Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 17
Histone Lactylation Participates in Psoriasis Progression by Regulating the Adiponectin Expression
Authors Zhao S, Wu T, Fu M, Zhang Z
Received 16 November 2023
Accepted for publication 18 January 2024
Published 26 January 2024 Volume 2024:17 Pages 219—227
DOI https://doi.org/10.2147/CCID.S450254
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Sicheng Zhao, Tingyan Wu, Mingjing Fu, Zhe Zhang
Department of Dermatology, Suzhou Kowloon Hospital, Shanghai Jiao Tong University School of Medicine, Suzhou, People’s Republic of China
Correspondence: Sicheng Zhao, Department of Dermatology, Suzhou Kowloon Hospital, Shanghai Jiao Tong University School of Medicine, No. 118 Wansheng Street, Suzhou Industrial Park, Suzhou, Jiangsu, 215028, People’s Republic of China, Email [email protected]
Background: Psoriasis is a chronic inflammatory skin disease characterized by erythema, papules, and plaques. Adiponectin (ADIPOQ) is an important protein hormone secreted by adipose tissue. Here, we aimed to explore the expression of ADIPOQ in psoriasis patients and the moderation effect of histone lactylation on ADIPOQ.
Methods: The GSE78097 data set was downloaded from GEO database to analyze the differentially expressed genes (DEGs) in psoriasis. A total of 36 psoriasis patients were recruited to obtain the skin samples. The ADIPOQ protein levels, global lactylation and histone lactylation (H3K18lac) levels were detected by Western blot assay. Chromatin immunoprecipitation (CHIP) assay was performed to detect the combination between H3K18lac and promoter regions of the ADIPOQ. The receiver operating curve (ROC) analysis was used to evaluate the diagnostic value of ADIPOQ in psoriasis.
Results: ADIPOQ was decreased in the skin tissues of psoriasis patients. In addition, the global lactylation and H3K18lac levels were significantly decreased in the skin tissues of psoriasis patients. In HaCaT cells, promoting the global lactylation and H3K18lac levels increased the ADIPOQ protein levels, while si-LDHA transfection decreased the ADIPOQ protein levels. The CHIP results indicated that lactylation promoted the binding of promoter regions of the ADIPOQ and H3K18lac. Finally, the ROC analysis showed that ADIPOQ exhibited diagnostic value in psoriasis.
Conclusion: This study demonstrated ADIPOQ was decreased in the skin tissues of psoriasis patients, and ADIPOQ has diagnostic value for psoriasis. Furthermore, down-regulation of H3K18lac levels inhibited the transcription of ADIPOQ, which was the key factor of decrease of ADIPOQ levels in psoriasis patients.
Keywords: psoriasis, ADIPOQ, histone, lactylation
Introduction
Psoriasis is a chronic inflammatory skin disease characterized by erythema, papules, and plaques.1 The incidence rate of psoriasis is 2%~3%.2 Psoriasis is most commonly occurred in the elbows, knees, scalp, and lower back, seriously affecting the patient’s quality of life, and even leading to depression.3 The clinical treatment of psoriasis is difficult and prone to recurrence, which seriously endangers the physical and mental health of patients and affects their quality of life.4 The study of psoriasis pathogenesis has always been a focus and difficulty in dermatology research.
Lactylation is a novel histone modification recently discovered. Research has found that incomplete oxidation of glucose in M1 macrophages under hypoxic conditions leads to an increase in the production of metabolite lactate. Lactate can generate lactoyl CoA, which provides a lactoyl group to the lysine tail of histones through acyltransferase, resulting in a histone modification called lysine lactylation.5 Many studies have demonstrated that histone lactylation participates in various disease progression, such as ocular melanoma,6 Alzheimer’s disease,7 lung fibrosis,8 etc. The latest research has found that lactate can participate in the regulation of gene expression through epigenetic modifications of histone lactylation.6,9,10 Therefore, the discovery of histone lactylation opens up a new window for research on the role of lactate in various pathophysiological conditions, including infection and cancer. In psoriasis progression, many studies demonstrated that elevated lactate or lactate dehydrogenase levels were key factors.11,12 Therefore, we speculate that in the development of psoriasis, high levels of lactate and lactate dehydrogenase may cause histone lactylation to regulate disease progression. However, as far as we know, the role or histone lactylation in psoriasis remains unclear.
