Back to Journals » Journal of Asthma and Allergy » Volume 9
Historical cohort study examining comparative effectiveness of albuterol inhalers with and without integrated dose counter for patients with asthma or chronic obstructive pulmonary disease
Authors Price DB , Rigazio A, Small MB, Ferro TJ
Received 22 April 2016
Accepted for publication 6 July 2016
Published 26 August 2016 Volume 2016:9 Pages 145—154
DOI https://doi.org/10.2147/JAA.S111170
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Luis Garcia-Marcos
David B Price,1,2 Anna Rigazio,2 Mary Buatti Small,3 Thomas J Ferro,3
1Academic Primary Care, Institute of Applied Health Sciences, University of Aberdeen, Aberdeen, UK; 2Research in Real-Life Ltd, Cambridge, UK; 3Teva Pharmaceuticals, Frazer, PA, USA
Background: Using a metered-dose inhaler (MDI) beyond the labeled number of actuations may result in inadequate dosing of medication, which can lead to poor clinical outcomes. This study compared respiratory-related emergency department (ED) visit rates in patients with asthma, chronic obstructive pulmonary disease, or both when they used albuterol MDIs with versus without dose counters.
Methods: This retrospective study used US claims data to identify patients (ages 4–64 years) with asthma, chronic obstructive pulmonary disease, or both, using albuterol MDIs with or without an integrated dose counter. The study comprised a 1-year baseline period for patient characterization and confounder definition and a 1-year outcome period following the first albuterol prescription. The primary end point was the incidence rate of respiratory-related ED visits, compared using a reduced zero-inflated Poisson regression model. We also compared severe exacerbation rates and rescue medication use.
Results: A total of 93,980 patients were studied, including 67,251 (72%) in the dose counter cohort and 26,729 (28%) in the non-dose-counter cohort. The cohorts were broadly similar at baseline (55,069 [59%] female patients; median age, 37 years). The incidence rate of respiratory-related ED visits during the outcome year was 45% lower in the dose counter cohort than in the non-dose-counter cohort (adjusted rate ratio: 0.55; 95% confidence interval: 0.47–0.64). Exacerbation rates and short-acting β-agonist use were similar between cohorts.
Conclusion: These findings suggest that dose counter integration into albuterol MDIs is associated with decreased ED visit rates. The presence of integrated dose counters on rescue inhalers can help patients avoid using an empty or near-empty inhaler during exacerbations, thereby ensuring available medication for relief of their symptoms. Integrated dose counters on rescue MDIs could represent a simple and effective tool to improve clinical outcomes during exacerbations, with a potential for cost savings to health care systems.
Keywords: albuterol, asthma, chronic obstructive pulmonary disease, dose counter, inhaler, effectiveness
Introduction
Albuterol delivered by conventional “press-and-breathe” metered-dose inhalers (MDIs) is the most common symptomatic and rescue therapy for managing reversible bronchospasm in asthma and chronic obstructive pulmonary disease (COPD). Although dose counters can reliably monitor inhaler use,1–5 they are not currently integrated into some rescue MDIs, making it difficult for patients to know when those inhalers are empty. In one study, only 8% of patients reported counting the number of actuations and replacing their inhalers at or before the manufacturer’s specified maximum number of actuations had been reached.6 Beyond the labeled number of MDI actuations, the amount of active medication available per actuation can vary, resulting in a lower than therapeutic dose.2,7 Surveys of patients with asthma who use an MDI indicate that >50% of patients do not know the number of therapeutic doses remaining in their inhalers.6,7 Furthermore, a recent review concluded that up to 40% of patients actually using an empty or nearly empty MDI believe they are taking their asthma medication as prescribed.8
In a recent survey of 224 pediatric and adult patients with asthma or COPD, 62% of patients reported feeling anxious about not knowing the quantity of medication remaining in their inhalers.2 Of the patients surveyed, 72% reported shaking their inhalers to assess the quantity of remaining medication, and almost one-half (42%) waited until they thought their MDI was no longer working before replacing it. The addition of an integrated dose counter to MDIs relieved anxiety about running out of medication for two-thirds of 272 adolescent and adult patients with asthma or COPD in a subsequent study.9 In a cohort study of 1,095 adult patients who utilized the emergency department (ED) for asthma, 324 (30%) ran out of their inhaled short-acting β-agonist (SABA) or corticosteroid medication during the week before their index ED visit.10
Asthma prevalence remains high, affecting almost 26 million Americans in 2010,11 with approximately two million asthma ED visits made each year.11–13 In addition, an estimated 13.7 million adults in the US reported having a COPD diagnosis in 2011,14 and ED visits for COPD exacerbations numbered 1.8 million in 2011, an increase from 1.5 million in 2006.15
This retrospective database analysis using claims data was designed to investigate the impact of integrated dose counter albuterol inhalers on the incidence of respiratory-related ED visits among patients with asthma, COPD, or both. We hypothesized that the dose information provided by an integrated dose counter could lead to a reduction in the number of respiratory-related ED visits by decreasing the use of empty or near-empty canisters.
