Back to Journals » Open Access Emergency Medicine » Volume 11
Histopathological evaluation of autopsy cases with isolated pulmonary fat embolism (IPFE): is cardiopulmonary resuscitation a main cause of death in IPFE?
Authors Turkmen Samdanci E, Reha Celik M , Pehlivan S, Celbis O, Turkkan D , Ozdemir Kara D , Pamukcu E
Received 12 November 2018
Accepted for publication 24 April 2019
Published 7 June 2019 Volume 2019:11 Pages 121—127
DOI https://doi.org/10.2147/OAEM.S194340
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Hans-Christoph Pape
Emine Turkmen Samdanci,1 Muhammet Reha Celik,2 Sultan Pehlivan,3 Osman Celbis,4 Dilhan Turkkan,3 Dogus Ozdemir Kara,3 Esra Pamukcu5
1Department of Pathology, School of Medicine, Inönü University, Malatya, Turkey; 2Department of Thoracic Surgery, School of Medicine, Inönü University, Malatya, Turkey; 3Pathology Laboratory, Council of Forensic Medicine, Ankara Group Chairmanship, Ankara, Turkey; 4Department of Forensic Medicine, School of Medicine, Inönü University, Malatya, Turkey; 5Department of Statistics, Faculty of Science, Fırat University, Elâziğ, Turkey
Background: Fat embolism (FE) may develop following many traumatic and atraumatic clinical conditions; however, fewer data exist regarding the occurrence of isolated pulmonary FE (IPFE). Cardiopulmonary resuscitation (CPR) is an emergency procedure for maintaining blood circulation and oxygenation during cardiac arrest. In this study, we aimed to evaluate the association of CPR with IPFE in autopsy cases.
Methods: A total 402 cases among 4,118 autopsies were diagnosed with IPFE, and the medical background of these cases was retrospectively evaluated. Diagnosis of FE and FE grading were performed with histopathological examinations of postmortem tissue samples, and injury-severity scores of traumatic cases were assessed. Data of traumatic and atraumatic cases were statistically compared.
Results: Of the IPFE cases, 298 (741%) were male and 104 (25.9%) female, with overall mean age 53.7 (2–99) years. Causes of death of studied subjects were traumatic for 302 (75.1%) and atraumatic reasons for 100 (24.9%) cases. CPR was performed for 277 cases of which 177 (63.9%) were traumatic and 100 (36.1%) were non-traumatic. . In comparison to traumatic cases, significantly higher CPR frequency was determined in atraumatic IPFE (P=0.001). High grade FE in the traumatic cases, and mild-moderate grade of FE in the nontraumatic cases were found statistically significant(P=0.001).
Conclusion: This study indicates that CPR may be one of the leading factors in the development of IPFE in atraumatic conditions, and this procedure was related to mild–moderate IPFE manifestations. Regardless of whether conditions were traumatic or atraumatic, in patients who survive following CPR for manifest ventilation/perfusion problems, it should be remembered that IPFE may have developed due to CPR.
Keywords: cardiopulmonary resuscitation, CPR, fat embolism, pulmonary embolism, autopsy
Introduction
Fat embolism (FE) is characterized by the presence of fat globules in the circulatory system. This situation is very common (47–100%), especially after long-bone fractures and in several other conditions, such as surgery, septicemia, sickle-cell anemia, parenteral lipid infusion, liposuction, pancreatitis, acute respiratory distress, osteomyelitis, fatty liver, and cardiopulmonary resuscitation (CPR).1–5 The lungs, central nervous system, skin, and kidneys are the most frequently involved organs in FE, and mortality due to this complication is about 5–15%.6
Isolated pulmonary FE (IPFE) is a particular manifestation of FE characterized by the presence of fat tissue components in pulmonary vessels, without any evidence of FE in other organs.7 The presumptive diagnosis of IPFE is generally difficult, due to obscure clinical symptoms, and laboratory and radiological findings are not always indicative.7,8 Therefore, definitive diagnosis of this complication is established by histopathological examinations of postmortem lung-tissue samples.8
Etiological factors that contribute to the development of IPFE have not been clearly documented, especially in atraumatic patients without known risk factors. Though studies have reported that a number of patients with pulmonary FE also had CPR history,9,10 more data are still required about the contributions of CPR to the development of IPFE.
