi Zhang,4 Yali Zhao3
1Clinical Laboratory, Hainan Hospital of Chinese PLA General Hospital, Sanya, Hainan, People’s Republic of China; 2Transfusion Medicine Department, Hainan Hospital of Chinese PLA General Hospital, Sanya, Hainan, People’s Republic of China; 3Central Laboratory, Hainan Hospital of Chinese PLA General Hospital, Sanya, Hainan, People’s Republic of China; 4Ultrasound Diagnosis Department, Hainan Hospital of Chinese PLA General Hospital, Sanya, Hainan, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yali Zhao, Central Laboratory, Hainan Hospital of Chinese PLA General Hospital, Sanya, Hainan, 572000, People’s Republic of China, Email [email protected]
Background: The measurement of serum biomarkers is a promising decision aid in the assessment of atherosclerosis. However, data on the levels and epidemiological distribution of serum biomarkers of carotid atherosclerosis (CAS) in the oldest-old are limited. This study aimed to investigate the characteristics of CAS serum biomarkers in the oldest-old over 80 and explore their predictive value for CAS.
Methods: As part of the China Hainan Centenarian Cohort Study, a total of 1565 individuals over 80 years old were included. Atherosclerosis was assessed by carotid plaque and carotid intima-media thickness. Serum biomarker levels, demographic indicators, and physical examination indicators were detected. Prediction factors correlated to the CAS were explored by logistic regression and verified by receiver operating characteristic curve analysis. Multivariate regression models were fitted, along with subgroup analysis and robustness tests.
Results: Among the oldest-old population, 83.5% (1306) had CAS. In a fully adjusted multivariate logistic regression model, systolic blood pressure (SBP), heart rhythm (HR), serum homocysteine (Hcy), and apolipoprotein B (ApoB) levels were significantly and positively associated with CAS in the oldest-old (PS < 0.001). ROC analysis indicated that the combination of serum Hcy, ApoB, SBP, and HR increased the predictive value for CAS in the oldest-old (area under the curve: 0.856, 95% CI: 0.803– 0.879; sensitivity: 81.8%; specificity: 85.9%).
Conclusion: SBP, HR, Hcy and ApoB are independent risk factors for CAS in the oldest-old. The specific set of biomarkers and their combination with other risk markers may be a promising strategy for assessing CAS in the elderly, especially in global aging.
Keywords: carotid atherosclerosis, carotid intima-media thickness, biomarker, homocysteine, apolipoprotein B
Introduction
Stroke and ischemic heart disease (IHD) are the two most common causes of death worldwide. Atherosclerosis is identified as the leading cause of these diseases.1 The early detection of atherosclerosis in asymptomatic individuals focuses on the carotid arteries.2 Notably, carotid atherosclerosis (CAS) is responsible for 8–15% of ischemic strokes.3,4 Additionally, the burden of carotid plaque load and characteristics is highly correlated with cardiovascular events.5,6 Therefore, estimating the epidemiological burden and risk factors of CAS serves as a significant basis for the prevention and management of cardiovascular diseases.7,8
Dyslipidemia and chronic systemic inflammation are key features of atherosclerosis and occur throughout all stages of pathophysiology, including initiation, progression, and complications.9 Vascular inflammation markers are highly correlated with the degree of carotid plaque stenosis and vulnerability.10,11 Atherosclerotic plaque contents continuously interact with circulating blood. During plaque progression, specific molecules may diffuse toward the serum and become serum biomarkers, thus providing predictive information about plaque presence, status, and risk of complications.12 Several serum biomarkers combined with imaging features can help vascular specialists identify high-risk stroke patients and those requiring intervention.13,14 Compared with ultrasonography, computed tomography, magnetic resonance imaging, and other new imaging techniques such as intravascular ultrasound and positron emission tomography, serum biomarker testing has the advantages of operator-independent diagnostic accuracy, non-invasiveness, and low cost.15 Serum biomarkers that are currently widely concerned include inflammatory markers, lipids, metabolic markers, hematologic markers, etc. Their rational use may help quantify the risk associated with carotid artery disease.16 However, there are currently no data evaluating the serum biomarkers of CAS in the elderly population over 80 years old.
This study involved data collection of demographics and physical examinations, as well as the examination of serum biomarkers of CAS in the China Hainan Centenarian Cohort Study (CHCCS). We performed additional analyses, including a comparison of serum biomarker concentrations in the oldest-old with and without CAS, alongside an analysis of the correlation between them. We also determined whether serum biomarkers are independent risk factors for CAS after controlling other confounding factors. Finally, we assessed the predictive value of serum biomarkers for CAS by combining physical examination indicators. Overall, this study provides insights into managing cardiovascular disease in the oldest-old, particularly in the context of global aging.
