Back to Journals » Journal of Blood Medicine » Volume 9
HIV infection has a profound effect on hematological factors but not on electrolyte profile of Malawian adults presenting with uncomplicated malaria and severe malaria
Authors Munyenyembe AU , Gausi K , Nyirenda TS, Hiestand J, Mallewa J, Mandala WL
Received 2 May 2018
Accepted for publication 6 July 2018
Published 4 October 2018 Volume 2018:9 Pages 153—162
DOI https://doi.org/10.2147/JBM.S172869
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Martin Bluth
Alinane U Munyenyembe,1 Kamunkhwala Gausi,1 Tonney S Nyirenda,2 Jasmin Hiestand,3 Jane Mallewa,3 Wilson L Mandala1,4
1Malawi-Liverpool Wellcome Trust, Malaria Immunology Department, Blantyre, Malawi; 2Pathology Department, College of Medicine, Blantyre, Malawi; 3Medicine Department, College of Medicine, Blantyre, Malawi; 4Academy of Medical Sciences, Biological Sciences Department, Malawi University of Science & Technology, Thyolo, Malawi
Aim: Although malaria and HIV infections independently affect the electrolyte and hematologic profiles, little is known of how these profiles are affected in individuals coinfected with malaria and HIV. We therefore conducted this study to investigate the electrolyte and hematologic profiles of Malawian adults presenting with either uncomplicated malaria (UM), severe malaria (SM), and those presenting with HIV and UM or HIV and SM.
Methods: Study participants were recruited at Queen Elizabeth Central Hospital, and malaria infection was confirmed by rapid diagnostic test and malaria slides, and full blood count, HIV, and wet chemistries were analyzed.
Results: Sodium, potassium, calcium, and chloride levels of all 4 study groups were similar to those of healthy controls. Both HIV-infected groups (UM and SM) had lower red blood cell counts and lower hemoglobin concentration than the reference range. Platelet counts were lower in both HIV-uninfected SM cases (64×109/L) and in the HIV-infected SM cases (66×109/L) compared to the reference range (115–290×109/L). HIV– UM cases had higher proportion and absolute counts of neutrophils and white blood cell counts compared to the HIV+ UM cases.
Conclusion: HIV infection did not affect the electrolyte profile of Malawian adults presenting with UM or SM but had an effect on red blood cells, Hb concentration, neutrophils, and platelet counts.
Keywords: Plasmodium falciparum malaria, HIV infection, electrolytes, hematologic values, HIV-malaria co-infection, Malawian Adults
Introduction
Annually, over a billion people are at risk of contracting malaria infection worldwide, and as many as 212 million clinical episodes of malaria were reported in 2016 alone resulting in 429,000 deaths, the majority of which were children from Sub-Saharan Africa (SSA) presenting with Plasmodium falciparum malaria.1 Clinical P. falciparum malaria presents either as uncomplicated malaria (UM) or as a severe form of the disease, with cerebral malaria (CM), severe malarial anemia (SMA), metabolic acidosis, or respiratory distress, or other complications including overlap syndromes.1,2 Of these severe forms of malaria, CM, respiratory distress, and SMA are associated with high mortality, with as many as 1 in 7 children dying from CM or SMA inSSA.1,3
Immunity to malaria disease, but not to infection, is both humoral and cell-mediated with various mechanisms involved.4 Antibodies that develop through exposure to P. falciparum play a role,4 and the involvement of different cell subsets has been implicated in both protection against and pathogenesis of malaria.5,6 Malaria-specific protective immunity develops with age and exposure.7 Thus, adults under continuous exposure to P. falciparum antigens should normally have an effective immunity against malaria disease.8 This being the case, much attention is given to investigating interventions aimed at preventing and treating malaria in children.9,10
Levels of electrolyte, renal and liver analytes, and enzymes are critical for management of patients with severe illness.11 Like in the case of severe bacterial sepsis, severe malaria (SM) is also associated with pathophysiological imbalances that affect a number of organs in African children.12 For instance, complicated cases with renal disease associated with malaria may lead to renal failure characterized by proteinuria, rise in blood urea, hyperkalemia, and metabolic acidosis.12 Renal function disturbances, especially increases in blood urea and creatinine, among malaria-infected patients worldwide occur in <5% of cases, but in these cases the mortality rate can be as high as 15%–40%.13
Although the effect of HIV infection on malaria and on some immune cells, especially CD4+ T cells, has been extensively studied in adult Africans, perturbations in levels of electrolyte and renal and liver function analytes among African adults with or without HIV presenting with acute P. falciparum malaria remain poorly characterized. We prospectively recruited Malawian adults who were either HIV infected or HIV uninfected and presenting with UM or SM and analyzed a venous blood sample to determine levels of serum electrolytes and renal and liver function analytes to describe organ-specific functionality. The findings of our study show that HIV infection partly affects the proportion of neutrophils (Neutros) and platelets among Malawian adults presenting with either UM or SM but does not affect the electrolyte profile as much.