Adiponectin (ADIPOQ) is an important protein hormone secreted by adipose tissue.13 Initial research showed that ADIPOQ was expressed in adipocytes. Subsequent studies found that ADIPOQ was also expressed in other nonadipose cells such as skeletal muscle cells, salivary gland epithelial cells, muscle cells, myofibroblasts, etc.14 Recently, ADIPOQ has been demonstrated to exhibit biological effects in various aspects such as anti-inflammatory,15 lipid-lowering, hypoglycemic,16 and insulin sensitization.17 In psoriasis development, researches have shown that the levels of ADIPOQ in psoriasis patients with metabolic diseases are significantly reduced compared to healthy individuals, and are correlated with the severity of psoriasis.18 However, the mechanism of ADIPOQ reduction in psoriasis patients is not yet clear, and whether ADIPOQ expression is regulated by histone lactylation has aroused our interest.
Therefore, this study was performed to explore the relationship between histone lactylation and ADIPOQ levels in psoriasis progression, and the diagnostic value of ADIPOQ in psoriasis. We hypothesized that low levels of histone lactylation inhibited the pomoter transcription of ADIPOQ, which resulted in the decrease of ADIPOQ expression.
Material and Methods
Bioinformatic Analysis
The GSE78097 data set was downloaded from GEO database (http://www. ncbi.nlm) and analyzed using R software. The differentially expressed genes (DEGs) were expressed as volcano and heat maps. In addition, KEGG enrichment analysis of DEGs was performed using online tools from the ConsonsusPathDB database (http://cpdb.olgen.mpg.de/), with P<0.05 as the standard for screening. The String (Search Tool for the Retrieval of Interaction Genes) database (http://string.db.org/) was applied to analyze the protein interaction of DEGs, and hub gene was selected using Cytoscape’s plugin (Cytohubba).
Clinical Tissue and Blood Samples Collection
A total of 36 psoriasis patients, visited our hospital, were recruited in this study. All skin tissue samples of psoriasis patients were taken from the excised material of skin biopsy surgery. In addition, 36 patients undergoing plastic surgery were recruited and excess skin tissue removed during the surgery was collected, which was used as normal control samples.
For blood sample collection, venous blood samples were drawn from the participants between 09:00 and 11:00 hr following a 12-hr fasting period. Following centrifugation of the blood samples at 1500 g for 15 min, plasma was collected for ADIPOQ levels determination by ELISA kit (Human Leptin Quantikine Elisa Kit, COINO BIO, Shanghai, China) according to the manufacturer’s instructions in duplicate.
The characteristics of study participants were showed in Table 1. The collection and use of all samples were approved by the Medical Ethics Committee of Suzhou Kowloon Hospital, Shanghai Jiao Tong University School of Medicine. The informed consent form signed by the patient was obtained. The samples were stored at −80 °C for further analysis.
 |
Table 1 Baseline Characteristics of Study Participants in Normal Group and Psoriasis Group |
Cell Culture and Treatment
HaCaT cells were purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). The cells were cultured in a complete medium (DMEM; Invitrogen, USA) supplemented with 10% heat-inactivated fetal bovine serum (FBS, Invitrogen), 100 U/mL penicillin, and 100 μg/mL streptomycin (Invitrogen) for 24 h. The cultivation conditions are set to 37 °C and 5% CO2. For lactate treatment, the medium was added with 20 mM Lactate and the cells were cultured for 24 h. For cell transfection, the lactate dehydrogenase A (LDHA) knockout siRNA (si-LDHA) and negative control (si-nc) were synthesized by Genepharma (Shanghai, China). The si-LDHA and si-nc was transfected into cells using Lipofectamine 3000 (Invitrogen, USA) following the instructions. Twenty-four h later, the culture medium was changed to normal complete medium, and the cells were used for further experiments.