Methods
Participants and Settings
This study is part of the China Hainan Centenarian Cohort Study (CHCCS), which aims to examine determinants of various diseases among centenarians and the elderly. The detailed procedures, including the interview process and sampling strategies, have been reported previously.17,18 This investigation was made possible through the support of The Department of Civil Affairs of Hainan Province of the People’s Republic of China. The design and implementation of the cohort involved a team of sociologists, psychologists, geriatricians, cardiologists, neurologists, and other experts. From 18 cities and counties in Hainan Province, 1782 elderly people who were eligible (aged 80–116 years) were enrolled and surveyed based on the participant selection process shown in Figure 1. The following inclusion criteria were used to recruit study participants: (1) were permanent residents of the survey area and over 80 years old through age verification; (2) volunteered to participate in the study and provided written informed consent; (3) was conscious and able to cooperate in completing questionnaire interviews, physical examinations and blood tests. The following were participant exclusion criteria: (1) history of stroke (defined as a history of ischemic or unspecified stroke but not hemorrhagic stroke), coronary disease, severe liver disease, chronic kidney disease, anemia, and malignant; (2) had taken folic acid, vitamin B6, vitamin B12 and lipid metabolism drugs continuously for a long time (more than three months); (3) missing data for anthropometric, demographic or laboratory testing serum biomarkers. Finally, a total of 1565 participants were included.
 |
Figure 1 Flowchart of participant enrollment. |
Carotid Ultrasound
Carotid ultrasound was performed by experienced physicians using a Philips CX50, 7.5 MHz linear array transducer. CIMT and plaques were assessed in B mode, following the criteria established by the European Stroke Organization.19 CIMT was measured three times in longitudinal sections of the left and right common carotid arteries located 1.0 cm proximal to the common carotid bulb, and the average value was calculated for subsequent analysis. When CIMT measured between 1.0 and 1.4 mm, it was considered thickening. Carotid plaque was diagnosed when a focal CIMT ≥1.5 mm or a lesion encroaches into the arterial lumen ≥0.5 mm or 50% of the surrounding IMT value. CAS was defined as CIMT≥1 mm and/or the presence of atherosclerotic plaques in the carotid.20–22 The carotid ultrasound examination results were subsequently reviewed by two independent operators.
Laboratory Measurements from Blood Samples
After an overnight fast, venous blood samples were collected from each participant and transported in chilled bio-transport containers at 4°C to our central laboratory within 4 hours. The Roche automatic biochemical analyzer (Cobas 8000-c701, Roche Diagnostics GmbH, Mannheim, Germany) was used to measure serum levels of albumin (ALB), total bilirubin (TB), aspartate transaminase (AST), High-sensitivity C-reactive protein (hs-CRP), homocysteine (Hcy), total cholesterol (TC), triglyceride (TG), low-density lipoprotein-cholesterol (LDL-C), high-density lipoprotein-cholesterol (HDLC), apolipoprotein A I (ApoA I), and apolipoprotein B (ApoB). Automatic Hematology Analyzer (Sysmex XT-2000i, Hitachi Ltd., Tokyo, Japan) was used to measure red blood cells (RBC), white blood cells (WBC), neutrophils, lymphocytes, monocytes and platelet (PLT). All test items were calibrated with matching calibrators and underwent two levels of quality control testing before analysis. All samples were tested within 8 hours of arrival at the laboratory. The inter-assay coefficient of variation (CV) of all inspection items is <6.5%.
Covariates
Self-reported information was obtained from each subject, including age, sex, race, smoking status, alcohol consumption, and medical history. Subjects were grouped according to their cigarette use: current smoker, former smoker (quit smoking one year ago or earlier), or never, as well as their alcohol consumption: current drinker, former drinker (quit drinking one year ago or earlier), or never. History of hypertension, dyslipidemia, and diabetes was defined as previously diagnosed with hypertension, dyslipidemia, or diabetes, or the medical history of antihypertensive, lipid-lowering, or hypoglycemic drugs before the study examination. Height (H), weight (W), waist circumference (WC), and hip circumference (HC) were measured. BMI and WHR were calculated using the standard formulas: BMI=W/H2, WHR=WC/HC. The estimated glomerular filtration rate (eGFR) was calculated using the chronic kidney disease Epi formula.23
Ethics Approval and Consent to Participate
This study followed the Declaration of Helsinki and was approved by the Ethics Committee of the Hainan Branch of the Chinese People’s Liberation Army General Hospital (No. 301hn11201601). Informed consent was obtained from all participants.
