Back to Journals » Hepatic Medicine: Evidence and Research » Volume 14
Histopathological Evaluation of Ethanolic Leaf Extract of Lippia adoensis on Liver, Kidney, and Biochemical Parameters in Swiss Albino Mice
Authors Boye AT , Ekanem PE, Hailu TB, Hordofa ID , Asfaw MS
Received 15 April 2022
Accepted for publication 7 September 2022
Published 20 September 2022 Volume 2022:14 Pages 123—133
DOI https://doi.org/10.2147/HMER.S370927
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Gerry Lake-Bakaar
Abayneh Tunta Boye,1 Peter Etim Ekanem,2 Tesfamichael Berhe Hailu,2 Ifa Dereje Hordofa,3 Mulu Shiferaw Asfaw1
1Department of Anatomy, College of Health Sciences, Woldia University, Woldia, Ethiopia; 2Department of Anatomy, College of Health Sciences, Mekelle University, Mekelle, Ethiopia; 3Department of Anatomy, College of Health Sciences, Salale University, Salale, Ethiopia
Correspondence: Abayneh Tunta Boye, Woldia University, Tel +251-92-871-5552, Email [email protected]
Background: Eighty percent of Ethiopians use traditional medicine, one of which is the leaf of Lippea adoensis.
Objective: To investigate subacute toxicity of aqueous extracts of L. adoensis leaves on the liver and kidney and biochemical parameters in Swiss albino mice.
Methods: LD50 was assessed with nine experimental groups and one control group of adult female Swiss albino mice (five in each group). In the subacute study, 40 mice of both sexes were randomly divided into four groups of ten mice (both sexes) per group. Group I served as controls and received distilled water and feed only. Groups II–IV were used as treatment groups. They received calculated doses of aqueous leaf extracts orally at doses of 500 mg/kg, 1000 mg/kg, and 2000 mg/kg body weight, respectively.
Results: Since 80% of deaths occurred at the 10,000 mg/kg body-weight dose in this experiment, LD50 was considered to be < 10,000 mg/kg. In the subacute test, general signs of toxicity like hypoactivity, piloerection, lethargy, and a single episode of convulsion were observed at the 2000 mg/kg dose. Beginning from the third week of administration, both male and female mice receiving 500 mg/kg and 2000 mg/kg and all treatment groups in the fourth week showed significant (P< 0.05) weight loss compared to controls. Biochemical parameters were found to increase in all groups treated with ethanolic leaf extracts. Several histopathological changes like congestion, hemorrhage, severe necrosis, and infiltration of inflammatory cells in both liver and kidney in the L. adoensis–treated rats were observed at all doses.
Conclusion: In the present study, the ethanolic leaf extracts of L. adoensis produced dose-dependent weight loss and histopathological and biochemical changes in Swiss albino mice.
Keywords: Lippea adoensis, subacute, toxicological, histopathology, ethanolic extract
Introduction
The World Health Organization (WHO) defines traditional medicine as health practices, approaches, knowledge, and beliefs incorporating plant-, animal-, and mineral-based medicines, spiritual therapies, manual techniques, and exercises, applied singularly or in combination to treat, diagnose, and prevent illnesses and maintain well-being.1 The popularity of traditional medicine has continued in all regions of the developing world, and its use is rapidly spreading in industrialized countries also. Many countries in the world use traditional medicine to meet some of their primary health-care needs.2 According to an estimate of the WHO, the annual global market of traditional medicine is approximately US$83 billion.3 In Africa, up to 80% of the population uses traditional medicine for primary health care.2 The same holds for Ethiopia, where there are reports of around 80% population dependence on traditional medicine for primary health needs.4 The increasing popularity of herbal medicines is based on perceived belief in their effectiveness in the treatment and prevention of disease, the cultural acceptability of healers, the relatively low cost of traditional medicine when compared to modern medicine, and difficult-to-access to modern health-care facilities.5
