Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Higher IL-9 Level is Associated with Psoriasis Vulgaris Complicated by Metabolic Syndrome
Authors Yan L , Yu C , Zhao Z, Zhang Y, Wang R, Li C
Received 22 May 2023
Accepted for publication 15 August 2023
Published 23 August 2023 Volume 2023:16 Pages 2297—2307
DOI https://doi.org/10.2147/CCID.S422355
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jeffrey Weinberg
Liang Yan,1,2 Chongli Yu,1 Zhenkai Zhao,1 Yuan Zhang,1 Rui Wang,1 Chengxin Li1
1Department of Dermatology, The First Medical Center, Chinese PLA General Hospital, Beijing, 100853, People’s Republic of China; 2Department of Dermatology, General Hospital of Central Theater Command of PLA, Wuhan, Hubei, 430070, People’s Republic of China
Correspondence: Chengxin Li; Rui Wang, Department of Dermatology, The First Medical Center, Chinese PLA General Hospital, Beijing, 100853, People’s Republic of China, Tel +86-01066939315 ; Tel +86-01066939314, Email [email protected]; [email protected]
Purpose: The underlying pathophysiology linking psoriasis vulgaris (PV) and metabolic syndrome (MetS) is not fully understood. The present study aimed to investigate the serum level of interleukin (IL)-9 and tissue levels of IL-9 and its receptor in PV patients with MetS and analyze the correlation of IL-9 levels with psoriasis disease severity and MetS.
Methods: This study enrolled 75 PV patients with MetS, 57 PV patients without MetS, 20 healthy blood donors, and 7 healthy skin donors. Clinical, socio-demographic, and anthropometric data were obtained from all individuals. Fasting blood glucose, insulin, lipid profile levels, and serum levels of IL-9 and IL-17A were measured. The expression of IL-9 and its receptor in skin specimens in PV patients and healthy controls was determined using immunohistochemistry. Normal human epidermal keratinocytes were stimulated with five pro-inflammatory cytokines (tumor necrosis factor-α, oncostatin M, IL-22, IL-17A, and IL-1α) to establish a psoriatic keratinocyte model and subsequently treated with IL-9. Their mRNA levels of antimicrobial peptides and chemokines were measured using quantitative real-time polymerase chain reaction.
Results: Serum level of IL-9 and tissue levels of IL-9 and its receptor were upregulated in PV patients with MetS. IL-9 level was positively correlated to IL-17A level; however, no significant correlation of IL-9 level with psoriasis area severity index was observed. IL-9 level had a positive correlation with the presence of MetS and its components. Correspondingly, IL-9 level positively correlated with waist circumference, body mass index, homeostasis model assessment-insulin resistance, blood pressure, and triglyceride level and negatively correlated with high-density lipoprotein cholesterol level. Additionally, IL-9 stimulated the expression of antimicrobial peptides and chemokines in a psoriatic keratinocyte model.
Conclusion: Our findings confirmed that higher IL-9 level is associated with PV complicated by MetS, suggesting that IL-9 may be a link between PV and MetS.
Keywords: interleukin-9, interleukin-17A, psoriasis vulgaris, metabolic syndrome
A Letter to the Editor has been published for this article.
Introduction
Psoriasis vulgaris (PV) is a prevalent chronic recurrent condition associated with systemic inflammation.1 The interleukin (IL)-23/Th17 pathway is a crucial element in its immunological pathogenesis,2 and IL-17A is a critical effector cytokine in this pathway.3 Metabolic syndrome (MetS) is a common and significant comorbidity in PV.4 It is a collection of metabolic factors that increase the risk of cardiovascular disease (CVD) and type 2 diabetes (T2D).5
MetS is more prevalent in psoriatic patients than in the general population.6,7 The worldwide prevalence of MetS in PV patients was estimated to be 29%.8 The risk of having MetS has a “dose-response” relationship with psoriasis disease severity.9 PV patients with MetS respond less well to treatment than those without MetS.10 Furthermore, MetS is related to an increased risk of incident psoriasis.11 These previous findings all demonstrate a close relationship between psoriasis and MetS. However, the underlying pathophysiology linking them remains unclear.