Statistical Analysis
The measurement data were assessed for normality. Normally distributed data were compared using unpaired Student’s t-test and reported as mean ± standard deviation (SD). Non-normally distributed variables were compared using the Mann–Whitney U-test and presented as medians (Q1, Q3). Categorical variables were tested using the chi-squared test and are presented as count and percentage. The association between serum biomarkers and covariates with CAS was investigated using Spearman correlation analysis. Multivariate logistic regression analyses were conducted to calculate adjusted odds ratios (OR) for the association of serum biomarkers with CAS. Multicollinearity was checked using the variance inflation factor (VIF) at a cut-off point of 10. The interaction between selected potential factors on CAS was evaluated using a generalized linear model. The diagnostic threshold, specificity, sensitivity, and predictive accuracy of CAS-related predictors were measured using the receiver operating characteristic (ROC) curve and the area under the curve (AUC) analysis. p < 0.05 indicated statistical significance. All probability values were two-tailed distributed, and confidence intervals (CIs) were calculated at a 95% level of accuracy. All analyses were conducted using the software SPSS 28.0 (IBM Corp., Armonk, NY, USA), and all graphs were created using OriginPro 2019b (OriginLab Inc., Northampton, MA, USA).
Results
Clinical Characteristics of the Participants
A total of 1782 participants who met the inclusion were enrolled in this study, among them, 217 met the exclusion criteria and finally 1565 participants were included in the analysis. There were significant differences in blood cell formed components (WBC, Neutrophil, PLT), liver and kidney function indicators (AST, TB, eGFR), and lipid metabolism indicators (TC, LDL-C) between included and excluded participants (P < 0.05 for all) (Supplementary Table 1). The demographic, clinical, and laboratory characteristics of the participants are shown in Table 1. Among the 1565 participants, the median age was 100 (84, 102) years, and 28.4% (n = 445) were male. The proportion of the oldest-old with CAS was 83.5% (n = 1306). The age, SBP, HR, and prevalence of metabolic syndrome in the CAS group were higher than those in the Non-CAS group (P < 0.05 for all). Additionally, significant differences were found in the past smoking and drinking history between the two groups (P < 0.05 for all). Notably, the levels of serum Hcy and ApoB in the CAS group were significantly higher than those in the control group (P < 0.05 for all).
 |
Table 1 Baseline Characteristics for Participants |
Correlations Between Clinical Profile and CAS in the Oldest-Old
Correlation analysis showed that age (r = 0.091, P < 0.001), SBP (r = 0.174, P < 0.001), HR (r = 0.189, P < 0.001), Hcy (r = 0.259, P < 0.001) and ApoB (r = 0.218, P < 0.001) were significantly correlated with CAS (Table 2). Moreover, Hcy and ApoB were stratified into quartiles according to serum concentrations. The prevalence of CAS stratified based on quartiles was shown in Figure 2, indicating that Hcy and ApoB concentrations were positively associated with CAS incidence, and CIMT thickened with increasing Hcy and ApoB.
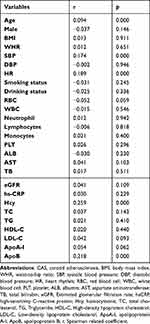 |
Table 2 Univariate Correlation Analysis of the Clinical Profile and CAS |
 |
Figure 2 Percentage of CIMT grade stratified by quartile of Hcy (A) and ApoB (B). Abbreviations: CAS, carotid atherosclerosis; Hcy, homocysteine; ApoB, apolipoprotein B. |
The Multiple-Variable Models for CAS and Associated Factors
To reduce the confounding effect of variables on CAS, multivariate logistic regression analysis was conducted to identify the independent variables associated with the presence of CAS in the oldest-old. As shown in Table 3, in the unadjusted model, SBP, HR, serum Hcy and ApoB levels were positively associated with CAS (P < 0.05 for all), while no association was observed between CAS and other serum biomarkers. Meanwhile, four regression models were tested to evaluate the robustness of the associations, and it was found that the associations remained significant in the adjusted models.
 |
Table 3 Multivariate Logistic Regression Analysis of Factors Associated with CAS in Long-Lived Elderly |
The Value of Single and Combined SBP, HR, Hcy, and ApoB Detection in the Prediction of CAS
ROC curve analysis showed that the AUCs of SBP, HR, Hcy, and ApoB alone were 0.558 (0.520, 0.595), 0.569 (0.530, 0.608), 0.678 (0.642, 0.713), and 0.600 (0.563, 0.636), respectively. Among these, Hcy had the highest sensitivity of 74.2%, while ApoB had the highest specificity of 75.4%. The AUC of the combined use of the four markers in predicting CAS was 0.856 (0.803, 0.879), with a sensitivity of 81.8%, a specificity of 85.9%, and a significantly higher predictive value than single detection (P < 0.05 for all). Additionally, we calculated the positive likelihood ratio (PLR) and negative likelihood ratio (NLR). Similarly, the results showed that combined detection had the highest PLR (5.80) and the lowest NLR (0.22), indicating a good diagnostic value (Table 4).
