Back to Journals » International Medical Case Reports Journal » Volume 16
Hilar Abnormality in the Left Lung: Left Pulmonary Artery Posterior to the Left Mainstem Bronchus
Authors Krylova SV, Glickman S, Kalam A, Chemakin K, Yi J, Forrester L, Mishall P, Pinkas A
Received 1 September 2022
Accepted for publication 7 March 2023
Published 10 March 2023 Volume 2023:16 Pages 135—139
DOI https://doi.org/10.2147/IMCRJ.S388320
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ronald Prineas
Sofia V Krylova, Sara Glickman, Ali Kalam, Katherine Chemakin, Joseph Yi, Linda Forrester, Priti Mishall, Adi Pinkas
Department of Pathology, Albert Einstein College of Medicine, Bronx, NY, 10461, USA
Correspondence: Adi Pinkas, Department of Pathology, Albert Einstein College of Medicine, 1300 Morris Park Avenue, F620S, Bronx, NY, 10461, USA, Email [email protected]
Abstract: The thoracic cavity contains vital cardiovascular and pulmonary structures. Few congenital anatomical variations in the bronchial tree and pulmonary vasculature have been reported. Understanding such variants is crucial during surgical procedures that involve the thorax. During routine dissection of an 89-year-old male cadaver as part of a first-year anatomy course, an anomaly of the bronchial tree was discovered. The left lung hilum was notable for the pulmonary artery being posterior to the mainstem bronchus. The case report describes normal lung development and anatomy and the significance of this novel variation in which has not been previously described in the literature.
Keywords: pulmonary artery, bronchus, lung, pulmonary vein, thoracic region
Introduction
Detailed knowledge and understanding of the normal anatomy of the thorax is essential for surgeons and radiologists alike. Furthermore, since the chest cavity contains many vital cardiovascular and pulmonary structures, it is critical for physicians to be well versed in abnormal anatomy of the region in order to minimize the risk of adverse intraoperative events. Abnormalities of thoracic organ morphology and arrangement can be caused by both acquired and developmental processes, with the latter leading to the more structurally unpredictable outcomes in patients. Some examples of acquired pathologies affecting anatomy of the thorax are neoplasms, infections, and trauma.1 The list of congenital defects leading to organ and vessel anomalies is much more extensive and variable in severity. One surgically relevant category is congenital branching abnormalities of the bronchial tree and pulmonary vasculature.
Thoracic structures most susceptible to anatomic and positional variabilities are the airways and the great vessels, pulmonary vasculature in particular.2 Normally, the trachea is located posteriorly to the great vessels from its start at the level of the C6 cervical vertebra until its bifurcation into the right and left mainstem bronchi at T4-T5 vertebral level. The bronchi pass in front of the descending aorta, and their positions in relation to the pulmonary vessels are variable based on the laterality.3 The pulmonary trunk arises from the right ventricle of the heart and initially progresses anteriorly and then posterolaterally on the left side of the ascending aorta. At the level of the aortic arch, the pulmonary trunk bifurcates into the right and left pulmonary arteries. The right pulmonary artery passes under the aortic arch and enters the hilum of the right lung in anterior position to the right bronchus. On the other hand, the left pulmonary artery enters the hilum of the left lung superiorly to the left bronchus.2
Branching abnormalities and malformations of the airways have been consistently reported in the literature. Variations in tracheobronchial tree have been estimated to appear in as much as 12% of patients on routine bronchoscopies and computer tomographies.4 Although the most common anatomical variability occurs at the level of lobar and segmental bronchi, abnormalities at the level of the mainstem bronchi and trachea, such as formations of tracheal bronchus, accessory cardiac bronchus, and bridging bronchi that supply the contralateral lobe, also exist.5–8 More extreme examples of pulmonary structure abnormalities are aberrant lung formations, including congenital pulmonary airway malformations and complete agenesis of the lung.9,10
Cases of anatomical variants of pulmonary vasculature have also been noted.2 One subset of described abnormalities is aberrant blood supply to the lungs by major systemic arteries, including left subclavian, internal mammary, and celiac.11 Other cases depicted variations in pulmonary venous drainage, such as right pulmonary veins passing behind, instead of in front of, the pulmonary artery.12 However, the greatest variability has been reported in segmental and subsegmental arterial branching, especially in the left lung.