Lippea adoensis is one of the medicinal plants commonly used in Ethiopia and is also distributed throughout Africa, South, and Central American countries.6 It is an erect woody shrub, which grows up to 1–3 m tall. It is an endemic medicinal plant, and cultivated varieties are commonly found in home gardens in different regions of Ethiopia at an altitudinal range of 1600–2200 m. L. adoensis var. koseret Sebsebe is widely grown in the central and southern highlands of Ethiopia.7 Most Lippea spp. are traditionally utilized as gastrointestinal and respiratory disease remedies. Some Lippia spp. have shown antimalarial, antiviral, and cytostatic activities. Besides this, the leaves from a majority of these species are utilized as seasoning for food preparation.6 In Ethiopia, the fragrant leaves of the variety koseret are used by the Gurage and Oromo tribes as a condiment in the preparation of spiced butter. The special taste and flavor of Gurage kitfo (minced meat with spiced butter) is attributed to the oils imparted by the leaves.8 L. adoensis extracts are used medicinally by a variety of Ethiopian communities for the treatment of skin infections, including eczema and superficial fungal infections. The dried leaves of koseret Sebsebe powdered together with barley are also used in treating stomach problems.9
Modern drugs generally undergo extensive formal testing for therapeutic and adverse effects before being licensed. No such controls in the risk of adverse effects exist for a majority of herbal remedies, which are being provided for their perceived use only.10 Despite the use of L. adoensis for traditional medicine in different parts of Ethiopia, there is sparse literature regarding its safety and toxicity. Though the pharmacological effect of L. adoensis and its essential oils have been addressed by scientific investigations, the toxicity profile of this plant is not known. The current study, which focuses on the subacute toxicity of ethanolic leaf extract of L. adoensis on the liver and kidney, as well as biochemical parameters of Swiss albino mice, aims to provide the needed information on the toxicity of this plant.
Methods
Study Design and Setting
A laboratory-based experiment was employed on Swiss albino mice of both sexes to investigate the toxic effects of leaf extracts of L. adoensis on the histopathology of the liver and kidney, as well as biochemical markers. The study was conducted at the histology laboratory of the Department of Anatomy, College of Health Sciences, Mekelle University.
Plant Collection and Authentication
The leaves of L. adoensis var. koseret were collected from the Wolaitta zone, which is located 343 km south of Addis Ababa, the capital of Ethiopia. A botanist in the Department of Biology authenticated the plant.
Preparation of Plant Extract
All parts of the plant were dried in open air, protected from direct exposure to sunlight to prevent loss of volatile components. The leaves were ground into a fine powder using an electric grinder, followed by the preparation of suspensions in ethanol 80%. The fine powder (300 g) was suspended in 450 mL ethanol 80% separately in sterilized screw-capped 500 mL glass beakers. The suspension was macerated with ethanol 80% for 2 hours with intermittent agitation by an orbital shaker at room temperature for 3 consecutive days. The supernatant was then decanted and filtered using a 0.1 mm2 mesh gauze from the undissolved portion of the plant. The suspension was then filtered three times using Whatman number 1 filter paper and evaporated using a vacuum evaporator to remove the solvent. The final stock solution was stored in a deep freezer at a temperature of −20°C until further use (6). Following maceration, sedimentation, filtration, 600 g of L. adoensis leaf powder using ethanol as solvent, 111.7 g crude extract was obtained with a yield of 18.6%.
Selection and Preparation of Experimental Animals
A total of 90 healthy adult Swiss albino mice were used in this study. To conduct the LD50 calculations, 50 adult female mice were used, and for the subacute test 40 mice of both sexes (20 male and 20 female) weighing 25–30 g were used. Mice in this experiment were aged 8–12 weeks and females were kept nulliparous and nonpregnant. The animals were kept in well-ventilated plastic cages under standard laboratory conditions (temperature of 22°C±3°C), and were exposed to photoperiods of 12 hours light/12 hours dark cycles (32). A conventional rodent laboratory diet was used for feeding with an unlimited supply of drinking water throughout the experiment. The animals were acclimatized to laboratory conditions for 1 week before the experiment.11
Method of Extract Administration
LD50 Determination
A total of ten groups of mice were used (nine treatment and one control), each consisting of five adult female Swiss albino mice. To increase the rate of absorption of the extract, all groups of mice were fasted overnight prior to administration. The doses were calculated and administered to the mice in the treatment groups based on their body weight. Each treatment group (groups I–IX) received designated doses of 2000–10,000 mg/kg body weight of the Lippea adoensis extract spaced by 1000 mg/kg to produce test groups with a range of toxic effects and mortality rates during 48 hours’ observation. The extract was administered orally by gavage.