Previous studies have also demonstrated the potential role of IL-9 in psoriasis. IL-9 is reportedly involved in the Th17 response and angiogenesis in psoriasis.12 A recent study showed that increased IL-9 level has a positive correlation with psoriasis disease severity in psoriatic patients.13 As a pleiotropic cytokine, IL-9 has been linked to MetS components. The serum concentration of IL-9 is elevated in patients with obesity and T2D.14,15 Although these findings suggest that IL-9 is a potential cytokine involved in the association between PV and MetS, no previous study has investigated the relationship between IL-9 and psoriasis complicated by MetS to date.
Hence this study aimed to estimate the serum IL-9 level and tissue levels of IL-9 and IL-9 receptor (IL-9R) in PV patients with MetS, and to analyze the relationship between IL-9 and MetS and the severity of psoriasis.
Materials and Methods
Study Groups
This is a cross-sectional study conducted in the Chinese PLA General Hospital from January 2019 to October 2022. Seventy-five adult PV patients with MetS (PVMS), 57 adult PV patients without MetS (PVNMS), 20 healthy blood donors, and 7 healthy skin donors from the Department of Dermatology were consecutively recruited.
The guideline proposed by the Chinese Dermatology Society was used to diagnose PV. PV patients aged ≥ 18 years who fulfilled the diagnostic criteria for MetS or met none of the criteria were included. Patients with prior systemic treatments such as steroids, immunosuppressants, retinoids, and biologics during the past 3 months and those with other clinical varieties of psoriasis, secondary dyslipidemia, malignancy, hepatic insufficiency, kidney dysfunction, HIV infection, nervous and mental disorders, and pregnancy were excluded.
Based on preliminary experiment outcomes, the sample size was calculated using PASS software (Version 21.0; NCSS Statistical Software, Kaysville, UT, USA). Sample sizes of 55 and 7 were estimated to ensure at least 80% power to detect a difference between the two PV groups and between PV patients with MetS and healthy controls, respectively.
Data and Sample Collection
Relevant data from all individuals, including age, sex, tobacco and alcohol consumption, disease duration, prior treatment, and family history of psoriasis, were collected. Height, weight, waist circumference (WC), body mass index (BMI, kg/m2), and blood pressure were recorded. The psoriasis area severity index (PASI) was applied to evaluate PV severity. The PASI score was calculated based on erythema, infiltration, desquamation, and lesion area.16
After an overnight fast, venous blood samples were collected from each participant for tests of fasting blood glucose (FBG), triglycerides (TG), and high-density lipoprotein cholesterol (HDL-C) levels. Fasting serum insulin level was also measured in PV patients, and the homeostasis model assessment-insulin resistance index (HOMA-IR) was calculated as HOMA-IR = fasting insulin (mU/L) × fasting glucose (mmol/L) / 22.5.17 Serum samples were isolated by centrifugation (1500 rpm, 15 min, 4°C) and kept at −80°C until analysis.
Sixty psoriatic skin tissue samples were collected from the PV patients who underwent skin biopsy for histopathological examination.
Participants with BMI > 25.0 kg/m2 were considered overweight/obese. The study applied the criteria for MetS proposed by the Chinese Diabetes Society (CDS) in 2021.18 Notably, MetS was diagnosed if three of the following criteria were met:
- Hypertension (blood pressure ≥ 140/90 mmHg and/or prior treatment for high blood pressure);
- Hyperglycemia (FBG ≥ 6.1 mmol/L and/or 2-h postprandial blood glucose ≥ 7.8 mmol/L and/or receiving therapy for diabetes mellitus);
- Low HDL-C level (fasting serum HDL-C < 1.04 mmol/L);
- Hypertriglyceridemia (fasting serum triglyceride ≥ 1.7 mmol/L); and
- Central obesity (WC ≥ 90 cm in males and ≥ 85 cm in females).
Enzyme-Linked Immunosorbent Assay (ELISA)
Serum IL-9 and IL-17A levels were tested utilizing ELISA Kits (Neobioscience Technology, Shenzhen, Guangdong, China).