Methods
Study design
Data for this study were extracted from the Clinformatics™ Data Mart retrospective claims database (OptumInsight Life Sciences, Eden Prairie, MN, USA), which includes anonymized data from an employed, commercially insured United States population, and collected between January 2006 and September 2012. Recorded data include medical claims (primary care and secondary care), pharmacy claims, and laboratory test results.
The study comprised a 1-year baseline period for patient characterization and confounder definition and 1-year outcome period for the effectiveness evaluation, with an index date defined as the date of the first prescription for albuterol (Figure 1). The study data were de-identified, thus written informed consent was not possible, and ethics committee review was not sought or deemed necessary by the authors.
  | Figure 1 Study design. Abbreviations: COPD, chronic obstructive pulmonary disease; SABA, short-acting β-agonist; HFA, hydrofluoroalkane. |
Patients
Included patients were aged 4–64 years at the index date and had a diagnosis of asthma and/or COPD and/or exercise-induced bronchoconstriction recorded at any time and at least one prescription for albuterol. All patients had a first recorded prescription for albuterol (index date) between January 1, 2010, and September 30, 2011, and continuous insurance coverage during the study period. Patients prescribed Ventolin® HFA (GlaxoSmithKline Inc., Research Triangle Park, NC, USA) were assigned to the dose counter cohort; and patients prescribed ProAir® HFA (Teva Respiratory LLC, Horsham, PA, USA) or Proventil® HFA (Merck & Co., Inc., Whitehouse Station, NJ, USA) were assigned to the non-dose-counter cohort. Each of these inhalers delivers 108 μg of albuterol sulfate (90 μg of albuterol base) from the mouthpiece per actuation. Both the fine-particle mass and the plume vary only slightly among the three different inhalers and are monitored by the manufacturer and the US Food and Drug Administration as part of the product’s commercial release.
The exclusion criteria were use of ProAir® HFA (Teva Respiratory LLC) or Proventil® HFA (Merck & Co., Inc.) (for the dose counter cohort) or the use of Ventolin HFA® (GlaxoSmithKline Inc.) (for the non-dose-counter cohort) during the outcome period and the use of any other SABA during the study period (all patients).
The asthma subpopulation was defined as patients who had at least one consultation, inpatient admission, or ED visit for asthma recorded at any time during the study period; the COPD subpopulation was defined as those who had at least one consultation, inpatient admission, or ED visit for COPD recorded at any time during the study period. The concomitant asthma and COPD subpopulation was defined as those patients having at least one consultation, inpatient admission, or ED visit for asthma and COPD and/or codiagnoses recorded at any time during the study period. We did not examine results separately for the subpopulation with exercise-induced bronchoconstriction.
Outcomes
The primary end point was the incidence rate of respiratory-related ED visits, defined as ED visits associated with a lower respiratory diagnostic code. Secondary end points for patients with asthma were the incidence rate of severe exacerbations (defined as respiratory-related inpatient admissions/ED visits or initiation of acute oral corticosteroids16) and rate of acute respiratory events (defined as occurrence of respiratory-related inpatient admission/ED visits or acute use of oral corticosteroids or antibiotics prescribed following a general practitioner visit for lower respiratory tract infection). For patients with COPD, exacerbations were defined as occurrence of respiratory-related inpatient admission/ED visits or acute use of oral corticosteroids or antibiotics prescribed following a general practitioner visit for lower respiratory tract infection. Additional end points included the average daily SABA dose and the probability of achieving asthma control (asthma subpopulation only) as risk-domain asthma control (defined as absence of acute respiratory events) and overall asthma control (defined as risk-domain asthma control and average daily albuterol dose ≤180 µg).