Subacute Test
Forty mice of both sexes were randomly assigned into four groups of ten mice (five male and five female) per group. Group I served as controls, and were administered distilled water and feed only. Groups II–IV were used as treatment groups and calculated doses based on LD50, ie, 500 mg/kg, 1000 mg/kg, and 2000 mg/kg body weight of L. adoensis aqueous leaf extractwas administered daily for 28 days. The extract was given once a day after the animals had fasted for 3–4 hours with free access to water. The plant extract was administered orally using gavages and animals fasted for 1–2 hours after the administration before routine feeding (32).11 The animals were treated daily throughout the experiment (28 days). All equipment used was cleaned and placed in an oven after each administration to prevent any contamination.
Data-Collection Techniques
Data were collected from the experimental animals before and after they were killed.
Body-Weight Measurement and Cage-Side Observations
Body weights of the experimental and control groups of animals were recorded using a digital electronic balance (PA4102C, China) before the beginning of the first day of administration, then weekly till the last day of administration of the extract. In each cage, mice were carefully observed individually before and after dosing the plant extract periodically for any changes in skin and fur, eyes, and respiration. Autonomic effects, such as salivation, diarrhea, and urination, and CNS effects, such as tremors, were also followed.
Blood Collection and Testing
At the end of the experimental period (on the 29th day), animals belonging to each group were weighed on the digital electronic balance and anesthetized with diethyl ether. Blood samples were collected by cardiac puncture after letting all animals fast overnight on the last day of the experiment. Samples of approximately 1.3–1.5mL were drawn quickly using 5 mL syringes and collected in vacutainers. For biochemical analysis, the blood samples in the plain vacutainers were allowed to stand for 3 hours for complete clotting and then centrifuged at 5000 rpm for 15 minutes using a benchtop centrifuge (HuMax-K, HUMAN, Germany). Concentrations of alanine aminotransferase (ALT), aspartate aminotransferase (AST), alanine aminophosphate (ALP), urea, and creatinine were automatically determined using an AutoLab 18 clinical chemistry analyzer.
Dissection and Tissue Collection
Each animal was killed by cervical dislocation. The abdominal cavity was opened and the liver and kidneys removed carefully. Organ samples were cleared from any surrounding tissue by washing in normal saline. They were put on clean paper and weighed immediately on a digital balance. Samples from the left and right kidneys and the liver were preserved in 10% neutral buffered formalin fixative for 24 hours and thoroughly rinsed under running tap water overnight.12
Tissue Processing
Isolated liver and kidneys were observed for gross pathological changes in color and texture. Randomly, longitudinal and transverse sections of the organs were taken and fixed for histological processing. The samples were then processed according to the standard technique of dehydration, clearing, infiltration, and embedding.12 Tissue blocks were then sectioned with a thickness of 5 μm using Leica rotary microtome (RM2125 RT Nussloch, Germany). Ribbons of the tissue sections were gently collected using a piece of brush and placed on the surface of a 40°C water bath. After the sections had been appropriately spread on the surface, they were mounted on tissue slides and stained regressively with routine Harris hematoxylin for 6 minutes and eosin for 17–20 seconds (H&E) and mounted with DPX.12
Light Microscopy and Photomicrography
Stained tissue sections of livers and kidneys were carefully examined under a compound light microscope in the histology laboratory of the Department of Anatomy. Tissue sections from the treated groups were examined for any evidence of histopathological changes compared with those of the controls. Photomicrographs of selected slides from both the treated and control groups were taken under magnification of 10× and 40× using an EVOS XI automated built-in digital camera to examine the liver and kidney tissue for any histopathological changes.