 |
Table 4 Comparison of the Predictive Ability of Hcy and ApoB Alone or in Combination on Carotid Atherosclerosis in the Elderly by ROC Curve |
Discussion
Whenever possible, accurate assessment of carotid atherosclerosis will be beneficial in reducing the risk of coronary heart disease and stroke.24,25 To the best of our knowledge, this study represents the first large-scale cross-sectional evaluation of serum markers of CAS in an elderly population older than 80. Our results indicated that serum Hcy and ApoB levels were significant independent risk factors for CAS in the elderly after adjusting for multiple potentially confounding factors. Nevertheless, we found no significant associations between hs-CRP, TC, LDL-C, HDL-C, ApoA-I levels, hematological indicators, and CAS in the oldest-old. Our findings support that serum Hcy combined with ApoB can serve as an effective target for the prevention and treatment of CAS in the elderly population.
According to the Hcy theory of atherosclerosis, elevated Hcy levels in the blood represent an important risk factor for developing atherosclerosis.26 Previous studies have reported that even mildly elevated serum Hcy level is an independent risk factor for increased carotid artery wall thickness.27,28 Additionally, Kim et al found that Hcy levels can predict asymptomatic carotid artery stenosis in patients undergoing coronary artery bypass grafting.29 Kim reported that elevated serum Hcy levels are independently associated with intracranial arterial calcification and atherosclerotic burden.30 Not only that, other studies have shown that Hcy is associated with carotid plaque densitometry, area, and its use as a subclinical marker of ischemic stroke risk.31,32 Studies have suggested several mechanisms through which Hcy can lead to vascular injury and subsequent cardiovascular diseases, including endothelial damage, DNA dysfunction, smooth muscle cell proliferation, increased oxidative stress, decreased glutathione peroxidase activity, and inflammation promotion.33 Nevertheless, some cohort studies have reported no significant correlation between elevated Hcy levels and CAS.34,35 Our findings support a positive correlation between serum Hcy and CAS in the oldest-old population. Notably, age differences in participants appear to be the primary cause of the varying conclusions observed. Hcy is more likely associated with CAS among older individuals, further substantiated by the NHLBI Family Heart study findings. Specifically, the study reports a significant positive correlation between Hcy levels and carotid artery wall thickness only in those aged 55 years or older.36 Collectively, these data suggest that age-based stratification is necessary when formulating medical strategies targeting Hcy metabolism.37,38
Studies suggested that ApoB and ApoA1 may be superior predictors of atherosclerosis and cardiovascular risk compared to traditional lipid parameters.39,40 Our study focused on assessing serum lipid markers for their ability to predict coronary artery stenosis (CAS) in the elderly. Our findings revealed that ApoB levels were an independent risk factor for CAS in the elderly, while ApoA1, TC, TG, LDL-C, and HDL-C did not demonstrate a significant correlation with CAS. Furthermore, ApoB exhibited the highest degree of specificity (75.4%) among all risk factors examined when used alone to predict CAS in the oldest-old population. ApoB is known to consist of ApoB-48 and ApoB-100. ApoB-48 forms complexes with free cholesterol and dietary TG. In contrast, ApoB-100 is present in LDL, intermediate LDL, very LDL, and small-dense LDL.41 A recent meta-analysis demonstrated that ApoB is a more precise marker for assessing cardiovascular risk than LDL and HDL.42 In a 9-year prospective study with a multiethnic cohort of participants who had atherosclerosis, CAS progression was associated with ApoB independent of cholesterol levels.43 Similarly, in a 13-year prospective study, Razavi et al found that lower LDL levels did not reliably predict the persistence of carotid plaque independently of ApoB. However, when ApoB levels were considered, a 10 mg/dL drop in ApoB was associated with an 11% higher likelihood of long-term absence of carotid plaque.44 Furthermore, proton nuclear magnetic resonance lipoprotein profiling data from three large cohorts identified ApoB as a positive correlate for cardiovascular events.45 This evidence suggests that ApoB is a clinically useful marker in the assessment of atherosclerosis risk.
Although CRP was the first identified biomarker of atherosclerosis, its association with carotid artery disease has been continuously debated. Multiple studies have revealed that CRP does not accurately predict the presence of carotid plaque46 and lacks a significant correlation with plaque inflammation.47,48 Our study showed that serum hs-CRP level is not an independent risk factor for CAS in the oldest-old. Furthermore, a reported large series found no significant association between the progression of cIMT and average hsCRP levels over a 2-year period; values were also not related in a dose-response manner.49 Indeed, serum hs-CRP, as an indicator of a chronic inflammatory state, is not organ-specific and displays a lognormal distribution throughout different study populations, which makes it difficult to obtain a threshold or diagnostic cutpoint for a specific disease. Additionally, CRP is closely linked to various factors, including blood pressure, age, sex, diabetes, smoking, LDL-C, HDL-C, ventricular hypertrophy, and atrial fibrillation,50 which limits its effectiveness as a marker for disease risk stratification.