Statistical analysis
Data were prepared for analysis by investigating outliers and the type and reason for missing data; skewed data were categorized if appropriate. Because of outliers, all patients with >10 ED visits were assigned a value of 10. No imputation was made for missing values.
We evaluated potential confounders, including those that were significantly different at baseline (independent sample t-Test, Mann–Whitney U-Test, χ2 test, P<0.10) and baseline predictors of outcomes (full multivariable model, P≤0.05). Collinearity analysis of confounders (Spearman’s correlation coefficients, ρ>0.3) was performed.
A multivariable model was used with stepwise reduction to derive the best-fitting model of noncollinear predictors (P<0.05). The incidence rate of ED visits was calculated using a reduced zero-inflated Poisson regression model. Exacerbation rates were compared using a reduced zero-inflated Poisson regression model, and the odds of achieving asthma control were analyzed using a logistic regression model. Average daily SABA use was analyzed using a reduced ordinal logistic regression model to determine the odds ratio (OR) for a higher categorized daily dose compared with a lower categorized daily dose (≤100, 101–200, 201–400, 401–800, or ≥800 µg albuterol).
Subanalyses were performed for patients with asthma only, COPD only, or concomitant asthma and COPD because of differences in prescribing indications and outcomes definitions.
Results
A total of 93,980 patients ages 4–64 years were included in the study (dose counter albuterol cohort: n=67,251 [72%]; non-dose-counter albuterol cohort: n=26,729 [28%]) (Figure 2). The dose counter and non-dose-counter cohorts were broadly similar at baseline (Table 1).
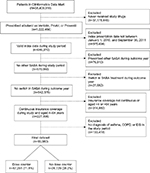  | Figure 2 Flow diagram of the patient selection process. Abbreviations: COPD, chronic obstructive pulmonary disease; EIB, exercise-induced bronchoconstriction; SABA, short-acting β-agonist. |
During the outcome period, 341 (0.5%) patients in the dose counter cohort overall had a respiratory-related ED visit, a significantly smaller proportion than in the non-dose-counter cohort (304 [1.1%]; P<0.001 for the comparison; Table 2). The adjusted rate ratio (RR) for respiratory-related ED visits, relative to the non-dose-counter cohort, was 0.55 (95% confidence interval [CI]: 0.47–0.65; adjusted for baseline respiratory-related ED visits, respiratory-related inpatient admissions, and asthma consultations; gastroesophageal reflux disease diagnosis, rhinitis diagnosis, short-acting muscarinic antagonist use, and β-blocker use).
  | Table 2 Health care resource utilization at baseline and during the outcome period Note: aχ2 test. Abbreviations: COPD, chronic obstructive pulmonary disease; ED, emergency department. |
Asthma subpopulation
A total of 75,787 (81%) patients had a diagnosis of asthma, including 53,964 (80%) of those in the dose counter cohort and 21,823 (82%) of those in the non-dose-counter cohort (Table 1). The treatment cohorts were comparable at baseline in terms of demographic characteristics, comorbidities, and medication use; a statistically significant difference in median age was not clinically significant (Table 1).
Respiratory-related ED visits were recorded during the outcome period for 270 (0.5%) and 244 (1.1%) patients with asthma in the dose counter and non-dose-counter cohorts, respectively (P<0.001; Table 2). Those in the dose counter cohort had 51% lower incidence of respiratory-related ED visits (adjusted RR: 0.49; 95% CI: 0.41–0.59) (Table 2, Figure 3). Average daily SABA dose, severe exacerbation rates, and acute respiratory event rates were similar between treatment cohorts (Tables 3 and 4, Figure 3). However, corresponding with the higher number of ambulatory visits resulting in antibiotic prescriptions (Table 3), patients with asthma in the dose counter cohort had 4% lower odds of achieving risk-domain or overall asthma control than those in the non-dose-counter cohort (Table 4, Figure 3).
COPD subpopulation
A total of 6,687 (7%) patients had COPD, including 4,953 (74%) in the dose counter cohort and 1,734 (26%) in the non-dose-counter cohort (Table 1). The treatment cohorts were comparable for patients with COPD with regard to demographic characteristics, comorbidities, and therapy.