Data Processing and Analysis
SPSS 20 was used to analyze the data, which are represented in numbers. Values of parameters are expressed as means ± SEM. One-way ANOVA was used to compare treatment over time between control and treated groups. P<0.05 was considered statistically significant.
Ethics
A clearance letter was obtained from the Health Research and Ethics Review Committee of the College of Veterinary Medicine, Mekelle University. Animals used for this study were handled according to OECD guidelines and kept from any unnecessary painful and terrifying situations.11 To keep the animals from pain and suffering during any surgical interventions, appropriate anesthesia was given. Animals were protected from pathogens and placed in an appropriate environment.
Results
Acute Toxicity and LD50 of Ethanolic Extract of L. adoensis
The administration of the ethanolic leaf extract of L. adoensis did not result in any deaths up to a dose of 6000 mg/kg body weight. General signs of toxicity were seen from 5000 mg/kg body weight. These included loss of appetite, hypoactivity, piloerection, lethargy, and a single episode of convulsion at 5000 mg/kg. As 80% death occurred at 10,000 mg/kg body-weight dose, LD50 was considered to be 10,000 mg/kg body weight.
Subacute Toxicity
Effect of L. adoensis Ethanolic Leaf Extract on General Body Weight
In the first 2 weeks of the experiment following administration of the extract, the mean body weight of both male and female mice at doses of 500 mg/kg, 1000 mg/kg, and 2000 mg/kg body weight did not show any significant changes compared with controls. However, starting from the third week of administration, both male and female mice in the 500 mg/kg and 2000 kg/kg and all treatment groups in the fourth week showed significant (P<0.05) weight loss compared with controls. Though both sexes showed weight loss with increasing doses, these weight changes were more prominent in female groups (P<0.01), except at 1000 mg/kg, as shown in Table 1.
 |
Table 1 Mean body weight of male and female mice treated with Lippea adoensis leaves for 4 weeks |
Effect of Ethanolic Leaf Extract of L. adoensis on Organ Weight
The mean weight of the liver increased in female mice at doses of 1000 mg/kg and 2000 mg/kg compared with the controls (P>0.05). However, an increase in mean liver weight among male mice in the treatment groups was not evident. The mean kidney weight increased in male mice at a dose of 1000 mg/kg and female mice at both 1000 mg/kg and 2000 mg/kg body weight (Table 2).
 |
Table 2 Mean organ weights of male and female mice treated with 500 mg/kg, 1000 mg/kg, and 2000 mg/kg body weights of L. adoensis leaves |
Effects of Subacute Administration of L. adoensis on Biochemical Parameters
The biochemical parameters under study were found to increase in the groups treated with ethanolic leaf extract of L. adoensis. There were significant (P<0.05) increases in the values of ALT, AST, and ALP in both male and female mice groups that received L. adoensis doses of 1000 mg/kg and 2000 mg/kg body weight compared with the controls, as shown in Table 3. Also, there were significant (P<0.05) increases in values of creatinine in mice of both sexes treated with 1000 mg/kg and 2000 mg/kg L. adoensis. Urea was found to increase in male groups at a dose of 2000 mg/kg and all doses in female groups treated with L. adoensis.
 |
Table 3 Biochemical parameters of female and male mice treated with 500, 1000, and 2000 mg/kg body-weight doses of L. adoensis leaves and controls |
Effects of Subacute Administration of L. adoensis on Liver Histology
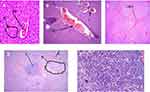
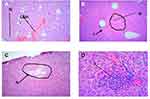
Histopathological analysis of liver sections from the control group showed normal histology of the liver, with normal hepatic cells, visible central veins, and thin sinusoids. The central vein and the portal area containing branches of the hepatic artery, biliary duct, and portal vein had normal appearance (Figure 1). In comparison to the controls, the general microscopic architecture of the liver-tissue sections of both male and female mice that received 500 mg/kg L. adoensis showed congestion of the portal vein (Figure 2A), mild focal necrosis and degeneration of hepatocytes (Figure 2B), and congestion and hemorrhage of the central vein (Figure 2C). Liver sections of mice from 1000 m/kg groups exhibited hemorrhage (Figure 2D), infiltration (Figure 2E), severe necrosis, and infiltration of inflammatory cells (Figure 3A and B). Similarly, sections of liver from 2000 mg/kg groups showed multifocal congestion and hemorrhage (Figure 3C), severe necrosis and infiltration of inflammatory cells (Figure 3D), and pale necrotized areas indicating severe cell death.