Therefore, we intended to analyze whether CPR was a potential factor that could be conducive to the development of IPFE. For this purpose, we evaluated postmortem lung-tissue samples obtained from cases who had died due to traumatic or atraumatic reasons, and the grade of IPFE was determined for these cases. According to our knowledge, this will be one of the larger studies in terms of included case numbers.
Methods
Study design and cases
Retrospective data analysis was performed on the medical files of 4,118 cases that had undergone autopsy within the last 2 years in two distinct facilities of provincial subunits of the Forensic Medicine Institute of Turkey in Malatya (n=1,705) and Ankara (n=2,413). The nature of events for the cases in both cities were similar.
All cases were autopsied in the morgue departments of the forensic medicine institutes in both cities, and tissue samples were obtained from various organs. A total of 402 (9.7%) IPFE cases identified from 4,118 autopsies were assessed in this study. Demographic data, such as age and sex, and medical data belonging to the studied cases, such as causes of mortality and premortal clinical parameters, including CPR, and brief medical histories were recorded.
Cases were divided into two main categories: traumatic (n=302) and atraumatic (n=100). Traumatic cases included traffic accidents (n=118), falls from height (n=60), assaults (n=32), firearm injuries (n=28), burns (n=14), poisoning (drug, insecticide, carbon monoxide, opioids or mushrooms; n=13), common falls (in toilet or bath or from bed; n=12), explosions (n=7), drowning (n=6), getting trapped under a landslide (n=6), death by hanging (n=2), and electrocution-related death (n=4).
The Abbreviated Injury Scale (AIS) and injury-severity score were used for evaluating the severity of trauma in the trauma cases.11 In accordance with the AIS, the body was divided into six regions: external neck, head and face, thorax, abdomen, pelvic organs, and extremities. The severity of injury in each region was measured (1–6). The sum of squares of values of the three injury regions in the AIS was calculated as the ISS value. Cases according to the ISS were evaluated in three categories: 0–14 points, minor injury; 16–66 points, major injury; and ≥75 points, fatal injury. Atraumatic cases included myocardial infarction (MI; n=81), liver failure (n=2), cancer (n=3), death at home without any sign of trauma (n=8), maternal death during delivery (partum, postpartum; n=2), myocarditis (n=1), bedridden mental retardation (n=1), toxic megacolon (n=1), and aortic aneurysm rupture (n=1).
Histopathological evaluation and FE grading
Tissue samples from the brain, cerebellum, lungs, liver, kidneys, heart, and pancreas obtained during the autopsies were fixed in 10% formalin solution. Tissue embedded in paraffin after routine tissue preparation were sliced at a thickness of 4 µm, stained with H&E, and examined under light microscopy. Because of the retrospective design of the study, oil staining (oil red O and Sudan black), which requires fresh tissue, was not convenient to use.
FE diagnosis was made based on the presence of lipocytes and/or components of bone marrow in the lumens of pulmonary vessels (alveolar capillaries, arteries, or veins) (Figures 1 and 2). FE was graded by a scoring system defined by Scully and Glass and modified by Mudd et al10. Briefly, grade 1 was no emboli (4×), grade 2 one to five emboli (10×), grade 3 one to five emboli (40×), and grade 4 five or more emboli in a majority of fields (40×).
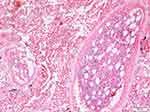 | Figure 1 Bone-marrow particles in the lumen of the vessel. H&E, 100×. |
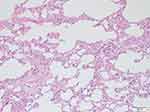 | Figure 2 Lipocytes in the lumen of alveolar capillaries. H&E, 100×. |
Data analysis
Data distribution was evaluated with histograms, Q–Q graphics, and Shapiro–Wilk tests. For comparison of two independent groups, Mann–Whitney U tests were performed. Pearson’s χ2 analysis was used for comparison of categorical variables. Data were analyzed using Turcosa statistical software. P<0.05 was regarded as statistically significant.