Methods
Study site and participants
This study was conducted in the Accidents, Emergency and Trauma Center and general medical male and female wards of Queen Elizabeth Central Hospital from July 2016 to March 2017. Queen Elizabeth Central Hospital is the main referral hospital in the southern part of Malawi, located in Blantyre, the business capital of Malawi. Of the 116 malaria-infected adults who were screened, 107 gave consent and participated in the study.
Study design
This was a prospective cross-sectional study which recruited Malawian adults diagnosed with UM or SM after obtaining consent from the patient or a legally able guardian. Case report forms were used to collect demographic information, history of malaria symptoms, HIV status, and history of travel. A 10 mL venous blood sample was collected from each participant for confirmation of malaria parasitemia using thick and thin films, for analysis of full blood count, liver function tests, renal function tests, and electrolytes, and to assess for end organ function; the same was also used for standard blood cultures.
Inclusion and exclusion criteria
Malaria was defined as a clinical syndrome without an apparent alternative cause, in the presence of a positive malaria rapid diagnostic test (MRDT) and thick blood film positive for P. falciparum asexual parasites on microscopy. The malaria cases were divided into 2 groups: those who had UM and those who had SM. The UM cases were those who had confirmed clinical malaria confirmed with positive malaria slides and MRDT but were not in an unarousable coma, or they did not have low hemoglobin (Hb) levels or liver or kidney complications associated with the malaria infection. Among the SM cases were those who had CM and SMA, and those participants who had liver or renal dysfunction were also included in the SM group. Based on the World Health Organization guidelines, study participants with CM had a Glasgow Coma Scale score of <11, with unarousable coma not attributable to any other cause, who were unable to localize stimuli and were incomprehensible to sounds, while those presenting with UM or other forms of SM had a Glasgow Coma Scale score of greater than 11 at both times.
Study participants presenting with SMA had a blood hemoglobin concentration of 7 g/dL or less or a hematocrit concentration of 20% or less together with a P. falciparum parasite count of more than 10,000/μL, while the rest had a hemoglobin concentration above this level. Study participants were checked clinically and neurologically monitored by regularly checking of vital signs during the time they were still in the admission wards until the day of discharge or death. All outcomes such as deaths and neurological sequelae for CM cases for the respective admissions were documented.
All study participants were adults (≥16 years) who were either out- or inpatients at Queen Elizabeth Central Hospital presenting with a temperature >37.5°C, a positive MRDT with a positive thick and thin blood smear microscopy test, and with signs consistent with malaria disease according to the World Health Organization guidelines.13 Women who declared that they were pregnant during recruitment stage and all potential participants who did not give consent were excluded from the study.