Research to date can demonstrate that biomarkers can help evaluate stroke risk and assist patients with carotid artery disease in selecting the best treatment.37 However, current research still focuses on the correlation between a single marker and pathological outcome.11 Recommending a specific set of biomarker tests for clinical use is cumbersome. First, some clinically proven associations are nonspecific; second, the clinical relevance of promising biomarkers is inconsistent across populations, and other clinically relevant biomarkers such as metalloproteinases, interleukins, or microRNAs require great economic resources and long determination times, limiting their use to really selected patients.16 Therefore, we believe that it is a valuable exploration to combine serum biomarkers with demographic and physical examination indicators to improve the positive predictive value. Although current reports are limited, this view has gradually attracted the attention of researchers. For example, a recent study found that combining co-existing hypertension and Hcy with carotid vulnerable plaque features has a stronger predictive value for subsequent vascular events than each measurement alone.51
Specificity is a weakness of serum biomarkers in regards to coronary and CAS. To address this, we should focus on a small group of clinically-proven biomarkers, then determine which ones are more cost-effective and widely available before ultimately proposing a set of biomarkers for clinicians. In this study, we screened out the physical examination indicators related to CAS in the elderly, HR, and SBP. When combined with serum biomarkers, the specificity of predicting CAS reached 85.9%. Our research focuses on the elderly over 80, who often have limited exercise capacity, making it difficult to undergo Doppler ultrasound or other imaging examinations regularly. However, physical examination data and blood samples are easily acquired. We believe that the combination of physical examination indicators and serum biomarkers with high-risk weights can be an effective strategy for stratifying the risk of carotid artery disease in elderly individuals.
It is important to note that this study had several limitations. Firstly, it was a single-center study that only included the elderly in China. A multicenter trial of different races or ethnicities is necessary to confirm the predictive value of serum Hcy and ApoB levels for CAS in the oldest-old. Secondly, we did not detect and classify carotid plaque components and thus could not confirm the associations between serum biomarkers and carotid plaque characteristics. Finally, the oldest-old in this study were not followed up for outcomes, nor were serum biomarkers measured multiple times over time, limiting our ability to make broad recommendations.
Our study not only substantiated previous research on the impact of Hcy and ApoB on CAS burden but also evaluated the predictive value of the combined application of serum biomarkers and physical examination indicators on CAS in the oldest-old. This will provide an important basis for the prevention and management of cardiovascular diseases using serum markers in the context of global aging.
Abbreviations
CAS, carotid atherosclerosis; BMI, body mass index; WHR, waist-to-hip ratio; SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rhythm; RBC, red blood cell; WBC, white blood cell; PLT, platelet; ALB, albumin; AST, aspartate aminotransferase; TB, total bilirubin; eGFR, Estimated glomerular filtration rate; hsCRP, high-sensitivity C-reactive protein; Hcy, homocysteine; TC, total cholesterol; TG, Triglyceride; HDL-C, High-density lipoprotein cholesterol; LDL-C, Low-density lipoprotein cholesterol; ApoA-I, apolipoprotein A-I; ApoB, apolipoprotein B; PLR, positive likelihood ratio; NLR, negative likelihood ratio; ROC, receiver operating characteristic; AUC, area under curve; OR, odds ratio.
Data Sharing Statement
In attempt to preserve the privacy of individuals, clinical data will not be shared; the data can be available from the corresponding author on reasonable request authors upon request.
Ethics Approval and Consent to Participate
The current study received the approval from Ethics Committee of Hainan Branch of Chinese People’s Liberation Army General Hospital (Sanya, Hainan; Number: 301hn11201601). Prior to the current study, informed consent were required from all participants.
Acknowledgments
We appreciate all the staff of CHCCS for their continued cooperation and contribution in field work.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by grants from National Natural Science Foundation of China (No. 81941021), Specific Research Fund of The Innovation Platform for Academicians of Hainan Province (No. YSPTZX202216), National Key R&D Program of China (No. 2018YFC2000400), National S&T Resource Sharing Service Platform Project of China (No. YCZYPT[2018]07), and General Hospital of PLA Medical Big Data R&D Project (No. MBD2018030).
Disclosure
The authors have nothing to disclose with regard to commercial support. The authors have no conflicts of interest relevant to this article to disclose.