Results
Of the 402 cases, 74.1% (n=298) were male and 25.9% (n=104) female. Mean ages were 52.6 (2–99) years for men and 56.8 (3–97) years for women. xxxxAmong all subjects, 75.1% (n=302) of FE cases in our study were traumatic and 24.9% (n=100) atraumatic (Figure 3). Traffic-accident cases were the largest group in the traumatic cases, and MI was the largest group in atraumatic cases. Of the traumatic cases, median ISS was 75 and ISS category 3. FE grades were 13 cases grade 1, 239 cases grade 2, and remaining 150 cases grade 3. CPR was performed for 277 cases of which 177 (63.9%) were traumatic and 100 (36.1%) were non-traumatic. (Table 1).
 | Table 1 Classification of cases according to etiology and CPR requirement |
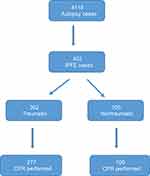 | Figure 3 Study-design flowchart. |
Statistical analyses showed that a significantly higher percentage of CRP was performed in atraumatic IPFE cases (P<0.001). Additionally, the percentage of grade 2 FE was significantly higher in atraumatic IPFE than traumatic cases (P<0.001); however, grade 1 and grade 3 FE were significantly higher in traumatic IPFE cases (P<0.001). Table 2 shows whether CPR was performed or not, FE grade, ISS, and ISS categories of overall cases.
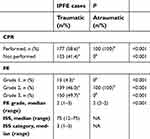 | Table 2 Distribution of cases according to whether CPR performed or not, FE grade, ISS, and ISS categories |
Discussion
The development mechanisms of fat globules appearing in the circulatory system, responsible for FE pathogenesis, have not been exactly explained yet. On the other hand, there are two mechanisms that have been emphasized.12 One of these is transient pressure increase in the bony trauma area causing the fat globules to migrate into the blood circulation from the bone marrow through ruptured medullary venous sinuses, consequently resulting in blockage of blood circulation in the lungs at the capillary level.13 Therefore, early-phase respiratory failure that develops due to posttraumatic FE can be explained.12 The other mechanism is biochemical: it is the FE that causes respiratory distress appearing within 24 hrs to 2 weeks in cases without bone fractures, such as burns, hyperthermia, liposuction, and pancreatitis.12,14–16 In this mechanism, increasing release of catecholamines due to trauma leads to an increase in lipase levels. As such, when fat is hydrolyzed, free fatty acids, such as oleic and linoleic acid, are released, giving rise to pulmonary damage and respiratory distress syndrome.12,14,17 Moreover, lipoprotein-lipase activity originating in the endothelium causes parenchymal damage that leads to local fatty-acid release in the pulmonary microcirculation.17,18
While FE is observed in a majority of trauma cases accompanied by bone fractures in varying proportions (40–100%), FE syndrome (FES) is present in only 0.9–5%.19–22 Since systemic FE and FES were among the exclusion criteria, a total of 402 cases with histopathological FE only in the lungs (IPFE) were included in the study. The number of cases (n=402) in the present study is the largest IPFE series in the literature. The fact that males accounted for 74.1% of the cases in this study, a larger proportion than female cases, can be interpreted as a higher rate of involvement of men in judicial situations.
CPR was performed for all cases with atraumatic etiology (100%, n=100), and rib fractures due to CPR were identified. Although CPR itself is a secondary trauma, the main causes of death in these cases were atraumatic, such as MI. It is possible that the intrathoracic pressure gradient during ventilation may facilitate the intake of fat particles (bone-marrow elements) from the broken ribs into the bloodstream during CPR. Bone-marrow elements were found in lung vessels (arteries, veins) in cases of bone fracture, while fat globules were detected in alveolar capillaries in such cases as burns or blunt trauma. However, the number of IPFE cases without bone fracture was low, and statistical evaluation was not possible.