Reverse Transcription-Quantitative Polymerase Chain Reaction (RT-qPCR)
Total RNA from skin samples and/or HaCaT cells was extracted by TRIzol reagent (Invitrogen, USA). Then, RNAs were reverse transcribed into cDNA using the PrimeScript RT kit (Takara, Dalian, China). The qPCR amplification experiment was performed using the Taq Pro Universal SYBR qPCR Master Mix kit (Takara) with the reaction conditions: 95°C, 30 sec; 95°C, 10 sec, 40 cycles; 60°C, 30 sec; 95°C, 15 sec; 60 °C, 60 sec; 95°C, 15 sec. The relative gene expressions were calculated using 2−ΔΔCT method. Primers used in this study are synthesized by Sangon Biotechnology Co., LTD (Shanghai, China) and listed as follows: ADIPOQ, forward, 5′-AACATGCCCATTCGCTTTA-3′ and reverse, 5′-TCTCCTTCCCCATACACCTG-3′; GAPDH, 5′-GACTCATGACCACAGTCCATGC-3′ and reverse, 5′-AGAGGCAGGGATGATGTTCTG-3′.
Western Blot
Skin samples and/or HaCaT cells were lysed using the RIPA lysis buffer on ice to extract proteins. After detecting protein concentration using a BCA kit (Beyotime, Shanghai, China), 30 μg of proteins were loaded on 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis for separation. Then, the proteins were transferred onto PVDF membranes. The membranes were incubated with primary antibodies (Anti-L-Lactyl Anti-L-Lactyl-Histone H3, anti-ADIPOQ, anti-LDHA and anti-GAPDH) at 4°C for one night, followed by with secondary antibody at room temperature for 1 h. A SuperPico ECL Chemiluminescence kit (Vazyma) was used to expose the protein bands.
Chromatin Immunoprecipitation (CHIP) Assay
A CHIP kit was purchased from Invitrogen. HaCaT cells were fixed in 1% formaldehyde (Sigma, USA) and crosslinked for 15 min. Crosslinked chromatin was ultrasonically fragmented. Anti-IgG and anti-Anti-L-Lactyl-Histone H3 were incubated with protein A/G, respectively, to make the antibody-beads complex. The DNAs were incubated with the antibody-bead complex for 6 h. The enrichment of H3K18lac on ADIPOQ DNA fragments was assessed using RT-PCR.
Statistical Analysis
Each experiment is conducted independently three times. Data analysis was performed using GraphPad Prism 7 software and is expressed as mean ± Standard Deviation. Student’s t-test or one-way analysis of variance was used for two groups or multiple group comparisons, respectively. Additionally, the receiver operating curve (ROC) analysis was used to analyze the area under the curve (AUC) of ADIPOQ, in order to evaluate the diagnostic value of ADIPOQ in psoriasis. Statistical significance was set at P < 0.05.
Results
ADIPOQ Was Down-Regulated in the Psoriasis Patients
Through the GSE78097 data set, we found that there were 382 DEGs in the psoriasis patients, of which 49 were upregulated and 333 were downregulated. The DEGs were expressed as Volcano (Figure 1A) and heat maps (Figure 1B). Then, the DEGs were used for KEGG pathway analysis. The results showed that the ADIPOQ was enriched in PPAR, Adipocytokine and AMPK signaling pathway, which was closely related to psoriasis progression (Figure 1C). In addition, the PPI diagram showed that among the DEGs, ADIPOQ was the Hub gene in psoriasis (Figure 1D). Therefore, ADIPOQ was selected for the next experiments. In the plasma of psoriasis patients ADIPOQ levels were significantly decreased compared with healthy person (Figure 1E). In the skin samples of psoriasis patients, the mRNA (Figure 1F) and protein (Figure 1G) levels of ADIPOQ was significantly decreased compared with healthy person.
Lactylation Level Was Up-Regulated in the Psoriasis Patients
Next, we performed the Western blot using Anti-L-Lactyl. The results showed that compared with the skin samples of healthy person, the global lactylation levels were significantly decreased in the skin samples of psoriasis patients (Figure 2A). In addition, through using the Anti-L-Lactyl-Histone H3, we found that the Histone lactylation (H3K18lac) levels in the skin samples of psoriasis patients were significantly decreased compared with the skin samples of healthy person (Figure 3A).