Investigations
HIV testing was performed using 2 rapid test kits: Determine (Abbott Laboratories, Tokyo, Japan) and Unigold (Trinity Brotch, Dublin, Ireland). Thick and thin blood smears for malaria microscopy investigation were prepared by standard methodology. White blood cell (WBC) counts and their respective differentials, Hb, hematocrit, mean corpuscular volume, and platelet counts were determined using a HMX Hematological Analyzer (Beckman Coulter, Brea, CA, USA) using the sample collected in the EDTA tube. All biochemistry samples were collected in plain tubes without any anticoagulant, and an AU480 Analyzer was used to process the biochemistry parameters such as creatinine and urea levels.
Data analysis
For statistical analysis, the participants were divided into 4 groups: HIV-uninfected with UM, HIV-uninfected with SM, HIV-infected with UM, and HIV-infected with SM. Kruskal–Wallis test was used to assess if there were statistically significant differences in the medians of hematological and electrolyte values among the 4 groups. Considering that comparisons for 4 groups were made, between-group comparisons of medians of various parameters for the 4 groups were assessed with Dunn’s multiple comparison test and P-value of <0.0125 was considered statistically significant. Both Kruskal–Wallis Test and Dunn’s test were performed in R-version 3.3.1 (The R Foundation, Vienna, Austria) using the Dunn test package. GraphPad Prism (GraphPad Software, La Jolla, CA, USA) was used for developing the figures. Medians were used instead of means since some of the variables were observed not to be normally distributed.
Ethical approval
The study was approved by the College of Medicine Research and Ethics Committee, University of Malawi. Each participant or an appropriate guardian provided informed written consent.
Results
Study participants’ demographic data
Overall, 107 participants were recruited who presented with UM and SM, of whom 57 (53%) were female. Of the participants, 30 (28%) were HIV infected (18 UM and 12 SM), 76 (71%) were HIV uninfected (58 UM and 18 SM), and the HIV status of 1 participant was unknown. The HIV-uninfected population was much younger (mean age of 25 years) compared to the HIV-infected population (mean age of 35.5 years). All 30 HIV-infected participants already knew their HIV status at the time of recruitment and were on antiretroviral treatment.
Comparison of different factors for the 4 groups against local reference ranges
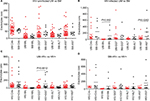
The median sodium levels of all 4 groups were within the reference range representing healthy Malawian adults (Table 1). HIV-uninfected UM and HIV-infected SM had slightly higher (4.5 mmol/L) median levels of potassium than the highest limit of the range of healthy adults (4.3 mmol/L). All malaria cases (UM and SM), whether HIV infected or not, had higher median calcium levels (ranging from 211 mmol/L in HIV-infected UM to 221 mmol/L in HIV-uninfected UM) than the levels in the reference ranges (105–116 mmol/L). HIV-infected SM was the only group which had a median chloride level (100 mEq/L) that was lower than the reference range (102–109 mEq/L).
The creatinine, bilirubin (BIL), and gamma glutamyl transferase (GGT) median levels for all 4 groups were within the reference ranges for healthy adults. HIV-infected SM had alanine transaminase (35 UL) and AST (105 IUL) levels that were higher than the highest limit in the reference range (8–32 UL and 13–37 IUL, respectively). HIV-infected UM only had a higher AST (45 IUL) level that was higher than the reference range.
Both HIV-infected groups (3.32×1012/L for UM and 3.22×1012/L for SM) had lower median red blood cell (RBC) count (RCC) compared to the reference range (4.32–5.66×1012/L) and also had lower hemoglobin concentration (9.15 g/dL for UM and 7.8 g/dL for SM) compared to the reference range (13.6–16.7 g/dL). Median platelet counts were lower in both HIV-uninfected SM cases (64×109/L) and in the HIV-infected SM cases (66×109/L) than the reference range (115–290×109/L). The proportion and absolute counts of Neutros, Lymphs, monocytes, basophils, and eosinophils for all 4 groups were within the reference ranges.