Respiratory-related ED visits were recorded during the outcome period for 16 (0.3%) and 23 (1.3%) patients with COPD in the dose counter and non-dose-counter cohorts, respectively (P<0.001; Table 2). Patients with COPD in the dose counter cohort had 60% lower adjusted respiratory-related ED visit rates versus patients in the non-dose-counter cohort (adjusted RR: 0.40; 95% CI: 0.22–0.75) (Table 2). Other COPD outcomes were comparable between cohorts, including COPD exacerbation rates (adjusted RR: 1.05; 95% CI: 0.97–1.13) and average daily SABA dose (adjusted OR: 1.08; 95% CI: 0.94–1.25) (Table 4).
Asthma and COPD subpopulation
A total of 6,425 (7%) patients had a codiagnosis of asthma and COPD, including 4,730 (7%) of those in the dose counter cohort and 1,695 (6%) of those in the non-dose-counter cohort (Table 1). The treatment cohorts were comparable with regard to demographic characteristics, comorbidities, and therapy.
There was no significant difference between the two cohorts in the unadjusted rate of outcome respiratory-related ED visit rates for patients with asthma and COPD (reported for 42 [0.9%] and 23 [1.4%] in dose counter and non-dose-counter cohorts, respectively; P=0.058; Table 2. The adjusted RR was 0.70 (95% CI: 0.43–1.16). Outcomes were comparable between treatment cohorts in terms of exacerbation rates (adjusted RR: 1.06 [0.97–1.16]) and average daily SABA dose (adjusted OR: 1.05; 95% CI: 0.92–1.20; Table 4).
Discussion
In this investigation of the impact of an integrated dose counter in albuterol inhalers, the incidence rate of respiratory-related ED visits during the outcome year was estimated to be 45% lower in the dose counter cohort than in the non-dose-counter cohort. This result remained significant after splitting the population by single diagnosis: a 51% lower incidence rate was seen in patients with asthma and a 60% lower rate in those with COPD. There was no statistically significant difference in ED visit incidence rate between cohorts for the small subpopulation of patients with codiagnosis of asthma and COPD.
Using a dose counter device was not associated with changes in indicators of disease control, including average daily SABA dose, which was not significantly different between the dose counter and non-dose-counter cohorts, for either the overall population or the subpopulations. We can speculate that the lack of difference in exacerbations may be because ED visits were relatively low in number as compared with the other components of the composite exacerbation variables, which included inpatient admissions and oral corticosteroid courses in the case of asthma exacerbations, and also antibiotics prescribed following a general practitioner visit for lower respiratory tract infection in the case of COPD exacerbations. Moreover, patients in the dose counter cohort of the asthma subpopulation had 4% lower odds of achieving overall asthma control in the outcome period. This was because they had a higher number of ambulatory visits for lower respiratory tract infections with antibiotic prescriptions, a component of the composite asthma control measures. We have no certain explanation for the latter finding.
Our data suggest that dose counters may enable patients to directly know when their rescue medication is empty and thus avoid using empty inhalers during exacerbations. This knowledge may help to reduce the need for acute health care interventions such as ED visits. However, although this significant difference (halving) in ED visits has important clinical consequences, ED visits were a relatively rare event (absolute rates were low) in a relatively mild disease population (those receiving first prescribed rescue medication in the study period and likely to be newly diagnosed patients). For patients with more severe disease, such a reduction in costly ED visits may be more important because higher health care costs are associated with greater asthma severity,17 and a substantial proportion of the health care costs associated with COPD accrue from patients with frequent ED visits for acute COPD exacerbations.18
Prior studies of patients with asthma or COPD have reported high levels of patient satisfaction (>90%) with the use of dose counters on MDIs containing rescue medication, specifically with regard to the ability to know when the inhaler should be replaced.1,2 In a recent online survey of 590 adults and children with asthma, many of whom were found to have empty or expired reliever inhalers, the addition of a dose counter was named most frequently as a means of improving satisfaction with their reliever inhalers.19 In the future, newer technologies may improve patient engagement with their therapy, and gains in disease management may be possible if the rescue dosing data are better integrated into practice. For example, in a recent study, telemonitoring of SABA use via a patient-facing smartphone app, with dose reporting to providers, was associated with decreased use of rescue medication and improved asthma control among those adults initially lacking asthma control.20
An important limitation of this study is the nonrandom treatment assignment, an issue common to all observational studies.21,22 Although adjustment for potential confounders was performed wherever feasible, there remains the possibility of unrecognized bias or confounding. In addition, detailed patient characterization was not possible from the data. For example, we could not determine whether patients with both asthma- and COPD-related claims should have been defined as having asthma-COPD overlap syndrome.23 Moreover, information on spacer use would have been of interest to consider.