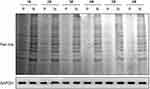 |
Figure 2 Lactylation level was up-regulated in the psoriasis patients. The global Lactylation levels in the skin samples were detecting by Western blot assay using Anti-L-Lactyl. |
 |
Figure 3 H3K18lac level was up-regulated in the psoriasis patients. The H3K18lac levels in the skin samples were detecting by Western blot assay using Anti-L-Lactyl-Histone H3. |
H3K18la Induced the Lactylation of ADIPOQ in HaCaT Cells
In the HaCaT cell, after lactate treatment, the lactylation of ADIPOQ, global lactylation and H3K18lac levels were significantly increased (Figure 4A). After si-LDHA transfection, the ADIPOQ levels, global lactylation and H3K18lac levels were significantly decreased (Figure 4B). In addition, the CHIP assay indicated that si-LDHA transfection inhibited the binding of promoter regions of the ADIPOQ and H3K18lac, while lactate treatment promoted it (Figure 4C).
ROC Curve Analysis of ADIPOQ in Psoriasis
Finally, we used ADIPOQ to detect psoriasis and plotted ROC curves for analysis (Figure 5A). The results showed that AUC value of ADIPOQ was 0.865, and 95% CI was 0.782–0.948. This result indicated that ADIPOQ has diagnostic value in psoriasis.
 |
Figure 5 ROC curve analysis of ADIPOQ in psoriasis. |
Discussion
Here, ADIPOQ was confirmed to be down-regulated in the psoriasis patients. In addition, global lactylation and H3K18lac levels were also down-regulated in the skin samples of psoriasis patients. Enhancement of lactylation levels of HaCaT cells increased the lactylation of ADIPOQ, which further promoted the combination of promoter regions of the ADIPOQ and H3K18lac and induced the ADIPOQ transcription.
ADIPOQ has been demonstrated to be involved in regulating various inflammatory response processes.19 In vitro experiments have confirmed that high levels of ADIPOQ reduces the production of interleukin-6 (IL-6), TNF-α, and interferon γ (IFN-γ), and increase the level of tissue inhibitors of metalloproteinases in macrophages by inducing IL-10 expression.20 ADIPOQ can also inhibit the production of IL-8 and the adhesion between monocytes and endothelial cells, thereby inhibiting the transduction of the NF-κB signaling pathway.21 Recently, accumulating researches have demonstrated that low levels of ADIPOQ are a key factor in psoriasis progression.18,22,23 Clinical research on psoriasis patients showed that the reduction of ADIPOQ was the main features, followed by decrease of IL-17 levels.24 The low level of ADIPOQ in blood and skin tissues aggravated the severity of skin lesions in psoriasis patients.25 However, there is some controversy over ADIPOQ expression in immune system diseases. Previous studies have suggested that plasma C-reactive protein (CRP) levels are negatively correlated with plasma ADIPOQ levels, which was also observed in adipose tissue.26,27 On the contrary, recent research demonstrated that ADIPOQ levels were positively correlated with the CRP levels,28,29 which was somewhat puzzling. We speculated that the expression of ADIPOQ may be related to the severity of the disease, leading to compensatory phenomena and changes in its expression. Previously, the relationship of ADIPOQ and CRP in psoriasis progression was unclear. However, it is confirmed that plasma ADIPOQ decreases and CRP increases as metabolic diseases progress nowadays.30,31 Therefore, the decrease of ADIPOQ is closely related to the psoriasis occurrence. Here, through the analysis of GSE78097 data set, ADIPOQ was found to be decrease in the psoriasis patients, which was further verified in the skin samples of psoriasis patients. In addition, the ROS analysis indicated that ADIPOQ has diagnostic value for psoriasis. These findings suggested that the expression of ADIPOQ may be a promising diagnostic biomarker for psoriasis patients. As reported by previous studies, the promotion of ADIPOQ expression alleviated the development of psoriasis.18,22 Combining with our findings, in future, developing drugs that can promote ADIPOQ expression may be a promising approach for treating psoriasis.