Interestingly, given the multitude of possible bronchial and vascular branching patterns, the spatial relationships of pulmonary arteries and bronchi at the hila of the lungs have been reported to the be constant.2 In the right lung, regardless of the number of branches, the arteries are said to be positioned anteriorly to the bronchi, and in the left lung – superiorly. In the present case, we report an anatomical variation which raises questions about the ubiquity of this assumption: left pulmonary artery enters the hilum of the left lung in the posterior position to the left main bronchus.
Materials and Methods
This project was completed according to the departmental and institutional ethical and research guidelines. Ethical exemption has been granted for collecting and publishing data regarding donor bodies at Albert Einstein College of Medicine to enable scholarship and dissemination of knowledge. The thoracic region of an 89-year-old male cadaver was dissected by students at Albert Einstein College of Medicine, Bronx, NY, as part of a routine first-year anatomy course. A bone saw was used to make a vertical incision through the anterior aspect of the ribs. The parietal pleura was dissected and the pulmonary cavity exposed. The heart and lungs were removed using a scalpel. At this time, students identified a left bronchus in an abnormal position – anteriorly to the left pulmonary artery. The pulmonary trunk originated from the right ventricle of the heart and divided under the aortic arch, as normal. However, the left pulmonary artery then travelled inferiorly to the left main bronchus and continued along its posterior side, eventually entering the hilum of the left lung in the posterior position to the bronchus.
This research is performed according to the Institutional guidelines on cadaver research.
Results
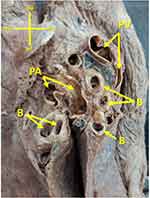
After the heart and lungs were removed from the thoracic cavity of the cadaver, the abnormalities of the left pulmonary artery and left bronchus, as well as hilum of the left lung were exposed. The spatial relationships between pulmonary and vascular structures on the right side of the body were normal: the right pulmonary artery travelled anteriorly to the right bronchus, entering the hilum of the right lung in the appropriate position. However, on the left, the pulmonary artery, instead of travelling above the main bronchus, travelled inferiorly and then posteriorly to it. Subsequently, the hilum of the left lung displayed a deviation from the expected anatomy: the entrance of the pulmonary artery was located in the posterior position to the bronchus, instead of the expected anterior (Figure 1.)
Discussion
The pulmonary system can be broken down into two major components – the vasculature, which consists of arteries, veins, and capillaries, and the airways. The airways can be further separated into organs of air conduction (trachea, bronchi, bronchiole) and gas exchange (alveoli). Such great functional complexity and variability of tissue types in the pulmonary system results from a tightly regulated multi-step process of embryogenesis.
Based on the morphological characteristics of the airways and pulmonary vasculature, prenatal development of the lung can be separated into four stages: embryonic, pseudoglandular, canalicular, and saccular.13 The two stages relevant to our anatomical abnormality of spatial relationship between the bronchus and the pulmonary artery are the embryonic and the pseudoglandular.
During the embryonic stage (weeks 4–7), the lung arises as a ventral bud from the foregut at the end of the fourth week of development. By week 41, the trachea and segmental bronchi are formed. The initial pulmonary vasculature is formed by vasculogenesis surrounding the early lung buds; as the airways branch and proliferate, the pulmonary arteries, arising from the sixth pair of aortic arches, follow the bronchi in their development.13,14
The pseudoglandular stage occurs between weeks 5 and 17. This is our main area of interest, since during this time period the formation of the bronchial tree, as well as further development of the pulmonary vasculature, takes place.13 Lobar portions of the airway are formed by the end of week six, and segmental branching becomes evident by the end of week seven. The vasculature continues to follow the airways in its branching pattern and by the end of pseudoglandular stage completes its development.14
In our dissected cadaver, we observed an abnormal anatomical relationship between the left pulmonary artery and its corresponding left main bronchus. Instead of the normal entry of the hilum anteriorly to the bronchus, the pulmonary artery entered posteriorly. This specific congenital abnormality points to an aberrant developmental process during either the embryonic or the pseudoglandular stage, since the bronchi and their vasculature develop within those time frames.