Strengths of the study include, 1) the large patient population, 2) the fact that all patients were members of an employed, commercially insured population (thus of similar socioeconomic status), and 3) the fact that the two cohorts (dose counter and non-dose-counter) were in the same insurance plan (thus affordability was likely similar for all). However, the generalizability of study findings is limited to patients receiving a first prescription for albuterol and to employed, commercially insured patients such as those who were included in the database. Further studies are needed to explore the use of integrated dose counters for other patient populations.
Conclusion
We found that the integration of dose counters into rescue inhaler devices is associated with decreased ED visit frequency. The presence of integrated dose counters on rescue inhalers can help patients avoid using an empty or near-empty inhaler during exacerbations, thereby ensuring available medication for relief of their symptoms. The integration of dose counters on rescue MDIs could represent a simple and effective tool to improve clinical outcomes during exacerbations, with a potential for cost savings to health care systems.
Acknowledgments
This study was supported financially by an unrestricted grant from Teva Pharmaceuticals, Frazer, PA, USA. The authors thank Jenny Fanstone of Fanstone Medical Communications Ltd., UK, and Elizabeth V Hillyer for medical writing support, funded by Research in Real-Life. We acknowledge with gratitude Dr Ruchir Parikh for his review of and contributions to the manuscript.
Author contributions
All authors were involved in the conception and design or analysis and interpretation of data, as well as revising the article critically for important intellectual content. All authors have approved the final version of the article for submission and agree to be accountable for all aspects of the work.
Disclosure
David B Price has board membership with Aerocrine, Almirall, Amgen, AstraZeneca, Boehringer Ingelheim, Chiesi, Meda, Mundipharma, Napp, Novartis, and Teva. Consultancy: Almirall, Amgen, AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Meda, Mundipharma, Napp, Novartis, Pfizer, and Teva; grants/grants pending with UK National Health Service, British Lung Foundation, Aerocrine, AstraZeneca, Boehringer Ingelheim, Chiesi, Eli Lilly, GlaxoSmithKline, Meda, Merck, Mundipharma, Novartis, Orion, Pfizer, Respiratory Effectiveness Group, Takeda, Teva, and Zentiva; payments for lectures/speaking: Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, GlaxoSmithKline, Kyorin, Meda, Merck, Mundipharma, Novartis, Pfizer, Skyepharma, Takeda, and Teva; payment for manuscript preparation: Mundipharma and Teva; patents (planned, pending or issued): AKL Ltd.; payment for the development of educational materials: GlaxoSmithKline, Novartis; stock/stock options: shares in AKL Ltd which produces phytopharmaceuticals and owns 80% of Research in Real-Life Ltd and its subsidiary social enterprise Optimum Patient Care; received payment for travel/accommodations/meeting expenses from Aerocrine, Boehringer Ingelheim, Mundipharma, Napp, Novartis, and Teva; funding for patient enrollment or completion of research: Almirall, Chiesi, Teva, and Zentiva; peer reviewer for grant committees: Medical Research Council (2014), Efficacy and Mechanism Evaluation program (2012), HTA (2014); and received unrestricted funding for investigator-initiated studies from Aerocrine, AKL Ltd, Almirall, Boehringer Ingelheim, Chiesi, Meda, Mundipharma, Napp, Novartis, Orion, Takeda, Teva, and Zentiva. Anna Rigazio was an employee of Research in Real-Life at the time of this study. Research in Real-Life conducted this study and has conducted paid research in respiratory disease on behalf of the following other organizations in the past 5 years: Aerocrine, AKL Ltd, Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Meda, Mundipharma, Napp, Novartis, Orion, Takeda, and Zentiva. Mary Buatti Small is a former employee and Thomas J Ferro is a current employee of Teva Pharmaceuticals. The authors report no other conflicts of interest in this work.
