Back to Journals » Neuropsychiatric Disease and Treatment » Volume 16
Higher Plasma APOC-III Was Associated with a Slower Reduction of β-Amyloid Levels in Cerebrospinal Fluid Among Older Individuals Without Dementia
Authors Zhang X
Received 17 November 2019
Accepted for publication 1 March 2020
Published 5 May 2020 Volume 2020:16 Pages 1139—1144
DOI https://doi.org/10.2147/NDT.S238985
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jun Chen
Xiaoyan Zhang On behalf of the Alzheimer’s Disease Neuroimaging Initiative
Department of Child Healthcare, The Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University, Wenzhou, People’s Republic of China
Correspondence: Xiaoyan Zhang
The Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University, 109 Xueyuan West Road, Wenzhou 325027, Zhejiang Province, People’s Republic of China
Email [email protected]
Purpose: Although emerging evidence has suggested that apolipoprotein C-III (APOC-III) is involved in the pathogenesis of Alzheimer’s disease (AD), the association of APOC-III with longitudinal changes in cerebrospinal fluid (CSF) AD pathologies (β-amyloid (Aβ 42) and tau proteins) is not clear. In the present study, we aimed to examine whether plasma APOC-III levels are associated with longitudinal changes in CSF Aβ 42, total-tau (t-tau), and phosphorylated-tau (p-tau) levels among older individuals without dementia.
Patients and Methods: Linear mixed models were fitted with plasma APOC-III used as a predictor for longitudinal changes in CSF AD biomarkers over a 7-year period. Data were obtained from the Alzheimer’s Disease Neuroimaging Initiative database, and 195 older individuals without dementia (47 subjects with normal cognition (NC) and 148 subjects with mild cognitive impairment (MCI)) with baseline plasma APOC-III measurements were included.
Results: Among older individuals without dementia, we found that the tertiles of plasma APOC-III were associated with changes in CSF Aβ 42, but not t-tau or p-tau. Specifically, the CSF Aβ 42 reduction for individuals in the highest plasma APOC-III tertile was significantly slower compared with those in the middle tertile, whereas no other pairwise difference was found to be statistically significant.
Conclusion: Among older individuals without dementia, higher plasma APOC-III levels were associated with slower declines in CSF Aβ 42.
Keywords: Alzheimer’s disease, apolipoprotein C-III, beta-amyloid, tau proteins, longitudinal study
Introduction
Emerging evidence has suggested that apolipoprotein C-III (APOC-III) is involved in the pathogenesis of Alzheimer’s disease (AD).1–9 For example, plasma APOC-III levels were found to be significantly reduced in patients with AD.6 In cross-sectional studies, higher APOC-III levels in plasma were correlated with better cognitive performance.3,6 Additionally, a recent longitudinal study found that higher APOC-III levels in CSF were associated with a slower cognitive decline in individuals with mild cognitive impairment (MCI),1 further supporting that APOC-III may be neuroprotective. A previous study suggested that this beneficial effect of APOC-III on cognition may be because APOC-III is an Aβ-binding protein that can promote Aβ efflux and indirectly reduce the accumulation of Aβ in brain.6 However, to our knowledge, no studies have attempted to examine the longitudinal associations of APOC-III levels in plasma with changes in CSF AD pathologies (including Aβ42, total tau (t-tau) and phosphorylated tau (p-tau)) in older individuals without dementia.
In the cross-sectional analysis, baseline plasma APOC-III levels were analyzed in relation to baseline CSF Aβ42, t-tau and p-tau levels in older individuals without dementia. Further, the longitudinal analysis was conducted with baseline plasma APOC-III used as a predictor for changes in CSF Aβ42, t-tau and p-tau levels over a 7-year period.
Patients and Methods
Alzheimer’s Disease Neuroimaging Initiative (ADNI)
This longitudinal study used data extracted from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu; 3 October 2018). The ADNI study was initiated in 2003 with the primary goal of examining whether a variety of markers, such as neuropsychological assessments, serial MRI, PET, and other fluid biomarkers, could be combined to predict the progression of MCI and early AD. Each participant of the ADNI study provided written informed consent, and each ADNI site obtained local institutional review board approval.