Immunohistochemistry (IHC)
All skin samples were 10% formalin-fixed, paraffin-embedded, and cut at 5 µm. After dewaxing and hydration, antigen retrieval was performed in Tris-ethylenediaminetetraacetic acid buffer (pH 9.0) in a pressure cooker. The slices were treated with a 3% H2O2 solution to block endogenous peroxidase activity and, subsequently, incubated with 5% goat serum (Proteintech, Wuhan, Hubei, China) for 1 h at 22°C. Subsequently, mouse IL-9 monoclonal antibody (1:400 dilution; Cat. no. 66144-1-Ig; Proteintech) and rabbit IL-9R polyclonal antibody (1: 200 dilution; Cat. no. PA5-86324; Invitrogen, Carlsbad, CA, USA) were applied overnight at 4°C. After incubation with an HRP-conjugated secondary antibody (Proteintech) at 22°C for 1 h, the tissue slides were stained with diaminobenzidine and photographed (Digital Slide Scanning System PRECICE 500B; UNIC Technologies, Inc., Suzhou, Jiangsu, China).
The results were quantitatively analyzed according to the extent and intensity of immunostaining by assessing the mean value of integrated optical density (IOD) with the support of image-pro plus software (version 16.0; Media Cybernetics, Inc., Rockville, MD, USA). Five areas of each tissue slide (400×) were randomly selected for IOD calculation, and calibration was done before calculation.
Cell Culture
Normal human epidermal keratinocytes (NHEKs; Cat. no. BNCC340593; BeNa Culture Collection, Beijing, China) were cultured in Dulbecco’s Modified Eagle Medium (Gibco, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (ExCell Bio, Suzhou, Jiangsu, China) and 1% penicillin/streptomycin at 37°C in a humidified chamber with 5% CO2. The psoriatic cell model was established by adding the M5 cocktail (tumor necrosis factor-α, oncostatin M, IL-22, IL-17A, and IL-1α; 10 ng/mL; PeproTech, Cranbury, NJ, USA) to the culture medium for 24 h.
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
Total RNA was extracted from NHEKs using a FastPure Cell Total RNA Isolation Kit (Vazyme, Nanjing, Jiangsu, China). Following reverse transcription, qRT-PCR was performed on a Bio-Rad CFX96 real-time PCR machine using a Taq Pro Universal SYBR qPCR Master Mix Kit (Vazyme). Table 1 lists the primers used for qRT-PCR. Relative quantification of mRNA levels was measured using the 2–ΔΔCt formula based on the expression of internal reference (β-actin).
 |
Table 1 Primer Sequences |
Statistical Analysis
Continuous data are presented as mean ± SD or median (IQR) depending on the data distribution. The Shapiro–Wilk test was used for normality testing. Categorical variables are shown as the number (percentage) of patients. As appropriate, intergroup differences were calculated using the Student’s t-test, Mann–Whitney U-test, or Chi-square test. The correlation between serum IL-9 concentration and other parameters was analyzed using Spearman correlation analysis. Data were considered significant at P < 0.05, and all statistical analyses were conducted using IBM SPSS Statistics for Windows version 26.0 (IBM Corporation, Armonk, N.Y., USA).
Results
Table 2 presents the clinical, socio-demographic, anthropometric, and biochemical characteristics of all participants. The median (IQR) PASI scores in PV patients with and without MetS were 12.3 (6.0–20.1) and 8.0 (5.4–18.1), respectively. No significant differences were found in sex, disease duration, family history of psoriasis, and PASI score between the two PV groups. Individuals in the PVMS group were older than those in the other two groups (both P < 0.001). Additionally, a larger proportion of participants in the PVMS group had a history of smoking and alcohol consumption than the PVNMS (both P < 0.05) and healthy controls (both P > 0.05) groups. As expected, there were significant differences in all metabolic parameters between PV groups with and without MetS.
 |
Table 2 Clinical, Sociodemographic, Anthropometric and Biochemical Characteristics of Psoriatic Vulgaris Patients and Controls |
IL-9 Level Was Higher in PV Patients with MetS Than in Those without MetS
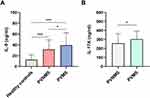
Serum IL-9 and IL-17A levels were significantly higher (both P < 0.05) in the PVMS group than in the PVNMS group (Figure 1A and B). In addition, a significantly higher serum IL-9 level (P < 0.001) was found in the PVNMS group in comparison with healthy controls (Figure 1A).