References
1. Ezzati M, Obermeyer Z, Tzoulaki I, Mayosi BM, Elliott P, Leon DA. Contributions of risk factors and medical care to cardiovascular mortality trends. Nat Rev Cardiol. 2015;12(9):508–530. doi:10.1038/nrcardio.2015.82
2. Piepoli MF, Hoes AW, Agewall S, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: the Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J. 2016;37(29):2315–2381. doi:10.1093/eurheartj/ehw106
3. Bonati LH, Jansen O, de Borst GJ, Brown MM. Management of atherosclerotic extracranial carotid artery stenosis. Lancet Neurol. 2022;21(3):273–283. doi:10.1016/S1474-4422(21)00359-8
4. Howard D, Gaziano L, Rothwell PM; Oxford Vascular Study. Risk of stroke in relation to degree of asymptomatic carotid stenosis: a population-based cohort study, systematic review, and meta-analysis. Lancet Neurol. 2021;20(3):193–202. doi:10.1016/S1474-4422(20)30484-1
5. Brunner G, Virani SS, Sun W, et al. Associations between carotid artery plaque burden, plaque characteristics, and cardiovascular events: the ARIC carotid magnetic resonance imaging study. JAMA Cardiol. 2021;6(1):79–86. doi:10.1001/jamacardio.2020.5573
6. Bos D, Arshi B, van den Bouwhuijsen Q, et al. Atherosclerotic carotid plaque composition and incident stroke and coronary events. J Am Coll Cardiol. 2021;77(11):1426–1435. doi:10.1016/j.jacc.2021.01.038
7. Song P, Fang Z, Wang H, et al. Global and regional prevalence, burden, and risk factors for carotid atherosclerosis: a systematic review, meta-analysis, and modelling study. Lancet Glob Health. 2020;8(5):e721–e729. doi:10.1016/S2214-109X(20)30117-0
8. Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation. 2007;115(4):459–467. doi:10.1161/CIRCULATIONAHA.106.628875
9. Libby P, Buring JE, Badimon L, et al. Atherosclerosis. Nat Rev Dis Primers. 2019;5(1):56.
10. Kamtchum-Tatuene J, Saba L, Heldner MR, et al. Interleukin-6 predicts carotid plaque severity, vulnerability, and progression. Circ Res. 2022;131(2):e22–e33. doi:10.1161/CIRCRESAHA.122.320877
11. Siniscalchi A, Murphy S, Gray C, De Sarro G, Gallelli L. Biomarkers in unstable carotid plaque: physiopathology and prediction. Cardiovasc Hematol Agents Med Chem. 2022;20(1):13–19. doi:10.2174/1871525719666210901131509
12. Hermus L, Lefrandt JD, Tio RA, Breek JC, Zeebregts CJ. Carotid plaque formation and serum biomarkers. Atherosclerosis. 2010;213(1):21–29. doi:10.1016/j.atherosclerosis.2010.05.013
13. Ammirati E, Moroni F, Norata GD, Magnoni M, Camici PG. Markers of inflammation associated with plaque progression and instability in patients with carotid atherosclerosis. Mediators Inflamm. 2015;2015:718329. doi:10.1155/2015/718329
14. Loftus I, Thompson M. Plaque biology: interesting science or pharmacological treasure trove. Eur J Vasc Endovasc Surg. 2008;36(5):507–516. doi:10.1016/j.ejvs.2008.06.002
15. Avgerinos ED, Kadoglou NP, Moulakakis KG, Giannakopoulos TG, Liapis CD. Current role of biomarkers in carotid disease: a systematic review. Int J Stroke. 2011;6(4):337–345. doi:10.1111/j.1747-4949.2011.00623.x
16. Martinez E, Martorell J, Riambau V. Review of serum biomarkers in carotid atherosclerosis. J Vasc Surg. 2020;71(1):329–341. doi:10.1016/j.jvs.2019.04.488
17. He Y, Zhao Y, Yao Y, et al. Cohort profile: the China Hainan Centenarian Cohort Study (CHCCS). Int J Epidemiol. 2018;47(3):694–695h. doi:10.1093/ije/dyy017
18. Chen C, Liu GG, Sun Y, et al. Association between household fuel use and sleep quality in the oldest-old: evidence from a propensity-score matched case-control study in Hainan, China. Environ Res. 2020;191:110229. doi:10.1016/j.envres.2020.110229
19. Touboul PJ, Hennerici MG, Meairs S, et al. Mannheim carotid intima-media thickness and plaque consensus (2004-2006-2011). An update on behalf of the advisory board of the 3rd, 4th and 5th watching the risk symposia, at the 13th, 15th and 20th European Stroke Conferences, Mannheim, Germany, 2004, Brussels, Belgium, 2006, and Hamburg, Germany, 2011. Cerebrovasc Dis. 2012;34(4):290–296. doi:10.1159/000343145