Participants
Patients inclusion criteria have been previously described elsewhere,10 and can be found at the ADNI website (adni.loni.usc.edu). In brief, participants with NC had a Mini-Mental State Examination (MMSE)11 score ≥24, and a Clinical Dementia Rating (CDR)12 score of 0. Participants with MCI had an MMSE ≥ 24, a CDR of 0.5, objective memory deficits as measured by delayed recall scores of the Wechsler Memory Scale Logical memory II, preserved activities of daily living, and an absence of dementia.
In this longitudinal study, we included subjects who met criteria for NC and MCI and had baseline plasma APOC-III samples and follow-up quantifications of CSF Aβ42, t-tau, and p-tau levels. At baseline, there was a total of 195 non-demented older individuals, including 47 participants with NC and 148 participants with MC. As shown in Table 1, annual levels of CSF Aβ42, t-tau, and p-tau were examined for up to 7 years.
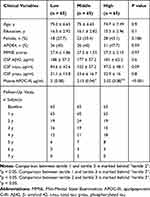 |
Table 1 Demographic and Clinical Data by Tertiles of Plasma APOC-III Levels |
Determination of APOC-III in Plasma
Plasma APOC-III levels were measured using xMAP multiplex panel (MyriadRBM),7 details of which can be found at the ADNI website (http://adni.loni.ucla.edu/). The file “Biomarkers Consortium ADNI plasma QC Multiplex data” was downloaded from the ADNI website (3 October 2018). To better approximate a normal distribution, the APOC-III analyte was natural log transformed. Study subjects were divided into tertile groups according to APOC-III levels. Plasma levels of APOC-III were [1.79, 2.09], [2.09, 2.22], and [2.22, 2.61] µg/mL in the lowest, middle and highest tertiles, respectively.
Determination of CSF Aβ42, t-tau, and p-tau
CSF levels of Aβ42, t-tau, and p-tau were determined with the multiplex xMAP Luminex platform,13 details of which can be found at the ADNI website (adni.loni.usc.edu). The file “UPENNBIOMK_MASTER” was extracted from the ADNI dataset in October 2018. In the present study, yearly CSF Aβ42, t-tau, and p-tau levels were examined for up to 7 years (Table 1).
Statistical Analyses
First, we applied Kruskal–Wallis tests and x2 tests to assess differences in demographic and clinical variables across the tertiles of APOC-III levels. Second, to examine the cross-sectional relationships between APOC-III and CSF AD biomarkers, Kruskal–Wallis tests were conducted. Third, to evaluate the association of baseline plasma APOC-III with changes in CSF AD biomarkers over time, linear mixed models were fitted for each CSF AD biomarker (CSF Aβ42, t-tau, and p-tau). All these models were adjusted for age, sex, education and APOE4 genotype. Further, each model included a random intercept for each subject. All statistical analyses were conducted using R software (V.3.6.0).
Results
Demographic and Clinical Variables
The demographic and clinical variables of the study participants by tertiles of plasma APOC-III are demonstrated in Table 1. We did not find significant differences in age or educational attainment across tertiles. Characteristics (sex, percentage of subjects with the APOE4 genotype or MMSE11 scores) did not differ by APOC-III tertiles (Table 1). In addition, we did not find a significant difference in plasma APOC-III levels between males and females (t = −1.68, p = 0.095). The numbers of subjects present at each follow-up visit are also listed in Table 1.
Associations of Plasma APOC-III Levels with CSF AD Pathologies
To examine the cross-sectional associations of tertiles of plasma APOC-III with CSF AD pathologies, Kruskal–Wallis tests were performed. However, no significant association of tertiles of plasma APOC-III with CSF AD pathologies was observed (all p > 0.05; Table 1, Figure 1).