Among the HIV-infected, SM had higher levels of BIL and AST but lower sodium levels than UM
When the study participants were stratified based on HIV infection, among those who were HIV uninfected, there was no difference in the median levels of any of the parameters analyzed between the UM and SM cases (Figures 1A and 2A). However among the HIV-infected participants, those who presented with SM had significantly (P=0.001 for BIL and 0.003 for AST) higher median BIL levels (8 μmol/L) and AST (105 IU/L) compared to the levels observed in the UM cases (2 μmol/L for BIL and 45 IUL for AST) (Figure 1B).
Among the UM, HIV-infected individuals had higher GGT and urea levels but lower calcium and RCC levels compared to the HIV-uninfected.
Once the study participants were stratified based on malaria severity, among those who presented with UM, HIV-uninfected individuals had significantly (P=0.004) higher median calcium levels (221 mmol/L) compared to the HIV-infected individuals (211 mmol/L) and significantly (P=0.007) higher RCC (4.73×1012/L) compared to HIV-infected (3.32×1012/L) (Figure 2C). In turn, HIV-infected individuals had significantly (P=0.0006) higher GGT levels (47 U/L) compared to the levels in HIV-uninfected individuals (24 U/L) (Figure 1C). Among those with SM, HIV-infected individuals had similar levels of all measured parameters as the HIV-uninfected study participants (Figures 1D and 2D).
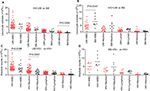
Among the HIV-infected participants, SM cases had higher WBC counts than UM
When the study participants were stratified by HIV status, among the HIV uninfected, UM and SM cases had similar proportions (Figure 3A) and absolute counts (Figure 4A) of various leukocyte subsets. Among the HIV infected, UM had similar percentages of various leukocyte subsets as those observed in SM (Figure 3B). However, SM cases had significantly (P=0.01) higher median WBC counts (6.2×109 cells/L) than UM cases (4.3×109 cells/L) (Figure 4B).
Among the UM cases, HIV-infected patients had higher proportion and absolute counts of Neutros than HIV-uninfected ones
When the participants were stratified by malaria severity, among the UM cases, those who were HIV uninfected had significantly (P=0.0037 for percentage and 0.0067 for absolute counts) higher median proportion of Neutros (60% compared to 49%) and absolute Neutro counts compared to those who were HIV infected (5.2×109 cells/L compared to 2.6×109 cells/L) (Figures 3C and 4C). HIV-uninfected UM cases also had significantly (P=0.01) higher WBC counts (5.6×109 cells/L) compared to HIV-infected UM cases (4.3×109 cells/L) (Figure 4C). Among the SM cases, there were no differences in leukocyte subsets between the HIV-infected and HIV-uninfected participants (Figures 3D and 4D).
Discussion
Although malaria is prevalent in almost all countries in the tropics, close to 90% of annual malaria cases occur in SSA,14 a region which is also known to have high prevalence of HIV infection.15 Either separately or in the case of coinfection, malaria and HIV infection continue to substantially contribute to the high morbidity and mortality in SSA.16 The geographical overlap observed between malaria and HIV infections has raised a number of questions on how one infection influences the other in terms of transmission, severity, and the hosts’ susceptibility to the other infection. In addition, studies have looked at the effect of coinfection on the host’s immunity and response to malaria treatment in the case of HIV infection.17 We have recently shown that HIV infection compounds the effect of acute CM infection on Malawian children’s cell-mediated immunity (unpublished data).