Considering the differences in age, smoking status, and alcohol use between the two PV groups, the correlations of serum IL-9 level with these three factors were assessed, and the results showed no correlation between IL-9 and age, smoking history or history of alcohol consumption (Table 3).
We investigated whether IL-9 and IL-9R were upregulated in skin specimens of PV patients. As indicated in Figure 2A–D, IL-9 was mainly expressed in inflammatory and endothelial cells in the skin of PV patients with or without MetS, whereas IL-9 expression was rarely detected in normal skin. Notably, higher IL-9 expression was found in PV patients with MetS than in those without MetS.
Similarly, we found strong IL-9R expression in the skin of PV patients with MetS, whereas weak and absent expression was observed in the skin of PV patients without MetS and in normal skin (Figure 3A–D). In addition to inflammatory cells, IL-9R was expressed in keratinocytes in the two subgroups of PV (Figure 3B and C).
Serum IL-9 Level Positively Correlated with IL-17A Level and the Presence of MetS
Table 3 shows the correlations of serum IL-9 level with IL-17A level, clinical features and MetS components in PV patients. Although the IL-9 level did not correlate with PASI (r = 0.136, P = 0.284), it had a positive correlation with the IL-17A level (r = 0.414, P < 0.001). Moreover, the presence of MetS and its components, including central obesity, overweight/obesity, hyperglycemia, high blood pressure, and dyslipidemia, positively correlated with serum IL-9 level (Table 3).
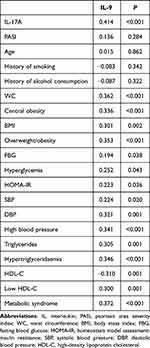 |
Table 3 Spearman Correlation Coefficients Between Serum IL-9 Level and Serum IL-17A Level, Clinical Features and Metabolic Syndrome Components in Psoriasis Vulgaris Patients |
IL-9 Stimulated Antimicrobial Peptides and Chemokines Expression in the Psoriatic Keratinocyte Model
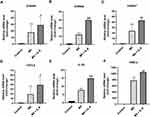
IL-9 promotes the differentiation and amplification of Th17 cells19 and activates the Th17 pathway in psoriasis.12 Interestingly, we also observed IL-9R expression in psoriatic keratinocytes. Thus, we explored whether IL-9 directly affects the psoriatic cell model to further understand the role of IL-9 in the pathogenesis of psoriasis and the association between PV and MetS. Consistent with previous studies,20,21 M5 treatment increased gene expression of antimicrobial peptides and chemokines, including S100 calcium-binding protein A7 (S100A7), S100 calcium-binding protein A8 (S100A8), S100 calcium-binding protein A9 (S100A9), C-X-C motif chemokine ligand 8 (CXCL8), interleukin 1 beta (IL-1β), and human beta-defensin 2 (HBD-2) in NHEKs. Moreover, IL-9 amplified this response by further stimulating antimicrobial peptides and chemokines expression at the transcriptional level (Figure 4A–F).
Discussion
In the current study, serum level of IL-9 and tissue levels of IL-9 and IL-9R were elevated in the PVMS group in contrast with the PVNMS group, whereas those in the PVNMS group were higher than those in healthy controls. Our findings showed that IL-9 is associated with PV complicated by MetS, suggesting that IL-9 may be involved in pathogenic mechanisms linking psoriasis and MetS.