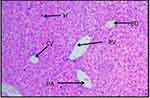 |
Figure 1 H&E (100×)–stained liver section of control mouse, showing central vein (CV), hepatocytes (H), hepatic artery (HA), portal vein (PV), and bile duct (BD). |
Effects of Subacute Administration of L. adoensis on Kidney Histology
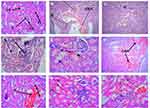
Examinations of sections of kidneys of mice of both sexes in the control group indicated normal kidney histology: renal corpuscles showed normal size of urinary space and tubular structures, proximal and distal convoluted tubules, and glomerulus (Figure 4A). However, sections of kidneys from both male and female mice in the 500 mg/kg group revealed focal congestion and hemorrhage (Figure 4B), interstitial congestion (Figure 4C), necrosis, and interstitial inflammatory cells (Figure 4D), At 1000 mg/kg administration, necrosis of the tubules and hemorrhage within the stromal tissue were observed (Figure 4E); multifocal congestion and necrosis (Figure 4F), and edema and hemorrhage (Figure 4G). Both male and female mice in the 2000 mg/kg groups indicated congestion and hemorrhage, necrosis with focal tubular destruction (Figure 4H), hemorrhage, and infiltration of inflammatory cells (Figure 4I).
Discussion
Despite being natural and biological, the phytoconstituents in this plant exert unanticipated toxicities that target visceral organs that are loaded with metabolic activities like the liver and the kidney.5 L. adoensis has such phytochemicals as alkaloids, tannins, terpenoids, saponins, and flavonoids, which have been reported to be toxic.13,14
According to the findings from this study, acute administration of L. adoensis did not show any mortality in the experimental mice with single oral doses up to 8000 mg/kg body weight. However, following single oral doses of 8000 mg/kg body weight and above, the animals showed more severe symptoms of toxicity and exhibited weakness, hypoactivity, poor appetite, piloerection, lethargy, and convulsions. The LD50 was considered to be 10,000 mg/kg body weight, as 80% death occurred at this dose. This dose is within the range of rodent oral LD50 values for linalool, which is one of the major constituents of L. adoensis reported by Bickers et al.15
Cage-side observations in the subacute study revealed that mice in the 1000 mg/kg and 2000 mg/kg groups had mild to severe signs of toxicity. Mice in the 1000 mg/kg group showed piloerection, hypoactivity, and loss of appetite. Observation of mice in the 2000 mg/kg group revealed the same signs plus some severe ones like lethargy and intermittent episodes of convulsions. This is in line with a study reported by Sit et al16 in which essential oils from Litsea elliptica leaves in rats resulted in hypoactivity, lacrimation, and piloerection. Hypoactivity and loss of appetite might be associated with compounds in the extract, which could have interfered with the intake and metabolism of dietary nutrients and their anorexic effects. Lethargy and convulsions were probably due to reported central depressive effects of monoterpens, specifically linalool, from the extract.17
Changes in the body and internal organ weights (of a significant magnitude) are considered good indices of toxicity.18 The findings from this study indicated a significant (P<0.05) decrease in body weight of mice treated with 500 mg/kg and 2000 mg/kg L. adoensis in the third week and all dose groups in the fourth week. This is in line with findings from Arika et al,13 who reported dose-dependent decreases in body weight following oral and intraperitoneal administration of Lippea javanica for 28 days in mice. Weight loss is a sensitive index of toxicity after exposure to a toxic substance.19 A decrease in body weight might be due to phytochemicals in the plant that could suppress food intake or interfere with its metabolism. Phytochemicals are known to affect body weight through manipulating energy expenditure, appetite suppression, satiety enhancement, and fat-glucose absorption blocking.20
Organ-weight data can also provide sensitive indices of toxicological change.21 In our study, the subacute administration of L. adoensis ethanolic leaf extract induced an increase in organ weights of livers and kidneys in mice of both sexes, which is congruent with the findings of Arika et al.13 This may be due to increased metabolic activity and facilitated protein synthesis in these organs induced by extracts.13 Another possibility of increased weight in kidneys and livers could indicate hypertrophy, related to underlying pathology like edema and congestion, as supported by the histopathological findings of this study. Hypertrophy of the organs is a first-hand indication of the toxicity of a chemical or biological substance.22 Linalool is also reported to cause epithelial hyperplasia in kidney.17