Although 47–100% of blunt-trauma cases have been shown to be related to FE, fatal FE cases are exceptionally rare in the literature.23–28 In our series, among the cases murdered by an assault (n=31), IPFE was identified in only one on which CPR was not performed and that had no bone fractures. In this case, spread ecchymoses were detected in the soft tissue of the arms, legs, hips, and back, covering 20–30% of the whole body. The definite cause of death was reported as crush syndrome, due to the acute tubular necrosis found in the kidneys, and FE developing through a biochemical mechanism was determined to have made a major contribution to death.
In some studies conducted on the correlation between FE and CPR, pulmonary FE refers to 65–86% of trauma cases and 88% nontrauma cases in which CPR was performed.29,30 In our study, CPR-performed cases accounted for 68.9% of all cases (277 of 402). Furthermore, IPFE was present in all cases and CPR performed on 53.4% (177 of 302) of traumatic and 100% (100 of 100) of atraumatic ones.
There was a statistically significant difference in the severity of FE between the trauma subgroups (P˂0.001). In groups with high ISS and AIS scores, FE grade was high as well. This was interpreted as an increase in circulatory bone-marrow elements migrated into the vessels due to an increased number of bone fractures. In addition, examination of cases who had both rib fractures and CPR revealed severe FE more than cases without CPR or rib fractures. On the other hand, all CPR-performed atraumatic cases, there were rib fractures present secondary to CPR, resulting in FE. As both respiratory and circulatory support have to be maintained during CPR, fat and/or bone-marrow particles migrating into the circulatory system to cause FE appear to develop following CPR. The FE in these cases was classified as grade 2, and was not considered to be the main cause of death.
When cases in this study were evaluated with respect to the precise cause of death, almost all were found to have a cause of death (bleeding, cardiovascular collapse, burns and complications, asphyxia) independent of IPFE. Although IPFE alone can be a definite cause of death, it was found to be a contributing factor for almost all cases in our study. Crush syndrome was suggested as the precise cause of death in one of the assault cases, where IPFE was only a contributing factor. It was reported that IPFE had an additional contribution in the burn-related respiratory and circulatory collapse identified as the precise cause of death.
The diagnosis of FE is very difficult to define clinically, unless it becomes syndromic (FES) in living patients with rib fractures due to CPR. Although studies on these issues are rare, in patients admitted to emergency units due to trauma with rib fractures who require inpatient management in thoracic surgery clinics, FES symptoms are either not observed or very rare. Silva et al reported that of 229 cases with rib fractures, FES developed in only five (2.2%).31 In the thoracic surgery clinic of our hospital, FES was not detected in any of the 709 patients with rib fractures monitored and treated due to trauma in 1998–2017.
The importance of the FE in CPR-survivors depends on the clinical condition of the patients (eg, severe hypoxia in blood-gas analysis). Although multislice thoracic computed tomography imaging is an approved method for diagnosis in pulmonary embolism, because of the deteriorated clinical condition of CPR survivors, it is usually not possible to obtain a computed tomography scan soon after resuscitation.
When it comes to evaluating findings regarding malpractice, no studies or data are available in the related literature either both medical or judicial dimensions of the development of FES following CPR. Moreover, there are very few, if any, reports of FE due to rib fractures following CPR. The grade of FE in CPR-performed nontrauma patients was statistically significantly lower than trauma cases with multiple bone fractures in our study. Besides this, lethal FES does not develop in patients who are followed up, particularly in thoracic surgery or orthopedics due to rib fractures or other bone fractures. Therefore, rib fractures secondary to CPR should not be regarded as malpractice, but physicians should be alert to identifying them as an indicator of FE when respiratory symptoms develop.
Limitations
This study was retrospective, and only autopsy cases with FE were examined. Although the FES-development rate is quite low in living FE patients, it is not possible to make any comments about the actual FE rate in the patients.
Conclusion
The precise cause of death must be determined with reference to clinical, autopsy, and forensic findings before reporting histopathologically identified IPFE at an autopsy as the real cause of death. It must be remembered that IPFE can develop at high rates in living patients with rib fractures following CPR. Also, clots can be added on fat globules and/or bone-marrow particles present in the circulatory system (especially in microcirculation), and thus FE can develop new clinical symptoms or deteriorate clinical behaviors of the present disease. IPFE should be taken into consideration for patients being monitored and treated for rib fractures when respiratory parameters get worse without any other reason. Although IPFE can develop due to rib fractures during CPR, FES and systemic FE rarely develop in clinical practice following CPR. Therefore, although rib fractures are common, effective CPR performance is mandatory, and one should not hesitate because of malpractice due to FE.