20. Chambless LE, Folsom AR, Clegg LX, et al. Carotid wall thickness is predictive of incident clinical stroke: the Atherosclerosis Risk in Communities (ARIC) study. Am J Epidemiol. 2000;151(5):478–487. doi:10.1093/oxfordjournals.aje.a010233
21. Rimmele DL, Borof K, Jensen M, et al. Association between carotid atherosclerosis and atrial fibrillation, cardiac, and renal function. Eur J Vasc Endovasc Surg. 2022;63(4):641–647. doi:10.1016/j.ejvs.2022.01.010
22. Boulos NM, Gardin JM, Malik S, Postley J, Wong ND. Carotid plaque characterization, stenosis, and intima-media thickness according to age and gender in a large registry cohort. Am J Cardiol. 2016;117(7):1185–1191. doi:10.1016/j.amjcard.2015.12.062
23. Michels WM, Grootendorst DC, Verduijn M, Elliott EG, Dekker FW, Krediet RT. Performance of the Cockcroft-Gault, MDRD, and new CKD-EPI formulas in relation to GFR, age, and body size. Clin J Am Soc Nephrol. 2010;5(6):1003–1009. doi:10.2215/CJN.06870909
24. Bytyçi I, Shenouda R, Wester P, Henein MY. Carotid atherosclerosis in predicting coronary artery disease: a systematic review and meta-analysis. Arterioscler Thromb Vasc Biol. 2021;41(4):e224–e237. doi:10.1161/ATVBAHA.120.315747
25. Gupta A, Baradaran H, Schweitzer AD, et al. Carotid plaque MRI and stroke risk: a systematic review and meta-analysis. Stroke. 2013;44(11):3071–3077. doi:10.1161/STROKEAHA.113.002551
26. McCully KS. Homocysteine metabolism, atherosclerosis, and diseases of aging. Compr Physiol. 2015;6(1):471–505.
27. Adachi H, Hirai Y, Fujiura Y, Matsuoka H, Satoh A, Imaizumi T. Plasma homocysteine levels and atherosclerosis in Japan: epidemiological study by use of carotid ultrasonography. Stroke. 2002;33(9):2177–2181. doi:10.1161/01.STR.0000026861.18199.89
28. McQuillan BM, Beilby JP, Nidorf M, Thompson PL, Hung J. Hyperhomocysteinemia but not the C677T mutation of methylenetetrahydrofolate reductase is an independent risk determinant of carotid wall thickening. The Perth Carotid Ultrasound Disease Assessment Study (CUDAS). Circulation. 1999;99(18):2383–2388. doi:10.1161/01.CIR.99.18.2383
29. Kim SJ, Song P, Park JH, et al. Biomarkers of asymptomatic carotid stenosis in patients undergoing coronary artery bypass grafting. Stroke. 2011;42(3):734–739. doi:10.1161/STROKEAHA.110.595546
30. Kim JM, Park KY, Shin DW, Park MS, Kwon OS. Relation of serum homocysteine levels to cerebral artery calcification and atherosclerosis. Atherosclerosis. 2016;254:200–204. doi:10.1016/j.atherosclerosis.2016.10.023
31. Alsulaimani S, Gardener H, Elkind MS, Cheung K, Sacco RL, Rundek T. Elevated homocysteine and carotid plaque area and densitometry in the Northern Manhattan Study. Stroke. 2013;44(2):457–461. doi:10.1161/STROKEAHA.112.676155
32. Zhang T, Jiang Y, Zhang S, et al. The association between homocysteine and ischemic stroke subtypes in Chinese: a meta-analysis. Medicine (Baltimore). 2020;99(12):e19467. doi:10.1097/MD.0000000000019467
33. Pushpakumar S, Kundu S, Sen U. Endothelial dysfunction: the link between homocysteine and hydrogen sulfide. Curr Med Chem. 2014;21(32):3662–3672. doi:10.2174/0929867321666140706142335
34. Liu C, Sun X, Lin H, et al. Association between hyperhomocysteinemia and metabolic syndrome with early carotid artery atherosclerosis: a cross-sectional study in middle-aged Chinese population. Nutrition. 2018;53:115–119. doi:10.1016/j.nut.2018.02.014
35. Li Y, Wang L, Zhang W, Fang Y, Niu X. No association between elevated homocysteine levels and carotid atherosclerosis in a rural population in China. Stroke Vasc Neurol. 2016;1(4):154–160. doi:10.1136/svn-2016-000037
36. Tsai MY, Arnett DK, Eckfeldt JH, Williams RR, Ellison RC. Plasma homocysteine and its association with carotid intimal-medial wall thickness and prevalent coronary heart disease: NHLBI Family Heart Study. Atherosclerosis. 2000;151(2):519–524. doi:10.1016/S0021-9150(99)00409-8