Recently, lactylation modification has been confirmed by increasing research to be involved in the progression of multiple diseases.32,33 Histone lactylation modification, a newly identified epigenetic modification, has been demonstrated to modulate the transcription of target gene through adding a lactoyl group to the lysine residue of the target gene.5,34 In the progress of immunoregulation, histone lactylation activates the endogenous lactate clock and enhances reparative gene levels, thereby promoting immune homeostasis.35 The regulatory roles of histone lactylation has been demonstrated in many diseases, such as myocardial infarction,33 cell renal cell carcinoma,36 pulmonary hypertension37 and other immune-related pathological conditions.38 These researches demonstrated that histone lactylation participated in the disease progression through modulating the GCN5, PDGFRβ, HIF-1α, etc. However, the function of histone lactylation in psoriasis remains unclear. In this study, we found that the global lactylation and histone lactylation (H3K18lac) levels were significantly decreased in the skin samples of psoriasis patients. Through the in vitro experiments, we further confirmed that inducing H3K18lac can increase ADIPOQ protein levels, and inhibiting H3K18lac exhibited an opposite effect. Through the CHIP assay, we further confirmed that lactylation modification promoted the combination of promoter regions of the ADIPOQ and H3K18lac. These results indicated that inducing the H3K18lac levels might promote the transcription of ADIPOQ, which eventually enhanced the ADIPOQ levels. Thus, it can be seen that promoting the H3K18lac is a promising method for increasing ADIPOQ expression. In the future, the availability of therapeutic drugs for psoriasis patients can focus on regulating histone lactate. However, further research is needed to explore how to increase the level of lactylation without affecting normal metabolism in the body.
However, there were still some limitations in this study. We did not conduct animal experiments to further explore the role of ADIPOQ in psoriasis. Whether increasing the expression of ADIPOQ through H3K18lac modification can alleviate the development of psoriasis is our future research focus.
In conclusion, this study demonstrated ADIPOQ was decreased in the skin tissues of psoriasis patients, and ADIPOQ has diagnostic value for psoriasis. Furthermore, down-regulation of H3K18lac levels inhibited the transcription of ADIPOQ, which was the key factor of decrease of ADIPOQ levels in psoriasis patients. This study provides new ideas for the diagnosis and treatment of psoriasis in the future.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Suzhou Kowloon Hospital, Shanghai Jiao Tong University School of Medicine.
Consent to Participate
Informed consent was obtained from all individual participants included in the study.
Consent to Publish
The authors affirm that human research participants provided informed consent for publication of the images in Figures.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Disclosure
The authors have no relevant financial or non-financial interests to disclose for this work.
References
1. Korman NJ. Management of psoriasis as a systemic disease: what is the evidence? Br J Dermatol. 2020;182(4):840–848. doi:10.1111/bjd.18245
2. Vaengebjerg S, Skov L, Egeberg A, Loft ND. Prevalence, incidence, and risk of cancer in patients with psoriasis and psoriatic arthritis: a systematic review and meta-analysis. JAMA dermatol. 2020;156(4):421–429. doi:10.1001/jamadermatol.2020.0024
3. Bronckers IM, Paller AS, Geel MJV, Kerkhof PCMV, Seyger MMB. Psoriasis in children and adolescents: diagnosis, management and comorbidities. Paediatric Drugs. 2015;17(5):373–384. doi:10.1007/s40272-015-0137-1