Ethics approval and consent
This study received ethical approval from the ethics committee of the Turkish Forensic Medicine Institute, including a statement covering patient-data confidentiality and compliance with the Declaration of Helsinki (2012/18). The ethics committee accepted that consent of participants was not asked for, as data were extracted from autopsy cases.
Consent for publication
This manuscript contains no individual person’s data, and thus can be published without consent from the participants. Availability of data and material generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author contributions
All authors contributed toward data analysis, drafting, and revision of the paper and agree to be accountable for all aspects of the work. All authors read and approved the final manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Brandt SE, Zeegers WS, Ceelen TL. Fatal pulmonary fat embolism after dorsal spinal fusion. Eur Spine J. 1998;7(5):426–428. doi:10.1007/s005860050102
2. Heyneman LE, Müller NL. Pulmonary nodules in early fat embolism syndrome: a case report. J Thorac Imaging. 2000;15(1):71–74. doi:10.1097/00005382-200001000-00014
3. Tsitsikas DA, Gallinella G, Patel S, Seligman H, Greaves P, Amos RJ. Bone marrow necrosis and fat embolism syndrome in sickle cell disease: increased susceptibility of patients with non-SS genotypes and a possible association with human parvovirus B19 infection. Blood Rev. 2014;28(1):23–30. doi:10.1016/j.blre.2013.12.002
4. Pastore L, Kessler S. Pulmonary fat embolization in the immunocompromised patient: its relationship to steroid medication. Am J Surg Pathol. 1982;6(4):315–322. doi:10.1097/00000478-198206000-00004
5. Costa AN, Mendes DM, Toufen C, Arrunátegui G, Caruso P, de Carvalho CR. Adult respiratory distress syndrome due to fat embolism in the postoperative period following liposuction and fat grafting. J Bras Pneumol. 2008;34(8):622–625. doi:10.1590/S1806-37132008000800013
6. Shaikh N. Emergency management of fat embolism syndrome. J Emerg Trauma Shock. 2009;2(1):29–33. doi:10.4103/0974-2700.44680
7. Saukko P, Knight B, editors. Knight’s Forensic Pathology. London, UK: Edward Arnold; 2004.
8. Cuculić D, Stemberga V, Coklo M, Sosa I, Stifter S, Bosnar A. Trauma related fat embolism syndrome in forensic practice. Coll Antropol. 2010;34(2):723–726.
9. Voisard MX, Schweitzer W, Jackowski C. Pulmonary fat embolism–a prospective study within the forensic autopsy collective of the Republic of Iceland. J Forensic Sci. 2013;58(Suppl1):S105–S111. doi:10.1111/1556-4029.12003
10. Mudd KL, Hunt A, Matherly RC, et al. Analysis of pulmonary fat embolism in blunt force fatalities. J Trauma. 2000;48(4):711–715.