Liver and renal function tests are of great importance to evaluate changes produced by a toxicant. Raised blood levels of hepatic biomarkers and nitrogenous wastes to be excreted by the kidney are usually considered in the toxicity, due to their spillage into the bloodstream as a result of necrosis of the tissue.23 In the present study, there were significant (P<0.05) increases in mean values of ALT, AST, and ALP in the 1000 mg/kg and 2000 mg/kg groups compared with controls. This is supported by findings from histological examinations of the liver, whereby increasing doses of ethanolic extract of L. adoensis caused mild to severe alterations in histomorpholgy. Such findings are in line with reports from other authors,13,18 which showed dose-dependent increase in ALT, AST, and ALP. An increase in serum levels of these enzymes might have resulted from damage to the liver cells. Another possibility could be enterohepatic circulation of linalool, which has the effect of prolonging the metabolic load on the liver, consequently leading to hepatocellular damage.24
Both urea and creatinine serum levels were found to increase in a significant manner in mice treated with 1000 mg/kg and 2000 mg/kg L. adoensis, except urea in male groups, which did not show significant changes. The rise in serum levels of these biomarkers might have resulted from dose-related damage to renal parenchyma. This is also supported by histopathological findings of the same mouse groups, which showed severe structural damage after receiving the extract at corresponding doses, which caused elevated levels of renal biomarkers.
Histopathological examinations provide synergistic information in addition to biochemical and hematological parameters in assessment of toxicity. The main morphological changes that may suggest mechanisms of liver injury include zonal necrosis, hepatitis, cholestasis, steatosis, granuloma, and vascular lesions.25 The findings of this study are in agreement with other authors15,18 who reported vascular congestion, vacuolated nuclei, congested sinusoids, moderate scars of necrosis, and edema in the livers of mice treated with 60-day subchronic administrations of Zingiber officinale. This finding is also supported by Fandohan et al,26 who observed hepatocyte hydrophic changes at 1500 mg/ kg and 2000 mg/kg involving several liver cell types and the interstitium around hepatocyte layers to be significantly diminished. These changes could be due to the presence of monoterpens, such as linalool, in L. adoensis, repeated dosing of which induces oxidative metabolic pathways that are potentially toxic.17 Furthermore, hepatocellular injury due to cytotoxic effects of other phytochemicals in the extract may have caused these kinds of alterations in the histoarchitecture of the liver in mice.
Microscopic examinations of tissue slides of kidneys from treatment groups revealed findings that were in agreement with the results of biochemical parameters. Mice in the 500 mg/kg group developed mild congestion, hemorrhage, and tubular cell necrosis. Sections of kidneys from both male and female mice in the 1000 mg/kg and 2000 mg/kg groups showed multifocal congestion and hemorrhage. These observations are in accordance with the findings from Ebeye et al,27 in which the kidneys of Ocimum gratissimum–treated rats revealed vascular congestion, unremarkable appearance of the interstitial spaces, and glomeruli with varying degrees of interstitial infiltration by inflammatory cells. Our study findings also included occluded lumen (PT), infiltration of inflammatory cells, edema, and tubular necrosis (PT), especially in mice in the 2000 mg/kg groups, in consonance with Noori et al,28 where A. deserti extract showed significant histopathological alterations, such as degeneration in walls of proximal and distal tubules, atrophied glomeruli, and inflammatory cells with increasing doses. It is also supported by Ahmad et al,19 where serious signs of toxicity were observed in the kidneys of rats treated with 2000 mg/kg cinnamon bark aqueous extract. This could be caused by the presence of compounds like terpenoids in the extract, which have been reported to increase membrane permeability, thereby causing congestion and edema.18
Conclusion
In the present study, the ethanolic leaf extract of L. adoensis at doses of 500 mg/kg, 1000 mg/kg, and 2000 mg/kg body weight produced dose-dependent changes in body weight and biochemical parameters when compared with controls. Moreover, the ethanolic leaf extract of L. adoensis also resulted in pathological changes in the histological structure of livers and kidneys in Swiss albino mice. In the acute-toxicity study, the plant produced 80% mortality at a dose of 10,000 mg/kg, so the LD50 may be <10,000 mg/kg.