Although no other studies have directly investigated the relationship between IL-9 and psoriasis with MetS, some evidence suggests that IL-9 is linked to psoriasis and MetS components. Consistent with our results, previous studies have shown higher serum IL-9 level in PV patients than those in healthy individuals.13,22,23 Singh et al reported increased IL-9 and IL-9R expression in the skin lesions of psoriasis-like mice and human psoriatic skin in contrast to normal skin.12 The upregulation of IL-9 and IL-9R expression has also been reported in leukocytic infiltrates and the synovium in psoriatic arthritis patients in comparison with osteoarthritis patients.24 In addition, rising serum IL-9 level and different IL-9 gene polymorphism have been described in T2D patients when contrasted with controls, highlighting the role of IL-9 in the pathogenesis of T2D.15,25,26 Moreover, increased IL-9 level has been reported in severely obese patients with T2D by comparison to those without T2D.27 There is also a significant difference in circulating IL-9 level between hypertensive patients and controls, and IL-9 knockdown improves vascular function and reduces blood pressure in an animal hypertension model.28 Zalm et al reported that obese patients (52% of whom met the diagnostic criteria for MetS) had higher serum IL-9 levels than that of healthy controls. However, few studies have focused on the relationship between IL-9 level and MetS. Although Pilatz et al performed an analysis on serum IL-9 level, the concentration of IL-9 in all serum samples was zero, probably because of the accuracy of the testing methods.29
We further analyzed the correlation of IL-9 with IL-17A, PASI and MetS. IL-9 positively correlated with IL-17A; however, there was no significant correlation between IL-9 concentration and PASI. However, a recent report stated that IL-9 has a significant association with both PASI and IL-17.13 IL-9 potentiates the differentiation of CD4+ T cells into Th17 cells30 and IL-17A production in psoriasis.31 Anti-IL-17 antibody treatment decreases IL-9 level in K5.hTGF-b1 transgenic mice.12 Altogether, these observations and our results together indicate a positive feedback loop between IL-9 and IL-17A. Additionally, we found that IL-9 level positively correlated with the presence of MetS and its components. Correspondingly, IL-9 level had a positive correlation with WC, BMI, HOMA-IR, blood pressure, and triglyceride level, and negatively correlated with HDL-C level. In line with our results, Yang et al reported a positive correlation between IL-9 levels and blood pressure.28 Summarily, our results suggest that IL-9 may be one of the cytokines involved in systemic inflammation in the context of PV with MetS and may serve as a link between PV and MetS.
Previous investigations have provided clues regarding the underlying mechanism. IL-17/Th17 cells have been demonstrated to play a critical function in obesity, insulin resistance, and T2D,32,33 and the direct effects of IL-17A on reprogramming adipocytes, which promote MetS, have been identified.34 The interplay between IL-9 and the Th17 pathway supports the possibility that IL-9 is involved in the pathogenesis of MetS and its components. Moreover, IL-9 enhances the polarization and proliferation of M1 macrophages in vivo and in vitro.35 The significance of M1 macrophages, the proinflammatory phenotype, has been highlighted in adipose tissue inflammation and the development and progression of MetS.36,37 Therefore, another potential mechanism for the association between IL-9 and MetS may be that IL-9 affects macrophage M1/M2 polarization in adipose tissue.
This study revealed that IL-9 stimulated antimicrobial peptides and chemokines expression in a psoriatic keratinocyte model. These inflammatory cytokines are involved in the pathogenesis of psoriasis by activating innate immunity or recruiting leukocytes.38 Enhanced vascular endothelial growth factor and CXCL8 secretion have been reported in primary normal human keratinocytes following IL-9 treatment.39,40 IL-9 is also associated with the immune-mediated development of psoriasis through its interaction with the Th17 pathway.12,13 Our findings add to the accumulating evidence that IL-9 is a significant cytokine in the development of psoriasis partly through its direct impact on keratinocytes.
Our study has certain limitations. First, this was a single-centered study; therefore, greater sample numbers collected from multiple centers would contribute to more precise results. Second, we could not match the two PV groups by age; however, no correlation between IL-9 and age was shown in the correlation analysis. Finally, we only explored the effect of IL-9 on the psoriatic keratinocyte model. Therefore, the effect of IL-9 on psoriasis in the context of MetS in vivo should be investigated in further studies.
Conclusion
Our study contributes to further understanding of the cytokine profiles underlying the association between PV and MetS. This study suggests that higher IL-9 level is associated with PV complicated by MetS and IL-9 has a positive correlation with IL-17A and the presence of MetS. These findings indicate that IL-9 may be a link between PV and MetS. However, more information on the relationship between IL-9 and PV with MetS is required.