Electrolytes and minerals (micro and macro), which are present both in the circulating blood of an individual as well as in other body fluids, serve physiological roles in the body and should be available within specific optimal ranges.18 Any imbalance or perturbation caused by either infection or overconsumption can easily affect the function of each mineral and can lead to death if not corrected.18
Sodium (Na) is a significant cation in the extracellular body fluids where it regulates the normal distribution of water and osmotic pressure in body fluids.12 Potassium (K) is involved in skeletal and smooth muscle contraction, whereas as calcium (Ca), in addition to being an important mineral in bone and teeth formation, also has a role to play in muscle contraction.12
Malaria, both in children and adults, has been shown to affect the electrolyte levels of the affected individuals, with the effect more pronounced in cases with severe disease.19,20 Several studies that have investigated the effect of malaria on electrolyte profiles have reported that the disease is characterized by hyponatremia (a significant decrease in sodium levels), hypokalemia (lower potassium levels than normal), hypomagnesium (lower magnesium levels than normal), and lower levels of calcium than in healthy controls.19–23 Although hyponatremia is related to the Syndrome of Inappropriate antidiuretic hormone and hypervolemia that are related to heart failure,24 the hyponatremia, hypokalemia, and hypomagnesium observed in malaria cases could be due to the ensuing hypovomia normally associated with SM during acute phase, which then improves once the patient is provided with fluids intravenously.19
The results of our study are inconsistent with those made in other studies which found malaria to be characterized by lower than normal levels of sodium, potassium, magnesium, and calcium, even in the case of SM.19,20,22 This difference could be due to the fact that in our study we recruited adults (older than 16 years), whereas the other studies mainly recruited children under the age of 9 years. It is also worth noting that hyperkalemia has previously been associated with renal failure,25 and the renal disturbance in some of the participants in our study could partly explain this difference in results.
On the other hand, HIV infection on its own has also been shown to be characterized by hyponatremia, hypokalemia (but not hyperkalemia)26 and higher than normal levels of urea and creatinine with the effects being more pronounced in those who were ART naïve.27 The results of our study are consistent with the observation of hyperkalemia in HIV-infected individuals, but in our study this was only the case in those coinfected with HIV and SM. A better comparator could have been a cohort of HIV-infected nonmalaria group.
There is paucity in studies that have looked at the electrolyte profiles in either adults or children coinfected with HIV and malaria. The few studies reported in this area either looked at the effect of coinfection on hematological factors such as WBCs, platelet counts, and hemoglobin28 or investigated some hematostatic parameters such as prothrombin and thrombin.29 To our knowledge, this is the first study that has investigated the effect of HIV and malaria coinfection on a wide range of electrolytes, liver enzyme test parameters, and some hematological parameters in adults presenting with either UM or SM.
Considering that we and others have previously shown that SM, especially CM, is characterized by lymphopenia and higher than normal levels of Neutros,30,31 it was surprising to note that even in either UM or SM alone in the absence of HIV infection, or even in those coinfected individuals, the levels of all cells did not differ from those of the controls reference ranges. Again, this could have been the case because this study, unlike the previous studies, recruited adults. The association of P. falciparum malaria with reduced RCC and Hb concentration is expected since the parasites develop and replicate in RBCs and the fully developed merozoites cause anemia when the infected RBCs go through hemolysis.9 The higher WBC counts observed in SM compared to those in UM have been linked to the higher Neutro counts that are known to characterize SM.26
The study had several limitations, one of which was the low sample sizes of the HIV-infected participants who also had SM. Although comparisons were made with already established reference ranges for various parameters, ideally the study should have recruited age-matched healthy controls whose results could have been a more realistic basis for comparison. Another limitation of the study was the lack of a fifth group, an age-matched, HIV-uninfected, malaria-uninfected healthy control population who could have served as an ideal comparator group. Although we had access to the normal reference ranges for the different parameters, it would have been ideal to have baseline values for the different parameters from healthy controls recruited at the same time. Lastly, although all HIV-infected participants were already on ART, they were not stratified based on the duration of the infection, nor were they classified based on CD4+ T-cell counts or the duration that they had been on ART. All 3 factors could have possible confounding effects on the various electrolyte and hematological factors we measured,26,32 and as such it is possible that the differences for some parameters observed between the HIV-infected and the HIV-uninfected groups could be due to other comorbidities.