Recommendations
The results raised the following concerns. Firstly, although the subacute study produced significant levels of adverse effects on the study animals, further toxicity studies are needed to extend the time of study to subchronic and chronic levels. Secondly, studies focusing on specific isolate compounds that are responsible for such adverse effects are needed in order to establish the toxicity profile of the plant. Thirdly, further investigations of toxicity on other parts of the plant should also be considered.
Data Sharing
All the data regarding this research work are available from the corresponding author.
Ethical Approval and Consent to Participate
A clearance letter was obtained from the Health Research and Ethics Review Committee of the College of Veterinary Medicine, Mekelle University. OECD guidelines were followed in the handling of all animals used in the study.
Acknowledgment
The authors would like to thank all the contributers to this work throughout the research paper and express our sincere gratitude to Mekelle University Department of Anatomy for their support in providing their laboratory for the experimental work of this study.
Author Contributions
All authors made a significant contribution to the work reported, whether in the conception, study design, execution, acquisition of data, analysis, and interpretation, or all these areas, took part in drafting, revising, or critically reviewing the article, gave final approval to the version to be published, have agreed on the journal to which the article has been submitted, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. World health Organisation. Traditional Medicine. Geneva: World Health Organization; 2008.
2. Kassaye KD, Amberbir A, Getachew B, Mussema Y. A historical overview of traditional medicine practices and policy in Ethiopia. Ethiop J Health Dev. 2006;20(2):127–134.
3. Wassie SM, Aragie LL, Taye BW, Mekonnen LB. Knowledge, attitude, and utilization of traditional medicine among the communities of merawi town, northwest Ethiopia: a cross- sectional study. Evid Based Complement Altern Med. 2015;2015. doi:10.1155/2015/138073
4. Lambert J. Ethiopia; Traditional Medicine and the bridge to better health, World Bank Ethiop. J Health Dev. 2006;20(2):5.
5. Debjit B, Pawan D, Margret C, Kumar K. Herbal drug toxicity and safety evaluation of traditional medicines. Arch Appl Sci Res. 2009;1(2):32–56.
6. Pascual M, Slowing K, Carretero E, Mata DS, Villar A. Lippia: traditional uses, chemistry and pharmacology: a review. J Ethnopharmacol. 2001;76(3):201–214. doi:10.1016/S0378-8741(01)00234-3
7. Zeleke WT. Identification of Lippia adoensis for Access and Benefit sharing. Genetic Resources Access and Benefit Sharing; 2016.
8. Demissew S. A confusion in Lippia (Verbenaceae) in tropical Africa. Kew Bulletin. 1993;48(2):375–379. doi:10.2307/4117945
9. DDaY M. Antibacterial activity of methanol, aqueous and N-hexane extract of Lippea adoensis. Afr J Microbiol Res. 2014;8(44):326–371.
10. Stickel F, Patsenker E, Schuppan D. Herbal hepatotoxicity. J Hepatol. 2005;43(5):901. doi:10.1016/j.jhep.2005.08.002
11. No OT. 407: repeated Dose 28-day oral toxicity study in rodents. OECD guidelines for the testing of chemicals, Section; 2008:4.