Abbreviations
PV, psoriasis vulgaris; MetS, metabolic syndrome; IL, interleukin; CVD, cardiovascular disease; T2D, type 2 diabetes; IL-9R, interleukin 9 receptor; PVMS, PV patients with metabolic syndrome; PVNMS, PV patients without metabolic syndrome; WC, waist circumference; BMI, body mass index; PASI, psoriasis area severity index; FBG, fasting blood glucose; TG, triglycerides; HDL-C, high-density lipoprotein cholesterol; HOMA-IR, homeostasis model assessment-insulin resistance index; CDS, Chinese Diabetes Society; ELISA, enzyme-linked immunosorbent assay; NHEKs, Normal human epidermal keratinocytes; IHC, immunohistochemistry; IOD, integrated optical density; qRT-PCR, quantitative real-time polymerase chain reaction; S100A7, S100 calcium-binding protein A7; S100A8, S100 calcium-binding protein A8; S100A9, S100 calcium-binding protein A9; CXCL8, C-X-C motif chemokine ligand 8; IL-1β, interleukin one beta; HBD-2, human beta-defensin 2.
Data Sharing Statement
The data are not available to the public because of participant confidentiality.
Ethics Approval and Informed Consent
Our study was approved by Chinese PLA General Hospital Ethics Committee (ethics number: S2018-223-02). The research was conducted in conformity with the Helsinki Declaration. Each participant completed written informed consent.
Funding
This research was supported by the Beijing Natural Science Foundation of China under Grant 7224344 and the National Natural Science Foundation of China under Grant 82273530.
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. Korman NJ. Management of psoriasis as a systemic disease: what is the evidence? Br J Dermatol. 2020;182(4):840–848. doi:10.1111/bjd.18245
2. Griffiths CEM, Armstrong AW, Gudjonsson JE, Barker J. Psoriasis. Lancet. 2021;397(10281):1301–1315. doi:10.1016/s0140-6736(20)32549-6
3. Blauvelt A, Chiricozzi A. The immunologic role of IL-17 in psoriasis and psoriatic arthritis pathogenesis. Clin Rev Allergy Immunol. 2018;55(3):379–390. doi:10.1007/s12016-018-8702-3
4. Hao Y, Zhu YJ, Zou S, et al. Metabolic syndrome and psoriasis: mechanisms and future directions. Front Immunol. 2021;12:711060. doi:10.3389/fimmu.2021.711060
5. Nilsson PM, Tuomilehto J, Rydén L. The metabolic syndrome - What is it and how should it be managed? Eur J Prev Cardiol. 2019;26(2_suppl):33–46. doi:10.1177/2047487319886404
6. Singh S, Young P, Armstrong AW. An update on psoriasis and metabolic syndrome: a meta-analysis of observational studies. PLoS One. 2017;12(7):e0181039. doi:10.1371/journal.pone.0181039
7. Rodríguez-Zúñiga MJM, García-Perdomo HA. Systematic review and meta-analysis of the association between psoriasis and metabolic syndrome. J Am Acad Dermatol. 2017;77(4):657–666.e8. doi:10.1016/j.jaad.2017.04.1133
8. Liu L, Cai XC, Sun XY, et al. Global prevalence of metabolic syndrome in patients with psoriasis in the past two decades: current evidence. J Eur Acad Dermatol Venereol. 2022;36(11):1969–1979. doi:10.1111/jdv.18296
9. Langan SM, Seminara NM, Shin DB, et al. Prevalence of metabolic syndrome in patients with psoriasis: a population-based study in the United Kingdom. J Invest Dermatol. 2012;132(3 Pt 1):556–562. doi:10.1038/jid.2011.365
10. Coimbra S, Oliveira H, Neuparth MJ, et al. Systemic inflammation and proinflammatory interleukin-17 signalling persist at the end of therapy in patients with metabolic syndrome and psoriasis, reducing the length of remission. Br J Dermatol. 2016;174(2):414–416. doi:10.1111/bjd.14013