Conclusion
We have shown that SM has a more remarkable effect on platelet counts and that HIV infection affects the RCC and hemoglobin concentration values more than the other hematologic values. Although the study has also shown that malaria, severe or uncomplicated, in the presence or absence of HIV infection, did not affect any of the tested electrolytes, an inclusion of an age-matched healthy control group could have been useful in drawing more appropriate conclusions from such findings.
Data sharing statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author contributions
WLM, JM, and JH conceived the study. JH, JM, and AM oversaw clinical aspects of the study. WLM, AM, and JH performed the investigations. AM and KG analyzed the data. WLM, AM, TSN, JH, JM, and KG wrote the report and all authors reviewed the report. All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Acknowledgments
We are grateful to the 2 research nurses, Norah Ndalapa and Lucia Mbulaje, for their assistance in the recruitment of the study participants. We also thank all the study participants without whom this study could not have been possible. This study was funded by the Malawi-Liverpool Wellcome Trust (MLW) Intern fellowship to Alinane Munyenyembe.
Disclosure
The authors report no conflicts of interest in this work.
References
World Health Organization. World Malaria Report 2016. Geneva: World Health Organization; 2016. Available from: http://www.who.int/malaria/publications/world-malaria-report-2016/report/en/. Accessed April 28, 2018. | ||
Marsh K, Forster D, Waruiru C, et al. Indicators of life-threatening malaria in African children. N Engl J Med. 1995;332(21):1399–1404. | ||
World Health Organization. Levels Trends in Child Mortality: Report 2014. Geneva: World Health Organization; 2014. Available from: http://www.who.int/maternal_child_adolescent/documents/levels_trends_child_mortality_2014/en/. Accessed April 28, 2018. | ||
Langhorne J, Ndungu FM, Sponaas AM, Marsh K. Immunity to malaria: more questions than answers. Nat Immunol. 2008;9(7):725–732. | ||
Riley EM. Is T-cell priming required for initiation of pathology in malaria infections? Immunol Today. 1999;20(5):228–233. | ||
Schofield L, Grau GE. Immunological processes in malaria pathogenesis. Nat Rev Immunol. 2005;5(9):722–735. | ||
Carneiro I, Roca-Feltrer A, Griffin JT, et al. Age-patterns of malaria vary with severity, transmission intensity and seasonality in sub-Saharan Africa: a systematic review and pooled analysis. PLoS One. 2010;5(2):e8988. | ||
Miller MJ. Observations on the natural history of malaria in the semi-resistant West African. Trans R Soc Trop Med Hyg. 1958;52(2):152–168. | ||
Jenkins R, Omollo R, Ongecha M, et al. Prevalence of malaria parasites in adults and its determinants in malaria endemic area of Kisumu County, Kenya. Malar J. 2015;14:263. | ||
Mayor A, Aponte JJ, Fogg C, et al. The epidemiology of malaria in adults in a rural area of southern Mozambique. Malar J. 2007;6:3. | ||
Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348(2):138–150. | ||
Maitland K, Pamba A, Fegan G, et al. Perturbations in electrolyte levels in Kenyan children with severe malaria complicated by acidosis. Clin Infect Dis. 2005;40(1):9–16. | ||
Marshall WJ, Bangert SK, Lapsley M. Clinical Chemistry. Edinburgh: Mosby Elsevier; 2012. | ||
Snow RW, Guerra CA, Noor AM, Myint HY, Hay SI. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature. 2005;434(7030):214–217. | ||