While the exact mechanisms of congenital lung abnormalities have not yet been fully elucidated, it has been proposed that the causes lie in dysregulations of early molecular signaling that guide proper development and differentiation of the tissues.15 Some of the mechanisms regulating fetal lung development during the embryogenic stage are Sonic Hedgehog (SHH), Bone Morphogenetic protein 4 (BMP4), Sprouty 2 (SPRY2), and Wnt signaling pathways. During the pseudoglandular stage, interactions between fibroblast growth factors, such as FBF10, FBF9, and FBF7, and their receptor, FGFR2b, control the growth of the bronchial tree.16 Further research in these molecular pathways might shed light on mechanisms of congenital pulmonary abnormalities in the future.
In order to gain a better understanding of clinical significance of pulmonary variations like the one presented here, we attempted to identify reports of similar cases in the literature. Most frequently noted abnormalities consisted of variable numbers of lung lobes and branches of the pulmonary vasculature.17–27 Fewer studies depicted anomalies in the pulmonary hilum, and even then, their focus remained on the numerical variations of the entering structures, rather than their topographical relationships.24–26
Given that variability of pulmonary structures is not a rare finding in the patient population, the fact that none of the studies reported abnormal locations of pulmonary arteries and bronchi in the hilum signifies uniqueness of our case. Therefore, our report serves a clinically meaningful function of questioning the common belief that the spatial relationship between arteries and bronchi in the hilum is constant. In the world of increasing preference for minimally invasive procedures, during which the regional anatomy becomes less clearly visible compared to open surgeries, it is important for thoracic surgeons to be aware of potential variability in hilar topography. For instance, Watanabe reported that a critical measurement in preventing unplanned vascular transections during video-assisted thoracoscopic surgeries is being well aware of anatomical structures in the surgical area.27 Amore et al further noted the importance of awareness of pulmonary artery anatomy abnormalities for avoiding intraoperative complications during major lung resections.28 Procedures implicated in surgical treatment of pulmonary emboli similarly require deep understanding of normal and abnormal anatomy by physicians and radiologists. Finally, unexpected positioning of pulmonary vasculature in the hilum can create additional challenges for surgeons performing hilar mass and lymph node resections and biopsies.
Conclusion
Information presented in this case report is a useful addition to the body of existent literature on variability of pulmonary anatomy, since it provides an evidence of an alternate observation to a commonly held assumption about topography of the hilum. To our knowledge, this is the first report of the pulmonary artery entering the hilum of the left lung in a posterior position to the bronchus. Being aware of this anatomical variability can be a helpful tool for thoracic surgeons to minimize the risk of intraoperative complications.
Acknowledgments
The authors would like to thank the individual whose body allowed for this discovery and helped advance physician training and scientific knowledge.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Stoddard HJ, Lowery DR. Anatomy, Thorax, Mediastinum. StatPearls; 2021.
2. Kandathil A, Chamarthy M. Pulmonary vascular anatomy & anatomical variants. Cardiovasc Diagn Ther. 2018;8(3):201–207. doi:10.21037/cdt.2018.01.04
3. Ugalde P, Miro S, Fréchette É, et al. Correlative anatomy for thoracic inlet; glottis and subglottis; trachea, carina, and main bronchi; lobes, fissures, and segments; hilum and pulmonary vascular system; bronchial arteries and lymphatics. Thorac Surg Clin. 2007;17(4):639–659. doi:10.1016/j.thorsurg.2007.04.004
4. Wooten C, Patel S, Cassidy L, et al. Variations of the tracheobronchial tree: anatomical and clinical significance. Clin Anat. 2014;27(8):1223–1233. doi:10.1002/ca.22351