37. Spence JD, Azarpazhooh MR, Larsson SC, Bogiatzi C, Hankey GJ. Stroke prevention in older adults: recent advances. Stroke. 2020;51(12):3770–3777. doi:10.1161/STROKEAHA.120.031707
38. Koklesova L, Mazurakova A, Samec M, et al. Homocysteine metabolism as the target for predictive medical approach, disease prevention, prognosis, and treatments tailored to the person. EPMA J. 2021;12(4):477–505. doi:10.1007/s13167-021-00263-0
39. Juonala M, Viikari JS, Kähönen M, et al. Childhood levels of serum apolipoproteins B and A-I predict carotid intima-media thickness and brachial endothelial function in adulthood: the cardiovascular risk in young Finns study. J Am Coll Cardiol. 2008;52(4):293–299. doi:10.1016/j.jacc.2008.03.054
40. Raitakari OT, Mäkinen VP, McQueen MJ, et al. Computationally estimated apolipoproteins B and A1 in predicting cardiovascular risk. Atherosclerosis. 2013;226(1):245–251. doi:10.1016/j.atherosclerosis.2012.10.049
41. Morita SY. Metabolism and modification of apolipoprotein B-containing lipoproteins involved in dyslipidemia and atherosclerosis. Biol Pharm Bull. 2016;39(1):1–24. doi:10.1248/bpb.b15-00716
42. Sniderman AD, Williams K, Contois JH, et al. A meta-analysis of low-density lipoprotein cholesterol, non-high-density lipoprotein cholesterol, and apolipoprotein B as markers of cardiovascular risk. Circ Cardiovasc Qual Outcomes. 2011;4(3):337–345. doi:10.1161/CIRCOUTCOMES.110.959247
43. Steffen BT, Guan W, Remaley AT, et al. Apolipoprotein B is associated with carotid atherosclerosis progression independent of individual cholesterol measures in a 9-year prospective study of multi-ethnic study of atherosclerosis participants. J Clin Lipidol. 2017;11(5):1181–1191.e1. doi:10.1016/j.jacl.2017.07.001
44. Razavi AC, Bazzano LA, He J, et al. Discordantly normal ApoB relative to elevated LDL-C in persons with metabolic disorders: a marker of atherogenic heterogeneity. Am J Prev Cardiol. 2021;7:100190. doi:10.1016/j.ajpc.2021.100190
45. Tzoulaki I, Castagné R, Boulangé CL, et al. Serum metabolic signatures of coronary and carotid atherosclerosis and subsequent cardiovascular disease. Eur Heart J. 2019;40(34):2883–2896. doi:10.1093/eurheartj/ehz235
46. Chapman CM, Beilby JP, McQuillan BM, Thompson PL, Hung J. Monocyte count, but not C-reactive protein or interleukin-6, is an independent risk marker for subclinical carotid atherosclerosis. Stroke. 2004;35(7):1619–1624. doi:10.1161/01.STR.0000130857.19423.ad
47. Duivenvoorden R, Mani V, Woodward M, et al. Relationship of serum inflammatory biomarkers with plaque inflammation assessed by FDG PET/CT: the dal-PLAQUE study. JACC Cardiovasc Imaging. 2013;6(10):1087–1094. doi:10.1016/j.jcmg.2013.03.009
48. Rudd JH, Myers KS, Bansilal S, et al. Relationships among regional arterial inflammation, calcification, risk factors, and biomarkers: a prospective fluorodeoxyglucose positron-emission tomography/computed tomography imaging study. Circ Cardiovasc Imaging. 2009;2(2):107–115. doi:10.1161/CIRCIMAGING.108.811752
49. Wang A, Huang X, Liu X, et al. No association between high-sensitivity C-reactive protein and carotid intima-media progression: the APAC study. J Stroke Cerebrovasc Dis. 2017;26(2):252–259. doi:10.1016/j.jstrokecerebrovasdis.2016.09.013
50. Shah T, Casas JP, Cooper JA, et al. Critical appraisal of CRP measurement for the prediction of coronary heart disease events: new data and systematic review of 31 prospective cohorts. Int J Epidemiol. 2009;38(1):217–231. doi:10.1093/ije/dyn217
51. Li D, Qiao H, Yang X, et al. Co-existing hypertension and hyperhomocysteinemia increases the risk of carotid vulnerable plaque and subsequent vascular event: an MR vessel wall imaging study. Front Cardiovasc Med. 2022;9:858066. doi:10.3389/fcvm.2022.858066
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