4. Armstrong AW, Read C. Pathophysiology, clinical presentation, and treatment of psoriasis: a review. JAMA. 2020;323(19):1945–1960. doi:10.1001/jama.2020.4006
5. Zhang D, Tang Z, Huang H, et al. Metabolic regulation of gene expression by histone lactylation. Nature. 2019;574(7779):575–580. doi:10.1038/s41586-019-1678-1
6. Yu J, Chai P, Xie M, et al. Histone lactylation drives oncogenesis by facilitating m6A reader protein YTHDF2 expression in ocular melanoma. Genome Biol. 2021;22(1):1–85. doi:10.1186/s13059-021-02308-z
7. Pan R, He L, Zhang J, et al. Positive feedback regulation of microglial glucose metabolism by histone H4 lysine 12 lactylation in Alzheimer’s disease. Cell Metab. 2022;34(4):634–648. doi:10.1016/j.cmet.2022.02.013
8. Cui H, Xie N, Banerjee S, et al. Lung myofibroblasts promote macrophage profibrotic activity through lactate-induced histone Lactylation. Am J Respir Cell Mol Biol. 2021;64(1):115–125. doi:10.1165/rcmb.2020-0360OC
9. Jiang J, Huang D, Jiang Y, et al. Lactate modulates cellular metabolism through histone lactylation-mediated gene expression in non-small cell lung cancer. Front Oncol. 2021;11:647559. doi:10.3389/fonc.2021.647559
10. Wang J, Liu Z, Xu Y, et al. Enterobacterial LPS-inducible LINC00152 is regulated by histone lactylation and promotes cancer cells invasion and migration. Front Cell Infect Microbiol. 2022;12:913815. doi:10.3389/fcimb.2022.913815
11. Koguchi-Yoshioka H, Watanabe R, Matsumura Y, et al. Serum lactate dehydrogenase level as a possible predictor of treatment preference in psoriasis. J Dermatol Sci. 2021;103(2):109–115. doi:10.1016/j.jdermsci.2021.07.007
12. Takeo N, Fujiwara S, Sakai T, Saito-Shono T, Ishikawa K, Hatano Y. Hereditary lactate dehydrogenase M-subunit deficiency with late-developing pustular psoriasis-like lesions. J Dermatol. 2016;43(12):1429–1432. doi:10.1111/1346-8138.13516
13. Achari AE, Jain SK. Adiponectin, a therapeutic target for obesity, diabetes, and endothelial dysfunction. Int J Mol Sci. 2017;18(6):1321. doi:10.3390/ijms18061321
14. Da SRS, Liu M, Sweeney G. Adiponectin synthesis, secretion and extravasation from circulation to interstitial space. Physiology. 2021;36(3):134–149. doi:10.1152/physiol.00031.2020
15. Jung HN, Jung CH. The role of anti-inflammatory adipokines in cardiometabolic disorders: moving beyond adiponectin. Int J Mol Sci. 2021;22(24):13529. doi:10.3390/ijms222413529
16. Ohashi T, Shibata R, Morimoto T, et al. Correlation between circulating adiponectin levels and coronary plaque regression during aggressive lipid-lowering therapy in patients with acute coronary syndrome: subgroup analysis of Japan-ACS study. Atherosclerosis. 2010;212(1):237–242. doi:10.1016/j.atherosclerosis.2010.05.005
17. Blandin A, Amosse J, Froger J, et al. Extracellular vesicles are carriers of adiponectin with insulin-sensitizing and anti-inflammatory properties. Cell Rep. 2023;42(8):112866. doi:10.1016/j.celrep.2023.112866
18. Ruiyang B, Panayi A, Ruifang W, Peng Z, Siqi F. Adiponectin in psoriasis and its comorbidities: a review. Lipids Health Dis. 2021;20(1):87. doi:10.1186/s12944-021-01510-z
19. Wang Y, Wang X, Lau WB, et al. Adiponectin inhibits tumor necrosis factor-α–induced vascular inflammatory response via caveolin-mediated ceramidase recruitment and activation. Circ Res. 2014;114(5):792–805. doi:10.1161/CIRCRESAHA.114.302439
20. Huang Q, Wang Y, Gu B, Xu Y. Whether the risk of gestational diabetes mellitus is affected by TNF-α, IL-6, IL-10 or ADIPOQ polymorphisms: a meta-analysis. Diabetol Metab Syndr. 2020;12(1):81. doi:10.1186/s13098-020-00582-8