World Health Organization. Joint United Nations Programme on HIV/AIDS (UNAIDS)/World Health Organization. Global AIDS Update. Geneva: World Health Organization; 2016. Available from: www.unaids.org/sites/default/files/media_asset/global-AIDS-update-2016_en.pdf. Accessed April 28, 2018. | ||
Hochman SE, Madaline TF, Wassmer SC, et al. Fatal pediatric cerebral malaria is associated with intravascular monocytes and platelets that are increased with HIV coinfection. MBio. 2015;6(5):e01390-15. | ||
Hewitt K, Steketee R, Mwapasa V, Whitworth J, French N. Interactions between HIV and malaria in non-pregnant adults: evidence and implications. AIDS. 2006;20(16):1993–2004. | ||
Perazella MA, Brown E. Electrolyte and acid-base disorders associated with AIDS: an etiologic review. J Gen Intern Med. 1994;9(4):232–236. | ||
Rani A, Akhtar S, Nawaz SK, Irfan S, Azam S, Arshad M. Electrolyte disturbance and the type of malarial infection. Iran J Public Health. 2015;44(11):1492–1497. | ||
Jasani JH, Sancheti SM, Gheewala BS, et al. Association of the electrolyte disturbances (Na+, K+) with type and severity of malarial parasitic infection. J Clin Diagn Res. 2012;6(4):678–681. | ||
English MC, Waruiru C, Lightowler C, Murphy SA, Kirigha G, Marsh K. Hyponatraemia and dehydration in severe malaria. Arch Dis Child. 1996;74(3):201–205. | ||
Hanson J, Hossain A, Charunwatthana P, et al. Hyponatremia in severe malaria: evidence for an appropriate anti-diuretic hormone response to hypovolemia. Am J Trop Med Hyg. 2009;80(1):141–145. | ||
van Wolfswinkel ME, Hesselink DA, Zietse R, Hoorn EJ, van Genderen PJ. Hyponatraemia in imported malaria is common and associated with disease severity. Malar J. 2010;9:140. | ||
Koza Y, Taş MH, Şimşek Z, Gündoğdu F. Hyponatremia and heart failure: the overlooked piece of the puzzle. Anatol J Cardiol. 2015;15(12):1033–1038. | ||
Lehnhardt A, Kemper MJ. Pathogenesis, diagnosis and management of hyperkalemia. Pediatr Nephrol. 2011;26(3):377–384. | ||
Musso CG, Belloso WH, Glassock RJ. Water, electrolytes, and acid-base alterations in human immunodeficiency virus infected patients. World J Nephrol. 2016;5(1):33–42. | ||
Ramesh N, Premkumar VT. To study the renal and electrolyte disturbances in HIV infected patients. IOSR J Dent Med Sci. 2017;16(8):91–94. | ||
Jegede FE, Oyeyi TI, Abdulrahman SA, et al. Effect of HIV and malaria parasites co-infection on immune-hematological profiles among patients attending anti-retroviral treatment (ART) clinic in Infectious Disease Hospital Kano, Nigeria. PLoS One. 2017;12(3):e0174233. | ||
Chukwuanukwu RC, Ukaejiofo EO, Ele PU, Onyenekwe CC, Chukwuanukwu TO, Ifeanyichukwu MO. Evaluation of some haemostatic parameters in falciparum malaria and HIV co-infection. Br J Biomed Sci. 2016;73(4):168–173. | ||
Mandala WL, Msefula CL, Gondwe EN, Drayson MT, Molyneux ME, Maclennan CA. Cytokine profiles in Malawian children presenting with uncomplicated malaria, severe malarial anemia, and cerebral malaria. Clin Vaccine Immunol. 2017;24(4):e00533-16. | ||
Hviid L, Kurtzhals JA, Goka BQ, Oliver-Commey JO, Nkrumah FK, Theander TG. Rapid reemergence of T cells into peripheral circulation following treatment of severe and uncomplicated Plasmodium falciparum malaria. Infect Immun. 1997;65(10):4090–4093. | ||
Manzar MD, Sony P, Salahuddin M, et al. Electrolyte imbalance and sleep problems during anti-retroviral therapy: an under-recognized problem. Sleep Sci. 2017;10(2):64–67. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.