5. Chassagnon G, Morel B, Carpentier E, et al. Tracheobronchial branching abnormalities: lobe-based classification scheme. Radiographics. 2016;36(2):358–373. doi:10.1148/rg.2016150115
6. Ghaye B, Szapiro D, Fanchamps J-M, et al. Congenital bronchial abnormalities revisited. Radiographics. 2001;21(1):105–119. doi:10.1148/radiographics.21.1.g01ja06105
7. Zhao X, Ju Y, Liu C, et al. Bronchial anatomy of left lung: a study of multi-detector row CT. Surg Radiol Anat. 2009;31(2):85–91. doi:10.1007/s00276-008-0404-8
8. Rishavy TJ, Goretsky MJ, Langenburg SE, et al. Anterior bridging bronchus. Pediatr Pulmonol. 2003;35(1):70–72. doi:10.1002/ppul.10205
9. Leblanc C, Baron M, Desselas E, et al. Congenital pulmonary airway malformations: state-of-The-art review for pediatrician’s use. Eur J Pediatr. 2017;176(12):1559–1571. doi:10.1007/s00431-017-3032-7
10. Palla J, Sockrider MM. Congenital lung malformations. Pediatr Ann. 2019;48(4):e169–e174. doi:10.3928/19382359-20190326-02
11. Fernandez-Martorell P, Yoo SJ, Benson LN. An unusual form of anomalous systemic arterial supply to the left lung. Cardiol Young. 2006;16(3):305–307. doi:10.1017/S1047951106000497
12. Otsuki Y, Go T, Chang SS, et al. Anomalous right upper lobe pulmonary veins draining posterior to the pulmonary artery. Gen Thorac Cardiovasc Surg. 2019;67(10):901–903. doi:10.1007/s11748-019-01078-7
13. Schittny JC. Development of the lung. Cell Tissue Res. 2017;367(3):427–444. doi:10.1007/s00441-016-2545-0
14. Smith LJ, McKay KO, van Asperen PP, et al. Normal development of the lung and premature birth. Paediatr Respir Rev. 2010;11(3):135–142. doi:10.1016/j.prrv.2009.12.006
15. Maeda Y, Dave V, Whitsett JA. Transcriptional control of lung morphogenesis. Physiol Rev. 2007;87(1):219–244. doi:10.1152/physrev.00028.2006
16. Swarr DT, Morrisey EE. Lung endoderm morphogenesis: gasping for form and function. Annu Rev Cell Dev Biol. 2015;31(1):553–573. doi:10.1146/annurev-cellbio-100814-125249
17. Polaczek MM. Anatomic variations of pulmonary vessels relevant with regard to lung tissue resections - literature review and personal experiences; 2013.
18. Prakash P, Bhardwaj AK, Shashirekha M, et al. Lung morphology: a cadaver study in Indian population. Ital J Anat Embryol. 2010;115(3):235–240.
19. Nene AR, Gajendra KS, Sarma MVR. Lung lobes and fissures: a morphological study. J Exp Clin Med. 2011;5:30–38.
20. Berkmen T, Berkmen YM, Austin JH. Accessory fissures of the upper lobe of the left lung: CT and plain film appearance. AJR Am J Roentgenol. 1994;162(6):1287–1293. doi:10.2214/ajr.162.6.8191982
21. Frija J, Schmit P, Katz M, et al. Computed tomography of the pulmonary fissures: normal anatomy. J Comput Assist Tomogr. 1982;6(6):1069–1074. doi:10.1097/00004728-198212000-00004
22. Medlar EM. Variations in interlobar fissures. Am J Roentgenol Radium Ther. 1947;57(6):723–725.
23. Otsuji H, Uchida H, Maeda M, et al. Incomplete interlobar fissures: bronchovascular analysis with CT. Radiology. 1993;187(2):541–546. doi:10.1148/radiology.187.2.8475304
24. Deepak Khedekar SH, Hattangdi S. Morphological variations of the lung: a cadaveric study in Mumbai population. Int J Anat Res. 2017;5(3):4313–4316. doi:10.16965/ijar.2017.320
25. George BM, Nayak SB, Marpalli S. Morphological variations of the lungs: a study conducted on Indian cadavers. Anat Cell Biol. 2014;47(4):253–258. doi:10.5115/acb.2014.47.4.253
26. Subhalakshmi Wahengbam HRD, Sharma GT, Gangmei ND, Gangmei G, Devi ND. Morphological study of human lungs. Int J Anat Res. 2019. 7. 2.1:6345–6352. doi:10.16965/ijar.2019.115
27. Watanabe A. Troubleshooting in thoracoscopic anatomical lung resection for lung cancer. Surg Today. 2021;51(5):669–677. doi:10.1007/s00595-020-02136-x
28. Amore D, Casazza D, Caterino U, et al. Variations in the branching patterns of pulmonary artery during thoracoscopic pulmonary resection. Surg Radiol Anat. 2021;43(8):1331–1336. doi:10.1007/s00276-021-02677-x
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.