12. Ayele M. Evaluation of acute and sub-chronic toxicity of aqueous leaves extracts of maytenus gracilipes Celastraceae (kombolcha) on some blood parameters and histopathology of liver and kidney in Swiss Albino Mice. Addis Abeba University; 2015.
13. Arika W, Ogola P, Abdirahman Y, Mawia A, Wambua F. In vivo safety of aqueous leaf extract of lippia javanica in mice models. Biochem Physiol. 2015;5(191):2.
14. Liebelt AG. Unique Features of Anatomy, Histology, and Ultrastructure Kidney, Mouse. Urinary system: Springer; 1998:37–57.
15. Bickers D, Calow P, Greim H, et al. A toxicologic and dermatologic assessment of linalool and related esters when used as fragrance ingredients. Food Chem Toxicol. 2003;41(7):919–942. doi:10.1016/S0278-6915(03)00016-4
16. Masran S, Salji MR, Othman H, Budin SB, Taib IS. Acute toxicity (oral) information of Litsea elliptica Blume essential oil. Int Conference Biosci Biochem Bioinf. 2011;5:399–403.
17. Powers K, Beasley VR. Toxicological aspects of linalool: a review. Vet Hum Toxicol. 1985;27:484–486.
18. Esther O, Hocm IO, Udom GJ, Ogbuagu EO, John A. Toxicological assessment of Zingiber officinale roscoe (ginger) root oil extracts in albino rats. Toxicol Digest. 2019;4(1):108–119.
19. Ahmad RA, Serati-Nouri H, Abdul Majid F, Sarmidi MR, Abdul Aziz R. Assessment of potential toxicological effects of Cinnamon bark aqueous extract in rats. Int J Biosci Biochem Bioinforma. 2015;5(1):36–44. doi:10.17706/ijbbb.2015.5.1.36-44
20. Pasman WJ, Heimerikx J, Rubingh CM, et al. The effect of Korean pine nut oil on in vitro CCK release, on appetite sensations and on gut hormones in post-menopausal overweight women. Lipids Health Dis. 2008;7(1):10. doi:10.1186/1476-511X-7-10
21. Yang X, Schnackenberg LK, Shi Q, Salminen WF. Biomarkers in toxicology. In: Hepatic Toxicity Biomarkers. Elsevier; 2014: 241–259.
22. Yuet Ping K, Darah I, Chen Y, Sreeramanan S, Sasidharan S. Acute and subchronic toxicity study of euphorbia hirta l. methanol extract in rats. Biomed Res Int. 2013;2013:1–14. doi:10.1155/2013/182064
23. Rahman M, Siddiqui MK, Jamil K. Effects of Vepacide (Azadirachta indica) on asp artate and al anine aminotransferase profiles in a subchronic study with rats. Hum Exp Toxicol. 2001;20(5):243–249. doi:10.1191/096032701678227730
24. Parke D, Rahman Kmq WR. Effect of Linalool on Hepatic Drug-Metabolizing Enzymes in the Rat. Portland Press Ltd; 1974.
25. Zárybnický T, Boušová I, Ambrož M, Skálová L. Hepatotoxicity of monoterpenes and sesquiterpenes. Arch Toxicol. 2018;92(1):1–13. doi:10.1007/s00204-017-2062-2
26. Fandohan P, Gnonlonfin B, Laleye A, Gbenou J, Darboux R, Moudachirou M. Toxicity and gastric tolerance of essential oils from Cymbopogon citratus, Ocimum gratissimum and Ocimum basilicum in Wistar rats. Food Chem Toxicol. 2008;46(7):2493–2497. doi:10.1016/j.fct.2008.04.006
27. Ebeye O, Ekundina O, Wilkie I. Histological and biochemical effects of aqueous extract of Ocimum gratissimum on the liver and kidney of adult Wistar rats. Afr J Cell Pathol. 2014;2:59–64. doi:10.5897/AJCPATH14.008
28. Noori A, Amjad L, Yazdani F. The effects of Artemisia deserti ethanolic extract on pathology and function of rat kidney. Avicenna J Phytomedicine. 2014;4(6):371.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.