Longitudinal Change Models
First, to evaluate the association of baseline plasma APOC-III levels (categorized as tertiles) with changes in CSF AD biomarkers over time, linear mixed models were fitted. We found that tertiles of plasma APOC-III were associated with changes in CSF Aβ42, but not t-tau or p-tau (Table 2 and Figure 2). In addition, a plot of individual CSF Aβ42 concentrations over time across the tertiles has also been displayed (Figure S1). Specifically, as shown in Table 3 and Figure 2, the CSF Aβ42 reduction for individuals in the highest APOC-III tertile was significantly slower compared with those in the middle (the middle tertile of APOC-III – the highest tertile of APOC-III: estimate = −2.151, SE = 0.909, p = 0.0484), whereas no other pairwise difference was found to be statistically significant (all p > 0.05, Table 3) after correcting for multiple comparisons using Tukey method.
 |
Table 2 Summary of Linear Mixed Models Examining the Associations of Plasma APOC-III with Changes in CSF AD Pathologies |
 |
Table 3 Multiple Comparisons Across the Plasma APOC-III Groups |
In addition, we examined the association of plasma APOC-III with changes in CSF biomarkers from baseline to 1-year follow-up due to the fact that this approach may seem to have the least “survival” bias (Please see supplementary information: Table S1 and Figure S2).
Discussion
In the present study, we investigated the association of APOC-III levels in plasma with changes in CSF AD biomarkers over time among older individuals without dementia. We found that the tertiles of plasma APOC-III were associated with changes in CSF Aβ42, but not t-tau or p-tau. Specifically, the CSF Aβ42 reduction for individuals in the highest plasma APOC-III tertile was significantly slower compared with those in the middle tertile, whereas no other pairwise difference was found to be statistically significant.
In terms of the biology of APOC-III, it is primarily expressed in the liver and intestine and has an important role in lipid metabolism.14 An increasing amount of evidence has suggested that APOC-III might also be involved in the pathogenesis of AD. For instance, the APOC-III 3017G allele was found to be associated with decreased risk of AD among individuals without the presence of the APOE4 genotype.9 In cross-sectional studies, levels of APOC-III in plasma were reduced in patients with AD6 and were positively correlated with cognitive performance.3,6 Additionally, one recent prospective and longitudinal study showed that higher CSF APOC-III levels were associated with a slower cognitive decline in individuals with MCI.1 However, the mechanisms underlying this potentially neuroprotective effect of APOC-III on cognition remain elusive. One approach for examining the mechanism by which APOC-III affects cognitive decline is to investigate the association of APOC-III with longitudinal reductions in CSF Aβ42. In the present study, we provided in vivo evidence that higher CSF APOC-III levels may decelerate reduction in CSF Aβ42 in non-demented older adults, supporting the notion that APOC-III may be a potential agent that can slow disease progression in the early stage of AD. However, the precise mechanism is not clear. Levels of Aβ42 in CSF demonstrated a substantial reduction in patients with AD as reported in previous studies,15–19 probably resulted from aggregation and accumulation of Aβ42 in brain or a failure of Aβ42 clearance which contributes to decreased amount of Aβ42 proteins transport to CSF.20 APOC-III, an amyloid-binding protein, may promote Aβ efflux and indirectly reduce the deposition of Aβ in the brain parenchyma.6 However, further preclinical studies are needed to clarify the precise mechanisms underlying the relationship between APOC-III and amyloid pathology. Taken together, our data supported the notion that APOC-III may be a major pathogenic factor of AD after Aβ and tau.
Several limitations of this longitudinal study should be noted. First, the findings of this study should be interpreted with caution because of a potential selection bias. Further studies, especially population-based studies, should be conducted to replicate our results. In addition, the observational nature of our study limits our ability to differentiate whether increased APOC-III leads to, results from, or is just correlated with changes in amyloid pathology.
In conclusion, among older individuals without dementia, higher plasma APOC-III levels were associated with slower declines in CSF Aβ42. It should be explored in further studies if modification of APOC-III level slows disease progression.
Acknowledgments
Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
Data used in preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. A complete listing of ADNI investigators can be found at: http://adni.loni.usc.edu/wp-content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf
Disclosure
The author reports no conflict of interest in this work.
References
1. Wang Q, Zhou W, Zhang J. Higher Apolipoprotein C-III levels in cerebrospinal fluid are Associated with slower cognitive decline in mild cognitive impairment. J Alzheimers Dis. 2019;67(3):961–969. doi:10.3233/JAD-181096
2. Koch M, DeKosky ST, Fitzpatrick AL, et al. Apolipoproteins and Alzheimer’s pathophysiology. Alzheimer Dement. 2018.
3. Muenchhoff J, Song F, Poljak A, et al. Plasma apolipoproteins and physical and cognitive health in very old individuals. Neurobiol Aging. 2017;55:49–60. doi:10.1016/j.neurobiolaging.2017.02.017
4. Das M, Gursky O. Amyloid-forming properties of human apolipoproteins: sequence analyses and structural insights. Adv Exp Med Biol. 2015;855(undefined):175–211.