11. Snekvik I, Nilsen TIL, Romundstad PR, Saunes M. Metabolic syndrome and risk of incident psoriasis: prospective data from the HUNT Study, Norway. Br J Dermatol. 2019;180(1):94–99. doi:10.1111/bjd.16885
12. Singh TP, Schön MP, Wallbrecht K, Gruber-Wackernagel A, Wang XJ, Wolf P. Involvement of IL-9 in Th17-associated inflammation and angiogenesis of psoriasis. PLoS One. 2013;8(1):e51752. doi:10.1371/journal.pone.0051752
13. Midde HS, Priyadarssini M, Rajappa M, et al. Interleukin-9 serves as a key link between systemic inflammation and angiogenesis in psoriasis. Clin Exp Dermatol. 2021;46(1):50–57. doi:10.1111/ced.14335
14. Dalmas E, Rouault C, Abdennour M, et al. Variations in circulating inflammatory factors are related to changes in calorie and carbohydrate intakes early in the course of surgery-induced weight reduction. Am J Clin Nutr. 2011;94(2):450–458. doi:10.3945/ajcn.111.013771
15. Hang H, Yuan S, Yang Q, Yuan D, Liu Q. Multiplex bead array assay of plasma cytokines in type 2 diabetes mellitus with diabetic retinopathy. Mol Vis. 2014;20:1137–1145.
16. Sun L, Guo X, Qin Y, et al. Serum intestinal metabolites are raised in patients with psoriasis and metabolic syndrome. Clin Cosmet Investig Dermatol. 2022;15:879–886. doi:10.2147/CCID.S351984
17. Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabet Care. 2004;27(6):1487–1495. doi:10.2337/diacare.27.6.1487
18. Zheng Q, Kuai L, Jiang W, et al. Clinical feature, lifestyle behavior and non-communicable diseases comorbidities among psoriasis patients in shanghai: gender disparity analysis based on a cross-sectional study. Clin Cosmet Investig Dermatol. 2022;15:2751–2762. doi:10.2147/ccid.S393697
19. Chakraborty S, Kubatzky KF, Mitra DK. An update on interleukin-9: from its cellular source and signal transduction to its role in immunopathogenesis. Int J Mol Sci. 2019;20(9):1.
20. Guilloteau K, Paris I, Pedretti N, et al. Skin inflammation induced by the synergistic action of IL-17A, IL-22, oncostatin M, IL-1α, and TNF-α recapitulates some features of psoriasis. J Immunol. 2010;184(9):5263–5270. doi:10.4049/jimmunol.0902464
21. Wang Z, Zhou H, Zheng H, et al. Autophagy-based unconventional secretion of HMGB1 by keratinocytes plays a pivotal role in psoriatic skin inflammation. Autophagy. 2021;17(2):529–552. doi:10.1080/15548627.2020.1725381
22. Cardoso PR, Lima EV, Lima MM, et al. Clinical and cytokine profile evaluation in Northeast Brazilian psoriasis plaque-type patients. Eur Cytokine Netw. 2016;27(1):1–5. doi:10.1684/ecn.2016.0371
23. Michalak-Stoma A, Bartosińska J, Raczkiewicz D, et al. Multiple cytokine analysis of Th1/Th2/Th9/Th17/Th22/Treg cytokine pathway for individual immune profile assessment in patients with psoriasis. Med Sci Monit. 2022;28:e938277. doi:10.12659/msm.938277
24. Ciccia F, Guggino G, Ferrante A, et al. Interleukin-9 overexpression and Th9 polarization characterize the inflamed gut, the synovial tissue, and the peripheral blood of patients with psoriatic arthritis. Arthritis Rheumatol. 2016;68(8):1922–1931. doi:10.1002/art.39649
25. Varshney P, Parveen R, Khan MA, Kohli S, Agarwal NB. Increased serum interleukin-9 and interleukin-1β are associated with depression in type 2 diabetes patients. Arq Neuropsiquiatr. 2020;78(5):255–261. doi:10.1590/0004-282x20190177