21. Lee YA, Choi HM, Lee SH, et al. Synergy between adiponectin and interleukin-1beta on the expression of interleukin-6, interleukin-8, and cyclooxygenase-2 in fibroblast-like synoviocytes. Exp Mol Med. 2012;44(7):440–447. doi:10.3858/emm.2012.44.7.049
22. Słuczanowska-Głabowska S, Staniszewska M, Marchlewicz M, et al. Adiponectin, leptin and resistin in patients with Psoriasis. J Clin Med. 2023;12(2):663. doi:10.3390/jcm12020663
23. Gerdes S, Pinter A, Biermann M, Papavassilis C, Reinhardt M. Adiponectin levels in a large pooled plaque psoriasis study population. J Dermatol Treat. 2020;31(5):531–534. doi:10.1080/09546634.2019.1621979
24. Suh JH, Lee Y, Ohn J, et al. Adiponectin-derived pentapeptide ameliorates psoriasiform skin inflammation by suppressing IL-17 production in gammadelta T cells. J Dermatol Sci. 2022;106(1):45–52. doi:10.1016/j.jdermsci.2022.03.003
25. Campanati A, Ganzetti G, Giuliodori K, et al. Serum levels of adipocytokines in psoriasis patients receiving tumor necrosis factor-alpha inhibitors: results of a retrospective analysis. Int J Dermatol. 2015;54(7):839–845. doi:10.1111/ijd.12706
26. Ouchi N, Kihara S, Nishizawa H, et al. Reciprocal association of C-reactive protein with adiponectin in blood stream and adipose tissue. Circulation. 2003;107(5):671–674. doi:10.1161/01.CIR.0000055188.83694.B3
27. Fantuzzi G. Adiponectin and inflammation: consensus and controversy. J Allergy Clin Immunol. 2008;121(2):326–330. doi:10.1016/j.jaci.2007.10.018
28. Kaza M, Tsentidis C, Vlachopapadopoulou E, et al. The effect of metabolic profile on leptin, adiponectin, and hs-crp in children and adolescents with type 1 diabetes. Children. 2022;9(8):1162. doi:10.3390/children9081162
29. Menezes AMB, Oliveira PD, Wehrmeister FC, et al. Association of modifiable risk factors and IL-6, CRP, and adiponectin: findings from the 1993 birth cohort, Southern Brazil. PLoS One. 2019;14(5):e0216202. doi:10.1371/journal.pone.0216202
30. Baran A, Flisiak I, Jaroszewicz J, Świderska M. Effect of psoriasis activity on serum adiponectin and leptin levels. Post Dermatol Alergol. 2015;32(2):101–106. doi:10.5114/pdia.2014.40960
31. Ghoshal K, Chatterjee T, Chowdhury S, Sengupta S, Bhattacharyya M. Adiponectin genetic variant and expression coupled with lipid peroxidation reveal new signatures in diabetic dyslipidemia. Biochem Genet. 2021;59(3):781–798. doi:10.1007/s10528-021-10030-5
32. Xiong J, He J, Zhu J, et al. Lactylation-driven METTL3-mediated RNA m6A modification promotes immunosuppression of tumor-infiltrating myeloid cells. Mol Cell. 2022;82(9):1660–1677. doi:10.1016/j.molcel.2022.02.033
33. Wang N, Wang W, Wang X, et al. Histone lactylation boosts reparative gene activation post-myocardial infarction. Circ Res. 2022;131(11):893–908. doi:10.1161/CIRCRESAHA.122.320488
34. Zhang Q, Cao X. Epigenetic remodeling in innate immunity and inflammation. Annu Rev Immunol. 2021;39(1):279–311. doi:10.1146/annurev-immunol-093019-123619
35. Liberti MV, Locasale JW. Histone lactylation: a new role for glucose metabolism. Trends Biochem Sci. 2020;45(3):179–182. doi:10.1016/j.tibs.2019.12.004
36. Yang J, Luo L, Zhao C, et al. A positive Feedback Loop between Inactive VHL-triggered histone lactylation and pdgfrβ signaling drives clear cell renal cell carcinoma progression. Int J Biol Sci. 2022;18(8):3470–3483. doi:10.7150/ijbs.73398
37. Chen J, Zhang M, Liu Y, et al. Histone lactylation driven by mROS-mediated glycolytic shift promotes hypoxic pulmonary hypertension. J Mol Cell Biol. 2023;14(12). doi:10.1093/jmcb/mjac073
38. Chen L, Huang L, Gu Y, Cang W, Sun P, Xiang Y. Lactate-lactylation hands between metabolic reprogramming and Immunosuppression. Int J Mol Sci. 2022;23:19.
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