11. Rau CS, Wu SC, Kuo PJ, et al. Same abbreviated injury scale values may be associated with different risks to mortality in trauma patients: a cross-sectional retrospective study based on the trauma registry system in a level I trauma center. Int J Environ Res Public Health. 2017;14:12. doi:10.3390/ijerph14121552
12. Hamood S, Hayek T, Munichor M, Michaelson M, Best LA, Bentur L. Fat embolism in a boy with minor nonfracture trauma. Pediatr Pulmonol. 1999;27(3):221–223. doi:10.1002/(SICI)1099-0496(199903)27:3<221::AID-PPUL13>3.0.CO;2-K
13. Matthews BD, Sing RF, Heniford BT. Fatal cerebral fat embolism after open reduction and internal fixation of femur fracture. J Trauma. 2001;50(3):585. doi:10.1097/00005373-200103000-00034
14. Bolliger SA, Muehlematter K, Thali MJ, Ampanozi G. Correlation of fat embolism severity and subcutaneous fatty tissue crushing and bone fractures. Int J Legal Med. 2011;125(3):453–458. doi:10.1007/s00414-011-0563-8
15. Inoue H, Ikeda N, Tsuji A, Kudo K, Hanagama M, Nata M. Pulmonary fat embolization as a diagnostic finding for heat exposure. Leg Med (Tokyo). 2009;11(1):1–3. doi:10.1016/j.legalmed.2008.05.002
16. Zeidman M, Durand P, Kundu N, Doumit G. Fat embolism after liposuction in Klippel-Trenaunay syndrome. J Craniofac Surg. 2013;24(4):1319–1321. doi:10.1097/SCS.0b013e3182953a63
17. Shapiro MP, Hayes JA. Fat embolism in sickle cell disease. Report of a case with brief review of the literature. Arch Intern Med. 1984;144(1):181–182. doi:10.1001/archinte.1984.00350130211039
18. Byard RW. The complex spectrum of forensic issues arising from obesity. Forensic Sci Med Pathol. 2012;8(4):402–413. doi:10.1007/s12024-012-9322-5
19. Blankstein M, Byrick RJ, Richards RR, Mullen JB, Zdero R, Schemitsch EH. Pathophysiology of fat embolism: a rabbit model. J Orthop Trauma. 2011;25(11):674–680. doi:10.1097/BOT.0b013e318206ed30
20. Day JD, Walden SM, Stuart SR, Hutchins GM, Hruban RH. Fatal fat embolism syndrome after numerous vertebral body compression fractures in a lung transplant recipient. J Heart Lung Transplant. 1994;13(5):785–790.
21. Eriksson EA, Rickey J, Leon SM, Minshall CT, Fakhry SM, Schandl CA. Fat embolism in pediatric patients: an autopsy evaluation of incidence and etiology. J Crit Care. 2015;30(1):
22. Habashi NM, Andrews PL, Scalea TM. Therapeutic aspects of fat embolism syndrome. Injury. 2006;37(Suppl 4):S68–S73. doi:10.1016/j.injury.2006.08.042
23. Duran L, Kayhan S, Kati C, Akdemir HU, Balci K, Yavuz Y. Cerebral fat embolism syndrome after long bone fracture due to gunshot injury. Indian J Crit Care Med. 2014;18(3):167–169. doi:10.4103/0972-5229.128707
24. Hulman G. The pathogenesis of fat embolism. J Pathol. 1995;176(1):3–9. doi:10.1002/path.1711760103
25. Karayel F, Arican N, Kavas G, Turan AA, Pakis I. Maternal death due to non-traumatic fat embolism. J Forensic Sci. 2005;50(5):1201–1203. doi:10.1520/JFS2004363
26. Sousa I, Janeiro J, Campos P, Távora I. Trauma patient with fat embolism detected on computed tomography. Acta Med Port. 2017;30(1):73–76. doi:10.20344/amp.7355
27. Li S, Zou D, Qin Z, et al. Nonfracture-associated pulmonary fat embolism after blunt force fatality: case report and review of the literature. Am J Forensic Med Pathol. 2015;36(2):61–65. doi:10.1097/PAF.0000000000000142
28. Mellor A, Soni N. Fat embolism. Anaesthesia. 2001;56(2):145–154. doi:10.1046/j.1365-2044.2001.01724.x
29. Bierre AR, Koelmeyer TD. Pulmonary fat and bone marrow embolism in aircraft accident victims. Pathology. 1983;15(2):131–135. doi:10.3109/00313028309084699
30. Eriksson EA, Pellegrini DC, Vanderkolk WE, Minshall CT, Fakhry SM, Cohle SD. Incidence of pulmonary fat embolism at autopsy: an undiagnosed epidemic. J Trauma. 2011;71(2):312–315. doi:10.1097/TA.0b013e3182208280
31. Silva JJAB, Diana DA, Salas VER, Zamboni C, Hungria Neto JS, Christian RW. Fat embolism syndrome in femoral shaft fractures: does the initial treatment make a difference? Rev Bras Ortop. 2017;52(5):535–537. doi:10.1016/j.rboe.2016.08.021
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