References
Given J, Taveras H, Iverson H, Lepore M. Prospective, open-label assessment of albuterol sulfate hydrofluoroalkane metered-dose inhaler with new integrated dose counter. Allergy Asthma Proc. 2013;34(1):42–51. | ||
Wasserman RL, Sheth K, Lincourt WR, Locantore NW, Rosenzweig JC, Crim C. Real-world assessment of a metered-dose inhaler with integrated dose counter. Allergy Asthma Proc. 2006;27(6):486–492. | ||
Weinstein C, Staudinger H, Scott I, Amar NJ, LaForce C. Dose counter performance of mometasone furoate/formoterol inhalers in subjects with asthma or COPD. Respir Med. 2011;105(7):979–988. | ||
Sheth K, Wasserman RL, Lincourt WR, Locantore NW, Carranza-Rosenzweig J, Crim C. Fluticasone propionate/salmeterol hydrofluoroalkane via metered-dose inhaler with integrated dose counter: performance and patient satisfaction. Int J Clin Pract. 2006;60(10):1218–1224. | ||
Shah S, White M, Uryniak T, O’Brien CD. The functionality of a budesonide/formoterol pressurized metered-dose inhaler with an integrated actuation counter. Allergy Asthma Proc. 2010;31(1):40–48. | ||
Ogren RA, Baldwin JL, Simon RA. How patients determine when to replace their metered-dose inhalers. Ann Allergy Asthma Immunol. 1995;75(6 Pt 1):485–489. | ||
Rubin BK, Durotoye L. How do patients determine that their metered-dose inhaler is empty? Chest. 2004;126(4):1134–1137. | ||
Conner JB, Buck PO. Improving asthma management: the case for mandatory inclusion of dose counters on all rescue bronchodilators. J Asthma. 2013;50(6):658–663. | ||
LaForce C, Weinstein C, Nathan RA, Weinstein SF, Staudinger H, Meltzer EO. Patient satisfaction with a pressurized metered-dose inhaler with an integrated dose counter containing a fixed-dose mometasone furoate/formoterol combination. J Asthma. 2011;48(6):625–631. | ||
Hasegawa K, Brenner BE, Clark S, Camargo CA. Emergency department visits for acute asthma by adults who ran out of their inhaled medications. Allergy Asthma Proc. 2014;35(3):42–50. | ||
Moorman JE, Akinbami LJ, Bailey CM, et al. National surveillance of asthma: United States, 2001–2010. Vital Health Stat 3. 2012(35):1–58. | ||
Barnett SB, Nurmagambetov TA. Costs of asthma in the United States: 2002–2007. J Allergy Clin Immunol. 2011;127(1):145–152. | ||
American Lung Association. Trends in asthma morbidity and mortality, 2012. Available from: http://www.lung.org/finding-cures/our-research/trend-reports/asthma-trend-report.pdf. Accessed 12 April 2016. | ||
Ford ES, Croft JB, Mannino DM, Wheaton AG, Zhang X, Giles WH. COPD surveillance – United States, 1999–2011. Chest. 2013;144(1):284–305. | ||
Ford ES. Hospital discharges, readmissions, and ED visits for COPD or bronchiectasis among US adults: findings from the nationwide inpatient sample 2001–2012 and Nationwide Emergency Department Sample 2006–2011. Chest. 2015;147(4):989–998. | ||
Reddel HK, Taylor DR, Bateman ED, et al. An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med. 2009;180(1):59–99. | ||
Bahadori K, Doyle-Waters MM, Marra C, et al. Economic burden of asthma: a systematic review. BMC Pulm Med. 2009;9:24. | ||
Hasegawa K, Tsugawa Y, Tsai CL, Brown DF, Camargo CA Jr. Frequent utilization of the emergency department for acute exacerbation of chronic obstructive pulmonary disease. Respir Res. 2014;15:40. | ||
Storms WW, Tringale M, Ferro TJ. The impact of expired and empty quick-relief asthma inhalers: The Asthma and Allergy Foundation of America’s Asthma Inhaler Design Survey. Allergy Asthma Proc. 2015;36(4):300–305. | ||
Merchant RK, Inamdar R, Quade RC. Effectiveness of population health management using the propeller health asthma platform: a randomized clinical trial. J Allergy Clin Immunol Pract. 2016;4(3):455–463. | ||
Roche N, Reddel H, Martin R, et al. Quality standards for real-world research. Focus on observational database studies of comparative effectiveness. Ann Am Thorac Soc. 2014;11(Suppl 2):S99–S104. | ||
Price D, Hillyer EV, van der Molen T. Efficacy versus effectiveness trials: informing guidelines for asthma management. Curr Opin Allergy Clin Immunol. 2013;13(1):50–57. | ||
van den Berge M, Aalbers R. The asthma-COPD overlap syndrome: how is it defined and what are its clinical implications? J Asthma Allergy. 2016;9:27–35. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