26. Mohammed H, Salloom DF. Evaluation of interleukin-9 serum level and gene polymorphism in a sample of Iraqi type 2 diabetic mellitus patients. Meta Gene. 2021;27:100845. doi:10.1016/j.mgene.2020.100845
27. D’Esposito V, Ambrosio MR, Liguoro D, et al. In severe obesity, subcutaneous adipose tissue cell-derived cytokines are early markers of impaired glucose tolerance and are modulated by quercetin. Int J Obes. 2021;45(8):1811–1820. doi:10.1038/s41366-021-00850-1
28. Yang Y, Tang S, Zhai C, et al. Interleukin-9 deletion relieves vascular dysfunction and decreases blood pressure via the STAT3 pathway in angiotensin II-treated mice. Mediators Inflamm. 2020;2020:5741047. doi:10.1155/2020/5741047
29. Pilatz A, Hudemann C, Wolf J, et al. Metabolic syndrome and the seminal cytokine network in morbidly obese males. Andrology. 2017;5(1):23–30. doi:10.1111/andr.12296
30. Elyaman W, Bradshaw EM, Uyttenhove C, et al. IL-9 induces differentiation of TH17 cells and enhances function of FoxP3+ natural regulatory T cells. Proc Natl Acad Sci USA. 2009;106(31):12885–12890. doi:10.1073/pnas.0812530106
31. Ruiz-Romeu E, Ferran M, de Jesús-Gil C, et al. Microbe-dependent induction of IL-9 by CLA(+) T cells in psoriasis and relationship with IL-17A. J Invest Dermatol. 2018;138(3):580–587. doi:10.1016/j.jid.2017.08.048
32. Zhang S, Gang X, Yang S, et al. The alterations in and the role of the Th17/Treg balance in metabolic diseases. Front Immunol. 2021;12:678355. doi:10.3389/fimmu.2021.678355
33. Abdel-Moneim A, Bakery HH, Allam G. The potential pathogenic role of IL-17/Th17 cells in both type 1 and type 2 diabetes mellitus. Biomed Pharmacother. 2018;101:287–292. doi:10.1016/j.biopha.2018.02.103
34. Teijeiro A, Garrido A, Ferre A, Perna C, Djouder N. Inhibition of the IL-17A axis in adipocytes suppresses diet-induced obesity and metabolic disorders in mice. Nat Metab. 2021;3(4):496–512. doi:10.1038/s42255-021-00371-1
35. Park SM, Do-Thi VA, Lee JO, Lee H, Kim YS. Interleukin-9 inhibits lung metastasis of melanoma through stimulating anti-tumor M1 macrophages. Mol Cells. 2020;43(5):479–490. doi:10.14348/molcells.2020.0047
36. Reddy P, Lent-Schochet D, Ramakrishnan N, McLaughlin M, Jialal I. Metabolic syndrome is an inflammatory disorder: a conspiracy between adipose tissue and phagocytes. Clin Chim Acta. 2019;496:35–44. doi:10.1016/j.cca.2019.06.019
37. Castoldi A, Naffah de Souza C, Câmara NO, Moraes-Vieira PM. The macrophage switch in obesity development. Front Immunol. 2015;6:637. doi:10.3389/fimmu.2015.00637
38. Zhou X, Chen Y, Cui L, Shi Y, Guo C. Advances in the pathogenesis of psoriasis: from keratinocyte perspective. Cell Death Dis. 2022;13(1):81. doi:10.1038/s41419-022-04523-3
39. Ma L, Xue HB, Guan XH, Shu CM, Zhang JH, Yu J. Possible pathogenic role of T helper type 9 cells and interleukin (IL)-9 in atopic dermatitis. Clin Exp Immunol. 2014;175(1):25–31. doi:10.1111/cei.12198
40. Hong CH, Chang KL, Wang HJ, Yu HS, Lee CH. IL-9 induces IL-8 production via STIM1 activation and ERK phosphorylation in epidermal keratinocytes: a plausible mechanism of IL-9R in atopic dermatitis. J Dermatol Sci. 2015;78(3):206–214. doi:10.1016/j.jdermsci.2015.03.004
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.