5. Lin Q, Cao Y, Gao J. Decreased expression of the APOA1-APOC3-APOA4 gene cluster is associated with risk of Alzheimer’s disease. Drug Des Devel Ther. 2015;9:5421–5431. doi:10.2147/DDDT.S89279
6. Shih YH, Tsai KJ, Lee CW, et al. Apolipoprotein C-III is an amyloid-beta-binding protein and an early marker for Alzheimer’s disease. J Alzheimers Dis. 2014;41(3):855–865. doi:10.3233/JAD-140111
7. Mattsson N, Insel P, Nosheny R, et al. Effects of cerebrospinal fluid proteins on brain atrophy rates in cognitively healthy older adults. Neurobiol Aging. 2014;35(3):614–622. doi:10.1016/j.neurobiolaging.2013.08.027
8. Song F, Poljak A, Crawford J, et al. Plasma apolipoprotein levels are associated with cognitive status and decline in a community cohort of older individuals. PLoS One. 2012;7(6):e34078. doi:10.1371/journal.pone.0034078
9. Sun Y, Shi J, Zhang S, et al. The APOC3 SstI polymorphism is weakly associated with sporadic Alzheimer’s disease in a Chinese population. Neurosci Lett. 2005;380(3):219–222. doi:10.1016/j.neulet.2005.01.038
10. Aisen PS, Petersen RC, Donohue MC, et al. Clinical core of the Alzheimer’s disease neuroimaging initiative: progress and plans. Alzheimer Dement. 2010;6(3):239–246. doi:10.1016/j.jalz.2010.03.006
11. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi:10.1016/0022-3956(75)90026-6
12. Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43(11):2412–2414. doi:10.1212/WNL.43.11.2412-a
13. Shaw LM, Vanderstichele H, Knapik-Czajka M, et al. Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Ann Neurol. 2009;65(4):403–413. doi:10.1002/ana.21610
14. Norata GD, Tsimikas S, Pirillo A, Catapano AL. Apolipoprotein C-III: from pathophysiology to pharmacology. Trends Pharmacol Sci. 2015;36(10):675–687. doi:10.1016/j.tips.2015.07.001
15. Motter R, Vigo-Pelfrey C, Kholodenko D, et al. Reduction of beta-amyloid peptide42 in the cerebrospinal fluid of patients with Alzheimer’s disease. Ann Neurol. 1995;38(4):643–648. doi:10.1002/ana.410380413
16. Leinenbach A, Pannee J, Dulffer T, et al. Mass spectrometry-based candidate reference measurement procedure for quantification of amyloid-beta in cerebrospinal fluid. Clin Chem. 2014;60(7):987–994. doi:10.1373/clinchem.2013.220392
17. Olsson A, Vanderstichele H, Andreasen N, et al. Simultaneous measurement of beta-amyloid(1-42), total tau, and phosphorylated tau (Thr181) in cerebrospinal fluid by the xMAP technology. Clin Chem. 2005;51(2):336–345. doi:10.1373/clinchem.2004.039347
18. Wiltfang J, Esselmann H, Bibl M, et al. Highly conserved and disease-specific patterns of carboxyterminally truncated Abeta peptides 1-37/38/39 in addition to 1- 40/42 in Alzheimer’s disease and in patients with chronic neuroinflammation. J Neurochem. 2002;81(3):481–496. doi:10.1046/j.1471-4159.2002.00818.x
19. Andreasen N, Hesse C, Davidsson P, et al. Cerebrospinal fluid beta-amyloid(1-42) in Alzheimer disease: differences between early- and late-onset Alzheimer disease and stability during the course of disease. Arch Neurol. 1999;56(6):673–680. doi:10.1001/archneur.56.6.673
20. Blennow K, Mattsson N, Scholl M, Hansson O, Zetterberg H. Amyloid biomarkers in Alzheimer’s disease. Trends Pharmacol Sci. 2015;36(5):297–309. doi:10.1016/j.tips.2015.03.002
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


